Abstract
A hallmark of voluntary motor control is the ability to stop an ongoing movement. Is voluntary motor inhibition a general neural mechanism that can be focused on any movement, including involuntary movements, or is it mere termination of a positive voluntary motor command? The involuntary arm lift, or ‘floating arm trick’, is a distinctive long-lasting reflex of the deltoid muscle. We investigated how a voluntary motor network inhibits this form of involuntary motor control. Transcranial magnetic stimulation of the motor cortex during the floating arm trick produced a silent period in the reflexively contracting deltoid muscle, followed by a rebound of muscle activity. This pattern suggests a persistent generator of involuntary motor commands. Instructions to bring the arm down voluntarily reduced activity of deltoid muscle. When this voluntary effort was withdrawn, the involuntary arm lift resumed. Further, voluntary motor inhibition produced a strange illusion of physical resistance to bringing the arm down, as if ongoing involuntarily generated commands were located in a ‘sensory blind-spot’, inaccessible to conscious perception. Our results suggest that voluntary motor inhibition may be a specific neural function, distinct from absence of positive voluntary motor commands.
Keywords: voluntary control, voluntary inhibition, involuntary contraction, Kohnstamm, arm movement, motor cortex
1. Introduction
Voluntary control of one's actions is a key feature of human behavioural control. Interestingly, the capacity to inhibit actions is considered an important element of voluntary control [1,2]. The most obvious example of such inhibition comes when we voluntarily decide to terminate a voluntary motor command. Several studies confirm that voluntary termination involves similar cortical preparation and programming to voluntary initiation of movement. For example, a readiness potential may precede the voluntary termination of an isometric muscle contraction [3]. These studies suggest the existence of a functional ‘negative motor command’, possibly related to the finding of negative motor areas where direct electrical stimulation leads to slowing or cessation of ongoing voluntary movement [4].
However, the neuronal mechanism for intentional inhibition of involuntary movement is not clear. In particular, it is not clear whether ongoing involuntary movement can be suppressed at will, in the same way that one can voluntarily terminate or withhold a voluntary action. The most relevant human data come from patients with involuntary movements. Some involuntary movements, such as Parkinsonian tremor and cerebellar tremor, appear to be immune from voluntary control [5,6]. Here a range of very different inhibitory strategies is found, according to the very different forms of involuntary movement that occur following different forms of neural damage. Patients with frontal release signs such as anarchic hand have great difficulty in voluntarily suppressing movements that are involuntarily triggered by environmental stimuli. Della Sala et al. [7] report a patient who resorted to voluntary movements of the unaffected hand in order to restrain the involuntary movements of their anarchic hand. Further, in ‘geste antagoniste’, dystonia patients may learn voluntary actions that produce tremor suppression as a by-product, because of sensory involvement in tremorgenic circuits [8]. Both these examples demonstrate a physical form of inhibition achieved by a positive voluntary motor command. On the other hand, tic disorders, such as Gilles de la Tourette's syndrome, provide evidence of a central voluntary inhibition of involuntary movements. Many patients are able to suppress their tics on demand [9]. The underlying mechanism is not well understood, but most studies agree that a widespread frontal network is involved in voluntary tic suppression [10].
Classic experimental models of involuntary movement are brief, while inhibitory control processes are typically slow [11,12]. For instance, the long latency stretch reflexes in the arm last for only 100 ms [13]. Studies show that intentional control presets the gain of these brief reflexes [13–15], but the time scale of the individual reflexes makes it impossible to test whether reflexes can be intentionally countermanded after initiation.
A long-lasting involuntary movement provides a better experimental model for investigating the voluntary inhibition of an ongoing involuntary movement. For instance, in Kohnstamm's manoeuvre, a sustained isometric voluntary effort involving the deltoid muscle is followed by an involuntary contraction in the same muscle that can last for more than half a minute [16]. The involuntary contraction results in a slow arm lift, experienced as if a ‘hidden force’ were lifting the arm. Interestingly, such long-lasting involuntary contractions have been observed in the wrist, ankle, neck, knee and the hip [17–19]. We know little about the functional role of these contractions, but due to their larger amplitude in the proximal versus distal muscles they presumably play a role in maintaining body posture [20].
The extent of peripheral as opposed to central contribution towards this movement remains contentious (see [21] for review). In the periphery, the sustained voluntary contraction used for the induction generates a barrage of asymmetric proprioceptive inputs from antagonist and agonist muscles that may contribute to the involuntary movement [19,22]. Furthermore, such prolonged sensory activations or alterations in ‘muscle history’ are also associated with after-effects such as in inaccurate conscious perception of limb position [23]. Conceptually, a continuous flow of sensory inputs from the altered peripheral receptors may provide sufficient drive for motor neurons associated with the involuntary arm lift. Still, the peripheral changes alone cannot fully explain the involuntary movement production. For instance, the involuntary movement may outlast the estimated duration of the peripheral sensory alterations induced by the voluntary contraction—by several minutes to hours [21,24,25]. Furthermore, sustained voluntary contraction in one muscle may trigger involuntary motor after-effects in other muscles [21]. Perhaps the most remarkable demonstration of a central contribution towards the Kohnstamm movement comes from a report on how imagining the effort necessary for the voluntary contraction induces a long-lasting involuntary arm lift [26]. In that case, the same neuronal circuits responsible for the Kohnstamm were presumably recruited by the motor imagery.
Non-invasive brain stimulation and imaging methods have shed some light on brain structures involved in the Kohnstamm phenomenon. Transcranial magnetic stimulation (TMS) of the motor cortex results in larger motor evoked potentials (MEPs) during the involuntary arm lift than at rest [27]. This suggests that neurons of the corticospinal pathway are involved in the involuntary movement production. Furthermore, fMRI studies reveal a wide network of brain structures associated with the Kohnstamm movement, including the motor cortex [17,28]. Based on these studies, it cannot be concluded that the generation is entirely central as the activations may reflect the brain areas involved in monitoring rather than producing the Kohnstamm movement. Still, these studies do suggest that Kohnstamm movement may interact with the products of higher brain function. Indeed, behavioural studies have shown that the involuntary Kohnstamm movement integrates with visual perception and attention [19,29].
