Abstract
In many animals, including humans, interactions with caring parents can have long-lasting effects on offspring sensitivity to stressors. However, whether these parental effects impact offspring fitness in nature is often unclear. In addition, despite evidence that maternal care can influence offspring behaviour via epigenetic alterations to the genome, it remains unclear whether paternal care has similar effects. Here, we show in three-spined sticklebacks, a fish in which fathers are the sole provider of offspring care, that the direct care provided by fathers affects offspring anxiety and the potential for epigenetic alterations to the offspring genome. We find that families are differentially vulnerable to early stress and fathers can compensate for this differential sensitivity with the quality of their care. This variation in paternal care is also linked to the expression in offspring brains of a DNA methyltransferase (Dnmt3a) responsible for de novo methylation. We show that these paternal effects are potentially adaptive and anxious offspring are unlikely to survive an encounter with a predator. By supplying offspring care, fathers reduce offspring anxiety thereby increasing the survival of their offspring—not in the traditional sense through resource provisioning but through an epigenetic effect on offspring behavioural development.
Keywords: behavioural programming, early life stress, methylation, survival, three-spined stickleback, transgenerational plasticity
1. Introduction
Parents can influence their offspring by providing parental care. In addition to the obvious influence of parental provisioning on offspring size and morphological traits, parental care can also exert substantial influence on offspring behavioural traits [1–4]. For example in rodents and non-human primates, offspring receiving low levels of parental care, or deprived of it altogether, develop more reactive stress response systems, thereby reducing their ability to cope with stressors and leading to increased anxiety [2,3,5,6]. This suggests that receiving adequate parental care is an important part of offspring development and its removal has long-lasting consequences for how offspring react to stressors. However, these studies are often far-removed from ecologically relevant conditions. Thus, although parental care affects offspring sensitivity to stressors, it remains unknown whether this impacts offspring fitness.
It is clear that vulnerability to early life adversity, such as inadequate parental care, and its associated consequences vary among individuals [1,3,7,8]. Thus, we might expect there to be variation among parents in the parental care they provide owing to differential susceptibility of offspring. Indeed, in some cases, offspring seem primed for the amount of care they will receive. For example, cross-fostering studies have demonstrated that mismatches between parental provisioning and offspring demand can negatively impact both parents and offspring [9–11]. However, it is unclear whether offspring vulnerability is linked to the quality of parental care they anticipate receiving, particularly when parent–offspring interactions do not involve resource provisioning.
There is accumulating evidence that the influence of parental care on offspring behaviour can occur epigenetically [1,3,4]. For example, variation in maternal licking and grooming behaviour in rats alters offspring gene expression via DNA methylation of multiple regions within the genome [2,4]. Mother rats showing low levels of licking and grooming have offspring with increased DNA methylation of particular genes such as the glucocorticoid receptor (GR) in the hippocampus, resulting in decreased GR expression and an increased stress response [2,4]. Despite evidence in mammals that maternal care can influence offspring behaviour via epigenetic alterations to the genome, it remains unclear whether paternal care has similar effects ([12], but see [13]).
Here, we examine the influence of paternal care on offspring in a fish species where fathers are the sole providers of the care that is necessary for offspring survival (three-spined stickleback, Gasterosteus aculeatus). Unlike in biparental species where mothers might compensate or differentially allocate depending on the behaviour of their mate [12], in this species, we can isolate the post-fertilization effects of fathers from the effects of mothers. During the approximately two weeks that fathers provide care, they defend their nest from predators, fan the nest with their pectoral fins to provide fresh oxygen to the embryos and once the embryos hatch, retrieve fry that stray from the nest. During this period, offspring rely on yolk reserves provisioned by their mother prior to fertilization. Fathers do not feed offspring, but there