In summary, the Kohnstamm generator is either a ‘tonogenic’ central network triggered by the peripheral inputs and/or effort signals, or it is a collection of central neurons that drive the involuntary movement based on persistent peripheral inputs [30]. What is important for this report is that long-lasting central motor outputs (cortical and/or spinal) undoubtedly drive the Kohnstamm movement and this permits new theoretical questions on how voluntary inhibitory commands interact with ongoing involuntary motor outputs.
In theory, intentional inhibition of an ongoing involuntary movement could be achieved in either of two ways. First, the brain could send a voluntary motor command to activate additional, antagonistic muscles to stop and overpower the involuntary movement. Several studies confirm voluntarily co-contraction to stabilize a joint or to stop an ongoing action (e.g. [31]). Contracting the antagonist is effectively similar to the physical form of inhibition used by some movement disorder patients, as described above. Second, intentional inhibition could switch off the agonist muscle generating the involuntary movement. This possibility would involve a ‘negative motor command’, either at the level of spinal motor neurons or above. The concept of a ‘negative motor command’ may seem paradoxical but is consistent with several neurophysiological observations. Stimulating the cortical motor areas can result in inhibition of ongoing movement [4,32]. Negative motor commands might cut the involuntary command at its source. Alternatively, they might leave the involuntary generator intact, but add an additional inhibitory command that sums with and overrides involuntary drive. Nevertheless, previous studies provide little insight into which of these mechanisms is used to return body movement to voluntary control during a reflex.
Previous studies have addressed how the Kohnstamm movement integrates with locomotion, and how drugs, limb position, visual inputs, weights and attention influence its amplitude [20,33–36]. Interestingly, according to a previous report the Kohnstamm movement and the corresponding deltoid muscular contractions is entirely prevented when the arm is voluntarily restrained prior to the involuntary arm lift, but the report remained inconclusive on whether the voluntary inhibition can act after the involuntary movement onset [37]. Here, we focused on the Kohnstamm manoeuvre to investigate the interaction between the slow processes of intentional inhibition and the involuntary movement. We examined whether continued involuntary movement of the arm could be intentionally inhibited, and consider the neural mechanisms and subjective consequences of this voluntary–involuntary motor interaction.
2. Material and methods
(a). Experimental volunteers
Male and female volunteers between the ages of 18 and 60 participated in this study on the basis of written informed consent. A total of 39 individuals were considered for this study. Twenty-six of them participated in the perceptual experiments and five of them did not have a Kohnstamm. From nine of the 21 volunteers who did display a Kohnstamm, we recorded muscular activity during voluntary inhibition of the Kohnstamm. In eight additional volunteers, we addressed whether voluntary inhibition of the Kohnstamm was long-lasting. Five volunteers were recruited specifically for TMS experiments.
(b). Induction of the Kohnstamm
Volunteers stood to push against a wall for 40–60 s with 40–60% of maximum voluntary force (forces measured using 12.7 mm force-sensitive resistor; SparkFun Electronics, USA; for contraction measurements see below). During the push, the arm was abducted with an angle of 15° at the shoulder joint. After the push, they were instructed to step away from the wall and ‘relax’, and ‘not to interfere with any upwards or downwards movement of the arm’. Volunteers were able to comfortably go through all this for six Kohnstamm movements (three on the right arm and three left) with a break of 5–10 min between each trial. Half of the trials were randomly chosen to obey the instruction ‘gently bring the arm back down and actively keep it down’ and the instruction was delivered only after the Kohnstamm was induced (as indicated by the lift up). Upon return, the arm was maintained at the rest angle between 5° and 12°. In the baseline voluntary up and down condition, the arm was first abducted without pushing the wall, and this posture was maintained for 40–60 s. The trials in this condition were randomly presented along with the Kohnstamm trials.
For the experiments with multiple Kohnstamm movements, after the initial Kohnstamm induction the volunteers were asked to ‘gently bring the arm back down and actively keep it down’ at the rest angle for 1–3 s.
(c). Muscular recordings, brain stimulation and kinematics
Electromyography was recorded using two pairs of disposable neonatal ECG electrodes placed over the deltoid and pectoralis muscles. The signal was amplified with a gain of 1000× in the presence of a 50 Hz notch filter (Cambridge Electronic Devices, UK). The data were digitized at 2000 Hz (NI USB 6008 DAQ, National Instruments, USA) and filtered off-line using a bandpass filter between 10 and 950 Hz. The electrode placements were functionally verified by instructing volunteers to ‘lift the arm’ for the deltoid and to ‘bring it back down’, countering an upward force from the experimenter for the pectoralis muscle.
TMS was applied over the motor hotspot for the deltoid muscle using a figure-of-eight coil with an outer diameter of 70 mm (Magstim, UK). This area was located using exploratory pulses while the subject voluntarily contracted the deltoid muscle lifting the arm up to shoulder level. Next, the coil was maintained over this spot and the stimulation threshold was determined at the resting posture (i.e. the smallest stimulation intensity at which MEPs of 1 mV were detected in at least three of five consecutive pulses). To probe the motor cortex during the Kohnstamm, we used single TMS pulses (300 µs) at 120% of the resting threshold, and consecutive pulses were separated by about 4 s. The data from the pulses when the Kohnstamm reached its maximum were compared to a matched voluntary lift of the arm in terms of signal amplitude and silent period. We also compared the mean rectified EMG signals from two 100 ms bins—one before TMS and another 200 ms after TMS. In each subject, the peak-to-peak amplitudes of the six MEPs, the rectified signal amplitudes pre–post-TMS and the duration of the six silent periods induced by TMS were averaged. Mean values ± inter-subject s.e.m. will be presented.
In experiments involving the voluntary suppression of Kohnstamm, the rectified EMG signals from the involuntary movement onset to offset (threshold +2 s.d. from baseline) were quantified. We used integrated EMG to quantify the effectiveness the inhibition (i.e. its net effect on the amplitude and duration of the signal). Mean EMG was used to quantify the overall muscular activity (i.e. to address whether the levels of the second involuntary contractions were altered after the inhibition of the first Kohnstamm). Essentially, the latter allowed us to compare EMG levels even though the duration of the involuntary contractions were distinct by design.
To measure limb movements we used a pair of visible kinematic markers (LEDs)—one placed on the shoulder joint and the other above the elbow joint. The LEDs were placed on the back such that they were invisible to the volunteer. The data were recorded using a 60 fps camera (Toshiba Camileo, Japan) and processed by using image-processing software (ImageJ, USA). Angular displacement around the shoulder joint was estimated by using an object tracker (SpotTracker, Switzerland; ImageJ plug-in) and custom written scripts (MATLAB, USA).
(d). Perceptual measurements
Volunteers verbally rated the perceived resistance and the descriptors of resistance while bringing the arm back down on 1–50 and 1–5 scales, respectively, at the end of each trial. For these experiments, volunteers were separated as Kohnstamm-positive (who showed an involuntary arm lift; n = 21) and Kohnstamm-negative (who did not show an involuntarily lift in identical conditions; n = 5).
The measurements on the 21 Kohnstamm-positive volunteers were conducted under three different conditions: (i) Kohnstamm alone—where volunteers were instructed to remain relaxed after pushing the wall. (ii) Kohnstamm followed by voluntary down—where subjects brought their arm down against the involuntary movement. (iii) Voluntary up and down—where subjects did not push the wall, lifted their arm up on their own and then brought it back down. In this condition, the amplitude and duration of the movement was matched to the Kohnstamm in condition (i) as closely as possible. In addition to these conditions 10 of the 21 subjects were also instructed to elevate the arm to the shoulder level and to maintain the posture for about a minute, and then instructed to bring the arm down as in condition (ii) and (iii). The data presented in the figure 2e–f are from the 21 participants who did express the Kohnstamm movement. Each condition consisted of three trials, and the ratings were averaged for each participant and condition. Mean values ± inter-subject s.e.m. will be presented.
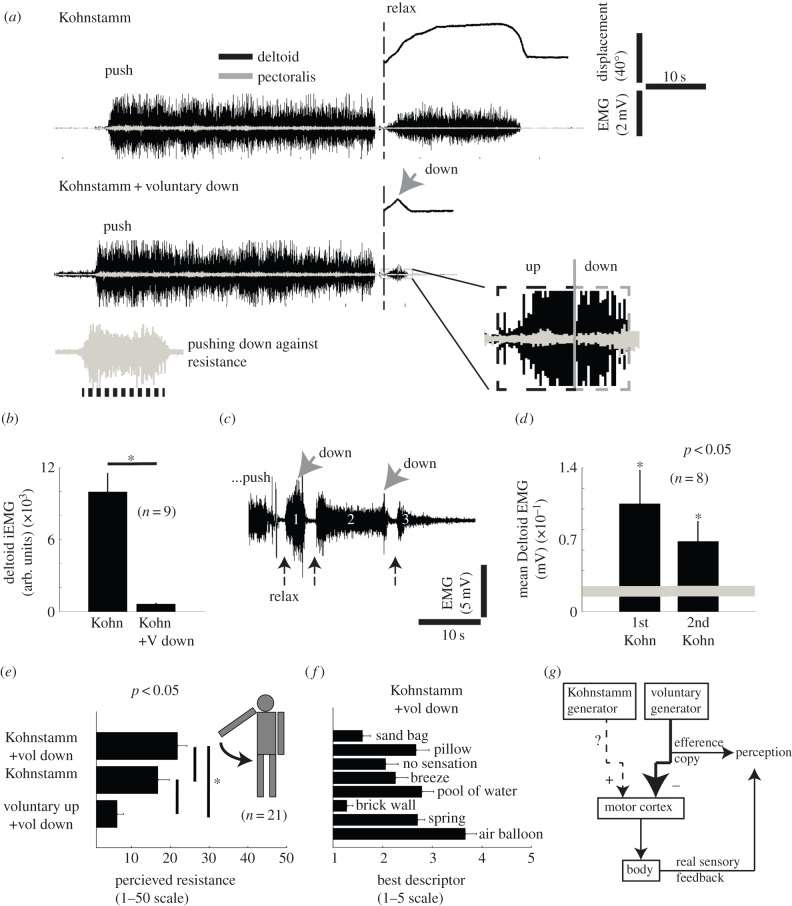
Figure 2.
Muscular activity and illusion of external resistance when voluntarily bringing the arm down against the Kohnstamm lift. (a) The muscular contraction in the deltoid muscle is reduced when bringing the arm down voluntarily against the Kohnstamm. Notice the absence of detectable change in the antagonistic Pectoralis muscle when the arm is brought down versus when the arm rises up. When pushing down against a resistance introduced by the experimenter, the Pectoralis muscle was clearly activated. (b) The integrated EMG of the deltoid muscle during a Kohnstamm lift (Kohn) and when instructed to bring the arm down after the induction of a Kohnstamm (Kohn + V down). (c) A representative deltoid EMG trace showing a second Kohnstamm lift re-occurring after the arm has been brought down voluntarily against the Kohnstamm. After a push (partial view), the instruction to relax (black arrow) induces the first Kohnstamm (1) and subsequently the muscular activity is reduced to bring the arm down voluntarily (grey arrow). Relaxing again induces a second (2) or even third (3) involuntary arm lift. (d) Mean EMG allowed us to compare the deltoid muscle activity in the first and the second Kohnstamm to the baseline (grey line, thickness represents s.e.m.). (e) Perceptual ratings for the resistance felt during the downward movement of the arm (n = 21). Scale: 1, no resistance; 50, strong resistance. (f) Volunteers also rated a list of options on a 1–5 scale. A rating of 1 indicates strong disagreement with the descriptor, and 5 indicates strong agreement. (g) A conceptual sketch based on the key findings presented here. The inhibitory voluntary commands (−) win over the weaker involuntary excitatory outputs (+) presumably (dashed line) in the motor cortex. The efference copy of the voluntary generator is available for perception. The involuntary movement generator does not produce efference copy available for perception, and its contribution is misattributed to external sources (*p < 0.05).
In the five volunteers who did not express a Kohnstamm movement, after the push against the wall an experimenter lifted the arm up and then the volunteer actively brought the arm down. The volunteers reported the perceived resistances while bringing the arm down. The data from these experiments are mentioned in the text. This group of volunteers without the Kohnstamm allowed us to directly address how pushing the wall alone and the peripheral changes associated with the push influences perception of external resistance during the downward arm movement.
(e). Statistics
In the experiments using TMS, MEPs were compared between voluntary and Kohnstamm conditions by using a paired Wilcoxon signed-rank test (MATLAB). The same test was also used to compare the silent periods between the two conditions. Mean EMG levels before and after the TMS, in the voluntary and Kohnstamm conditions, were tested by using the Kruskal–Wallis non-parametric analysis of variance (MATLAB). In the experiments involving the voluntary suppression of the Kohnstamm movement, the integrated EMG in the uninterrupted and suppressed conditions was compared using the paired Wilcoxon signed-rank test. The first and the second Kohnstamm post-suppression were compared against the same baseline using Kruskal–Wallis non-parametric analysis of variance followed by paired Wilcoxon signed-rank tests. The same two-stage non-parametric tests were used to test for the impact of Kohnstamm on perceived resistance. The level of significance was set at p < 0.05 for all the tests.
3. Results
(a). Transcranial magnetic stimulation of the motor cortex during Kohnstamm
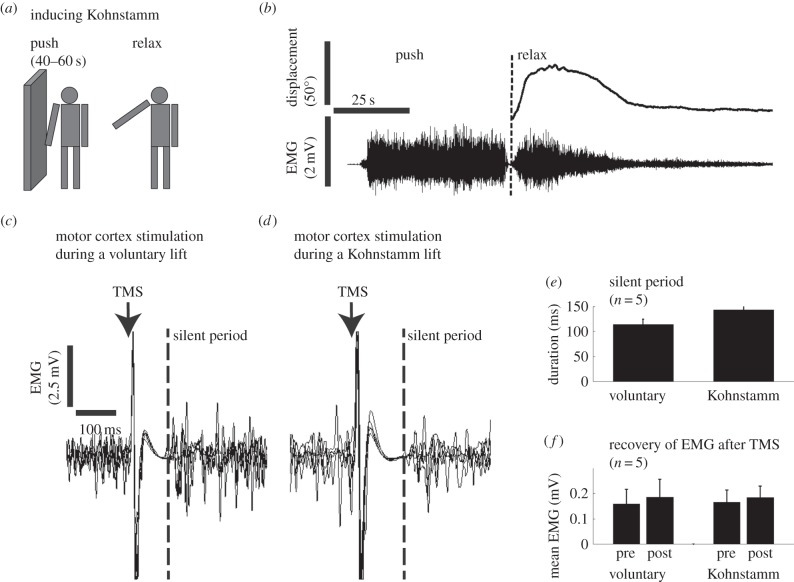
We used TMS to investigate the relationship between the neural centres generating the Kohnstamm and the primary motor cortex. The motor cortex sends voluntary motor commands to the spinal cord but can also be involved in involuntary movements [17,38–42]. We found that the motor cortical TMS had similar effects during both the voluntary and Kohnstamm arm lifts (figure 1c–f). First, TMS produced MEP in the deltoid muscle (peak to peak amplitudes: voluntary 4.8 mV ±1.6 s.e.m. and Kohnstamm 5.2 mV ±1.4 s.e.m., n = 5, p = n.s.). The MEP was followed by a period of reduced muscle activity or ‘silent period’ (voluntary 114 ms ±11 s.e.m. and Kohnstamm 143 ms ±13 s.e.m., n = 5, p = n.s.). Interestingly, the Kohnstamm EMG rebounded to its previous level after the silent period (pre-TMS mean rectified EMG 0.19 mV ±0.05 s.e.m., 200 ms post-TMS 0.19 mV ±0.07 s.e.m., n = 5, p = n.s.). In sum, artificially stimulating the motor cortex inhibited expression of the Kohnstamm, yet the Kohnstamm generator was not reset, but continued to work in the background.
Figure 1.
TMS of the motor cortex during a Kohnstamm lift and voluntary arm lift. (a) The Kohnstamm was induced by pushing against a wall and then stepping away to relax the deltoid muscle. (b) Kinematic and EMG traces of the Kohnstamm lift. The surface EMG electrodes were placed on the deltoid muscle. (c,d) TMS of the motor cortex during (c) voluntary and (d) Kohnstamm lift results in a prolonged silent period, suggesting a cortical origin (representative volunteer's data; six traces are superimposed. (e) Mean silent period. (f) The muscular contractions made a full recovery after the silent period. Pre and post measures based on 100 ms bins 10 ms before and 200 ms after TMS.
(b). Intentional inhibition of the Kohnstamm
Next, we instructed volunteers to ‘bring the arm back down’ during the involuntary arm lift. In theory, this could be done by activating antagonists, or by voluntarily inhibiting the involuntary contraction of the deltoid, thus letting the arm fall. Interestingly, we found no evidence for activation of the antagonist (pectoralis), and a clear reduction of deltoid EMG, suggesting the latter mechanism was used (figure 2a,b; pectoralis mean rectified EMG when going up 6.8 μV ±0.6 s.e.m. and 6.6 μV ±0.6 s.e.m. down, n = 9, p = n.s.). Interestingly, intentional inhibition of the Kohnstamm was not achieved by physical resistance using the antagonist, but by reducing the command finally emitted from the spinal cord.
Since the Kohnstamm generator persisted through the brief cortical silent period induced by motor cortical TMS, we investigated whether its activity also persisted during voluntary inhibition. When we instructed volunteers to relax after voluntarily bringing the floating arm down, the arm began to involuntarily lift up again (figure 2c,d). Thus, intentional inhibition of the Kohnstamm could override the involuntary arm lift, but did not suppress the Kohnstamm generator itself.
Thus, voluntary inhibition had similar inhibitory effects on the deltoid muscle to cortical stimulation.
(c). Perceptual consequences of inhibiting the Kohnstamm
The Kohnstamm generates a strange experience of automatic, involuntary movement, quite different from normal agency. Early reports suggested that the floating arm feels ‘lighter’ than the same arm during voluntary movement [16,43]. However, the subjective experience of voluntarily inhibiting the Kohnstamm has not been studied systematically. We found in pilot testing that people regularly perceived and spontaneously reported a feeling of external physical resistance when trying to voluntarily bring the arm down. We tested this experience systematically in a perceptual experiment (n = 26). We could induce a Kohnstamm in 21 of these participants, and the five Kohnstamm participants who did not express the involuntary movement were analysed separately (see below). We asked the 21 participants to rate the perceived external resistance they felt when voluntarily bringing the arm down against the Kohnstamm (figure 2e), during the arm drop as the Kohnstamm fades, and in a baseline condition involving a voluntary downward arm movement without any Kohnstamm. Numerical ratings showed that strongest resistance was felt when voluntarily bringing the arm down against the Kohnstamm. In addition, participants rated eight descriptors of the external movement environment, while voluntarily bringing the arm down against the Kohnstamm. Sixteen of the 21 volunteers gave strongest agreement to the feeling that the descending arm was pressing against a soft air balloon (figure 2f). By contrast, in the baseline condition involving fully voluntary up and down arm movements in the absence of Kohnstamm, no participant strongly agreed with this statement. Instead they reported ‘no sensation’.
Interestingly, five of the 26 volunteers did not display a Kohnstamm. We could compare data between the volunteers with and without the Kohnstamm to investigate whether the perceptual illusion is simply due to the history of muscle contraction, or to the recruitment of a central Kohnstamm generator. We asked the ‘Kohnstamm-negative’ participants to push against the wall, as before. We then passively lifted their arm to a location characteristic of the Kohnstamm in Kohnstamm-positive participants, and asked them to voluntarily bring the arm down. This situation is physically equivalent to the voluntary inhibition condition in a ‘Kohnstamm-positive’ participant. In this situation, Kohnstamm-negative volunteers reported resistance values (8 ± 1 s.e.m., on the 1–50 scale) that were similar to their baseline values (8 ± 2 s.e.m.). Moreover, Kohnstamm-negative participants gave their strongest agreement to ‘no sensation’ to describe bringing the arm down against resistance. Therefore, the perceptual experiences reported by Kohnstamm-positive participants depended critically on recruitment of a central Kohnstamm generator, and not on any other aspect of the task.
To further investigate whether muscle contraction alone could produce illusions of resistance, we additionally instructed 10 of the 21 Kohnstamm-positive volunteers to voluntarily maintain the arm at the level of the shoulder for 60 s. When volunteers brought the arm down in a controlled manner and were instructed to relax, no reflexive muscular contraction could be detected. In other words, merely activating the muscle for a period of time did not induce a Kohnstamm (data not shown). Importantly, none of these 10 volunteers experienced a sense of resistance when bringing the arm down and they reported ‘no sensation’ to describe bringing the arm down.
4. Discussion
Integration of voluntary and involuntary motor commands has been a focus of recent neuroscientific interest [13], but remains poorly understood. Here, we first induced an involuntary, excitatory motor command, using the Kohnstamm manoeuvre. We then asked participants to voluntarily control the involuntary movement, and ‘bring the arm down’. Instead of inhibiting with a competing positive motor command, we found that they were able to produce a voluntary ‘negative motor command’ that reduced the Kohnstamm contraction in the agonist. The voluntary reduction in involuntary agonist activity was sufficient for gravity to bring the arm back down. Thus, the Kohnstamm manoeuvre produced a long-lasting contraction of the deltoid. Deltoid contraction effectively ceased when participants were instructed to voluntarily bring the floating arm down, while the end of the voluntary inhibition resulted in the rebound of the involuntary contraction.
The rebound demonstrates that the Kohnstamm motor generator itself was not reset by voluntary inhibition of the involuntary contraction. We also showed that the Kohnstamm generator continues to operate after a brief period of muscular inactivity induced by the stimulation of the motor cortex. The persistence of the Kohnstamm generator may be due to either of the following two reasons, dependent on the exact nature of the generator. If the generator were peripherally driven, as mentioned in the Introduction, then the persistence suggests that the voluntary commands did not permanently alter the sensory-motor loop necessary for the driving the generator. Alternatively, and in the more likely case of an endogenous Kohnstamm motor generator, the persistence suggests that the generator continued to operate in the background in spite of voluntary countermanding. Therefore, the voluntary inhibitory signals must converge with the involuntary processes in the central nervous system to prevent (by suppressing the sensory-motor loop) or cancel (by suppressing the output of the endogenous motor generator) the excitatory motor outputs.
The idea that the Kohnstamm movement is associated with a persistent motor generator is not entirely new. For instance, a previous theory designed to explain the Kohnstamm movement states that the movement arises due to the resetting of the proprioceptive target [28,44]. Essentially, the sustained voluntary contraction in the induction phase creates a new sensory target or ‘default’ posture and after the withdrawal of the voluntary contraction the involuntary circuits operate to maintain the ‘default’ posture. Essentially, this theory relies on a persistent Kohnstamm motor generator for postural control [30]. Our results also suggest a persistent generator in the sense that the generator remains operational in spite of a period of voluntary inhibition. Apart from the Kohnstamm, similarly persistent automatic processes have been suggested for anticipated postural adjustments, and this persistence may make it difficult to voluntarily control multiple body parts at once [45].
A particularly intriguing result was the illusion of external resistance when voluntarily moving the arm down against the Kohnstamm upwards lift. This perceptual experience cannot readily be explained by changes in peripheral inputs. Similar peripheral sensory inputs are activated by voluntary inhibition of the Kohnstamm and by the voluntary arm-lowering movement that we tested in Kohnstamm-negative participants. Moreover, the change in muscle status, from contracting and pushing against the wall to induce the Kohnstamm, to subsequently relaxing again, cannot explain our results. First, this sense of resistance was absent in a voluntary control condition where participants voluntarily lifted the arm up for 60 s, and then relaxed. This condition neither induced a Kohnstamm nor resulted in a sense of resistance when bringing the arm down again. Second, the sense of resistance was absent in the small number of our volunteers who pushed against the wall but did not display a Kohnstamm lift movement. We conclude that the illusion of resistance during voluntary inhibition of the Kohnstamm is of central rather than peripheral origin.
The illusion of resistance during the voluntary downward movement is perhaps related to the feeling of ‘lightness’ experienced during the Kohnstamm arm lift. According to a theory proposed by Gurfinkel et al., this light feeling is explained by the absence of voluntary effort, converse to the feeling of a heavy arm when the muscles are curarized [30]. In general, the experiences of voluntary action differ strongly from those of similar passive body movements. Efferent cortical motor commands are thought to underlie the distinctive ‘sense of agency’ that accompanies voluntary movement [46]. Our data suggest that the sensory consequences of the upward Kohnstamm lift are not perceived as self-generated but are incorrectly attributed to external forces. Thus, when an upward Kohnstamm lift is combined with a voluntary command to bring the arm down, the Kohnstamm generator continues to contribute an excitatory motor drive, though the muscular expression of this drive is suppressed by the over-riding negative motor command. Nevertheless, the excitatory drive from the persisting Kohnstamm generator means that the arm moves down less readily than it otherwise would during the period of voluntary inhibition. Our participants perceived this lack of responsiveness as external resistance, even though it was in fact due to the persistent operation of the Kohnstamm generator. These externalizing attributions are consistent with the hypothesis that the hypothetical Kohnstamm generator does not transmit an efference copy to the neural centres that compute conscious awareness.
Efference copy signals are also involved in the sensory attenuation that accompanies voluntary movement. Previous TMS studies of sensory attenuation show that the relevant efference copy arises upstream of the motor cortex [47,48]. One previous TMS study found that Kohnstamm contractions and voluntary contractions produced similar facilitation of corticospinal excitability [27]. Those authors assumed a subcortical generator for the Kohnstamm contraction, and therefore interpreted their data as evidence for a spinal locus for MEP facilitation. However, both neuroimaging data [17,28] and our own silent period results question their assumption, and suggest a cortical involvement in the Kohnstamm phenomenon.
In particular, our TMS data revealed a silent period over 100 ms in the involuntarily contracting muscle. Silent periods over 100 ms following TMS during voluntary contraction are an established indicator of cortical inhibition of motor outputs [49–51]. Silent periods during involuntary contraction have not previously been reported, to our knowledge. Therefore, caution is required in applying the same logic as for voluntary movements. The involuntary contraction rebounded after the silent period, again similar to the rebound after voluntary contraction. Therefore, we speculate that the Kohnstamm commands, like voluntary motor commands, may arise upstream of the motor cortex. Nevertheless, our perceptual data suggest that the Kohnstamm generator does not generate efference copy signals in the same way as voluntary motor commands. Thus, by studying the time course of the respective motor commands and their perceptual consequences, we showed that the Kohnstamm generator and the voluntary command generator are dissociable. We speculate that the two distinct drivers of Kohnstamm excitation and voluntary inhibition may converge in the motor cortex. While the former does not contribute an efference copy signal for predicting the outcome of an action, the latter does (figure 2g).
In conclusion, this report provides fresh evidence for integration of voluntary and involuntary motor control, complementing the growing body of work on the intentional modulation of reflexes [15,52]. Unlike most reflexes, the Kohnstamm lift is relatively slow and long-lasting. These properties provided us with a unique opportunity to study the voluntary inhibition of an ongoing involuntary movement. We identified a form of interaction between voluntary and involuntary movement control that seems to go beyond the mere regulation of reflex gains studied previously. Using this model, we have provided two novel insights into mechanistic motor control. First, the voluntary inhibition of involuntary actions may take the form of a ‘negative motor command’, which summates with the involuntary excitatory drive. Although not investigated here, the same mechanism could potentially be used in voluntary inhibition of other classes of action, including stimulus-triggered movements. Second, the excitatory motor drive of the Kohnstamm generator does not enter conscious awareness. Taken together with the illusions, there is a perceptual blind spot in the brain at the point where the involuntary processes associated with the Kohnstamm have their effect. However, these involuntary processes can be overridden by voluntary commands to achieve motor control.
Acknowledgement
We thank Mr Jack De Havas Gwenlan for useful discussions and Ms Sofie Strunge Meyer for assistance in data collection.
Ethics statement
The University College London Research Ethics Committee approved the experiments (project ID, 3025/001) and our conduct also conformed to the Declaration of Helsinki.
Funding statement
A.G. was supported by the Society in Science Branco Weiss Fellowship and a research grant from Vontobel Stiftung. P.H. was supported by ERC Advanced Grant HUMVOL, by a Professorial Fellowship from ESRC, and by an ESRC-ESF ECRP grant on ‘Intentional Inhibition of Human Action’.
References
- 1.Filevich E, Kuhn S, Haggard P. 2012. Intentional inhibition in human action: the power of ‘no’. Neurosci. Biobehav. Rev. 36, 1107–1118. ( 10.1016/j.neubiorev.2012.01.006) [DOI] [PubMed] [Google Scholar]
- 2.Kuhn S, Haggard P, Brass M. 2009. Intentional inhibition: how the ‘veto-area’ exerts control. Hum. Brain Mapp. 30, 2834–2843. ( 10.1002/hbm.20711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pope PA, Holton A, Hassan S, Kourtis D, Praamstra P. 2007. Cortical control of muscle relaxation: a lateralized readiness potential (LRP) investigation. Clin. Neurophysiol. 118, 1044–1052. ( 10.1016/j.clinph.2007.02.002) [DOI] [PubMed] [Google Scholar]
- 4.Filevich E, Kuhn S, Haggard P. 2012. Negative motor phenomena in cortical stimulation: implications for inhibitory control of human action. Cortex 48, 1251–1261. ( 10.1016/j.cortex.2012.04.014) [DOI] [PubMed] [Google Scholar]
- 5.Kunesch E, Schnitzler A, Tyercha C, Knecht S, Stelmach G. 1995. Altered force release control in Parkinson's disease. Behav. Brain Res. 67, 43–49. ( 10.1016/0166-4328(94)00111-R) [DOI] [PubMed] [Google Scholar]
- 6.Wing AM. 1988. A comparison of the rate of pinch grip force increases and decreases in parkinsonian bradykinesia. Neuropsychologia 26, 479–482. ( 10.1016/0028-3932(88)90100-5) [DOI] [PubMed] [Google Scholar]
- 7.Della Sala S, Marchetti C, Spinnler H. 1991. Right-sided anarchic (alien) hand: a longitudinal study. Neuropsychologia 29, 1113–1127. ( 10.1016/0028-3932(91)90081-I) [DOI] [PubMed] [Google Scholar]
- 8.Poisson A, Krack P, Thobois S, Loiraud C, Serra G, Vial C, Broussolle E. 2012. History of the ‘geste antagoniste’ sign in cervical dystonia. J. Neurol. 259, 1580–1584. ( 10.1007/s00415-011-6380-7) [DOI] [PubMed] [Google Scholar]
- 9.Franzkowiak S, et al. 2010. Altered pattern of motor cortical activation-inhibition during voluntary movements in Tourette syndrome. Mov. Disord. 25, 1960–1966. ( 10.1002/mds.23186) [DOI] [PubMed] [Google Scholar]
- 10.Roessner V, Wittfoth M, Schmidt-Samoa C, Rothenberger A, Dechent P, Baudewig J. 2012. Altered motor network recruitment during finger tapping in boys with Tourette syndrome. Hum. Brain Mapp. 33, 666–675. ( 10.1002/hbm.21240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olk B, Kingstone A. 2003. Why are antisaccades slower than prosaccades? A novel finding using a new paradigm. Neuroreport 14, 151–155. ( 10.1097/00001756-200301200-00028) [DOI] [PubMed] [Google Scholar]
- 12.Terada K, Ikeda A, Nagamine T, Shibasaki H. 1995. Movement-related cortical potentials associated with voluntary muscle relaxation. Electroencephalogr. Clin. Neurophysiol. 95, 335–345. ( 10.1016/0013-4694(95)00098-J) [DOI] [PubMed] [Google Scholar]
- 13.Pruszynski JA, Kurtzer I, Scott SH. 2011. The long-latency reflex is composed of at least two functionally independent processes. J. Neurophysiol. 106, 449–459. ( 10.1152/jn.01052.2010) [DOI] [PubMed] [Google Scholar]
- 14.Gerilovsky L, Struppler A, Altmann H, Velho F. 1983. Spindle activity and monosynaptic reflex excitability during foreperiod. Electroencephalogr. Clin. Neurophysiol. 56, 487–493. ( 10.1016/0013-4694(83)90233-X) [DOI] [PubMed] [Google Scholar]
- 15.Rothwell JC, Traub MM, Marsden CD. 1980. Influence of voluntary intent on the human long-latency stretch reflex. Nature 286, 496–498. ( 10.1038/286496a0) [DOI] [PubMed] [Google Scholar]
- 16.Kohnstamm O. 1915. Demonstration einer Katatonieartigen Erscheimung beim Gesunden (Katatonusversuch). Neurology Zentral. 34S, 290–291. [Google Scholar]
- 17.Duclos C, Roll R, Kavounoudias A, Roll JP. 2007. Cerebral correlates of the ‘Kohnstamm phenomenon’: an fMRI study. Neuroimage 34, 774–783. ( 10.1016/j.neuroimage.2006.06.050) [DOI] [PubMed] [Google Scholar]
- 18.Forbes A, Baird P, Hopkins AM. 1926. The involuntary contraction following isometric contraction of skeletal muscle in man. Am. J. Physiol. 78, 81–103. [Google Scholar]
- 19.Gilhodes JC, Gurfinkel VS, Roll JP. 1992. Role of Ia muscle spindle afferents in post-contraction and post-vibration motor effect genesis. Neurosci. Lett. 135, 247–251. ( 10.1016/0304-3940(92)90447-F) [DOI] [PubMed] [Google Scholar]
- 20.Ghafouri M, Thullier F, Gurfinkel VS, Lestienne FG. 1998. Muscular after-contraction and ongoing postural reactions in standing and sitting humans. Neurosci. Lett. 250, 61–65. ( 10.1016/S0304-3940(98)00335-8) [DOI] [PubMed] [Google Scholar]
- 21.Duclos C, Roll R, Kavounoudias A, Roll JP. 2004. Long-lasting body leanings following neck muscle isometric contractions. Exp. Brain Res. 158, 58–66. ( 10.1007/s00221-004-1871-8) [DOI] [PubMed] [Google Scholar]
- 22.Hagbarth KE, Nordin M. 1998. Postural after-contractions in man attributed to muscle spindle thixotropy. J. Physiol. 506, 875–883. ( 10.1111/j.1469-7793.1998.875bv.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gregory JE, Morgan DL, Proske U. 1988. Aftereffects in the responses of cat muscle spindles and errors of limb position sense in man. J. Neurophysiol. 59, 1220–1230. [DOI] [PubMed] [Google Scholar]
- 24.Ribot-Ciscar E, Tardy-Gervet MF, Vedel JP, Roll JP. 1991. Post-contraction changes in human muscle spindle resting discharge and stretch sensitivity. Exp. Brain Res. 86, 673–678. ( 10.1007/BF00230541) [DOI] [PubMed] [Google Scholar]
- 25.Wilson LR, Gandevia SC, Burke D. 1995. Increased resting discharge of human spindle afferents following voluntary contractions. J. Physiol. 488, 833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craske B, Craske JD. 1986. Oscillator mechanisms in the human motor system: investigating their properties using the aftercontraction effect. J. Mot. Behav. 18, 117–145. ( 10.1080/00222895.1986.10735374) [DOI] [PubMed] [Google Scholar]
- 27.Mathis J, Gurfinkel VS, Struppler A. 1996. Facilitation of motor evoked potentials by postcontraction response (Kohnstamm phenomenon). Electroencephalogr. Clin. Neurophysiol. 101, 289–297. ( 10.1016/0924-980X(96)95599-X) [DOI] [PubMed] [Google Scholar]
- 28.Parkinson A, McDonagh M, Vidyasagar R. 2009. Brain activation in an involuntary human action. Brain Res. 1304, 57–65. ( 10.1016/j.brainres.2009.09.092) [DOI] [PubMed] [Google Scholar]
- 29.Hick WE. 1953. Some features of the after-contraction phenomenon. Q. J. Exp. Psychol. 5, 166–170. ( 10.1080/17470215308416639) [DOI] [Google Scholar]
- 30.Gurfinkel VS, Levik Iu S, Lebedev MA. 1989. [Immediate and remote postactivation effects in the human motor system]. Neirofiziologiia 21, 343–351. [PubMed] [Google Scholar]
- 31.Osu R, Gomi H. 1999. Multijoint muscle regulation mechanisms examined by measured human arm stiffness and EMG signals. J. Neurophysiol. 81, 1458–1468. [DOI] [PubMed] [Google Scholar]
- 32.Brown TG, Sherrington CS. 1912. On the instability of a cortical point. Proc. R. Soc. Lond. B 85, 250–277. ( 10.1098/rspb.1912.0050) [DOI] [Google Scholar]
- 33.Adamson G, McDonagh M. 2004. Human involuntary postural aftercontractions are strongly modulated by limb position. Eur. J. Appl. Physiol. 92, 343–351. ( 10.1007/s00421-004-1091-8) [DOI] [PubMed] [Google Scholar]
- 34.Allen F, O'Donoghue CH. 1927. The post-contraction proprioceptive reflex, its augmentation and inhibition. Exp. Physiol. 18, 199–242. [Google Scholar]
- 35.Ivanenko YP, Wright WG, Gurfinkel VS, Horak F, Cordo P. 2006. Interaction of involuntary post-contraction activity with locomotor movements. Exp. Brain Res. 169, 255–260. ( 10.1007/s00221-005-0324-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sapirstein MR, Herman RC, Wallace GB. 1936. Effect of certain drugs on after-contraction. Exp. Biol. Med. (Maywood). 35, 163–165. ( 10.3181/00379727-35-8894C) [DOI] [Google Scholar]
- 37.Forbes A. 1926. The involuntary contraction following isometric contraction of skeletal muscle in man. Am. J. Physiol. 78, 81. [Google Scholar]
- 38.Kimura T, Haggard P, Gomi H. 2006. Transcranial magnetic stimulation over sensorimotor cortex disrupts anticipatory reflex gain modulation for skilled action. J. Neurosci. 26, 9272–9281. ( 10.1523/JNEUROSCI.3886-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ortu E, Deriu F, Suppa A, Tolu E, Rothwell JC. 2008. Effects of volitional contraction on intracortical inhibition and facilitation in the human motor cortex. J. Physiol. 586, 5147–5159. ( 10.1113/jphysiol.2008.158956) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pruszynski JA, Kurtzer I, Nashed JY, Omrani M, Brouwer B, Scott SH. 2011. Primary motor cortex underlies multi-joint integration for fast feedback control. Nature 478, 387–390. ( 10.1038/nature10436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pruszynski JA, Scott SH. 2012. Optimal feedback control and the long-latency stretch response. Exp. Brain Res. 218, 341–359. ( 10.1007/s00221-012-3041-8) [DOI] [PubMed] [Google Scholar]
- 42.Shibasaki H. 2011. Cortical activities associated with voluntary movements and involuntary movements. Clin. Neurophysiol. 123, 229–243. ( 10.1016/j.clinph.2011.07.042) [DOI] [PubMed] [Google Scholar]
- 43.Sapirstein MR, Herman RC, Wallace GB. 1937. A study of after contraction. Am. J. Physiol. 19, 549–556. [Google Scholar]
- 44.Levik YS, Lebedev M. 1989. Immediate and remote postactivation effects in the human motor system. Neurophysiology 21, 247–253. [PubMed] [Google Scholar]
- 45.Esposti R, Baldissera FG. 2013. The role of anticipatory postural adjustments (APAs) in interlimb coordination of coupled arm movements in the parasagittal plane. I. APAs associated with fast discrete flexion and extension movements of one arm or of both arms ISO- and ANTI-directionally coupled. Exp. Brain Res. 228, 527–539. ( 10.1007/s00221-013-3584-3) [DOI] [PubMed] [Google Scholar]
- 46.Blakemore SJ, Frith C. 2003. Self-awareness and action. Curr. Opin. Neurobiol. 13, 219–224. ( 10.1016/S0959-4388(03)00043-6) [DOI] [PubMed] [Google Scholar]
- 47.Chronicle EP, Glover J. 2003. A ticklish question: does magnetic stimulation of the primary motor cortex give rise to an ‘efference copy’? Cortex 39, 105–110. ( 10.1016/S0010-9452(08)70078-9) [DOI] [PubMed] [Google Scholar]
- 48.Voss M, Bays PM, Rothwell JC, Wolpert DM. 2007. An improvement in perception of self-generated tactile stimuli following theta-burst stimulation of primary motor cortex. Neuropsychologia 45, 2712–2717. ( 10.1016/j.neuropsychologia.2007.04.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen R, Lozano AM, Ashby P. 1999. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp. Brain Res. 128, 539–542. ( 10.1007/s002210050878) [DOI] [PubMed] [Google Scholar]
- 50.Fuhr P, Agostino R, Hallett M. 1991. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr. Clin. Neurophysiol. 81, 257–262. ( 10.1016/0168-5597(91)90011-L) [DOI] [PubMed] [Google Scholar]
- 51.Terao Y, Ugawa Y. 2002. Basic mechanisms of TMS. J. Clin. Neurophysiol. 19, 322–343. ( 10.1097/00004691-200208000-00006) [DOI] [PubMed] [Google Scholar]
- 52.Spieser L, Meziane HB, Bonnard M. 2010. Cortical mechanisms underlying stretch reflex adaptation to intention: a combined EEG-TMS study. Neuroimage 52, 316–325. ( 10.1016/j.neuroimage.2010.04.020) [DOI] [PubMed] [Google Scholar]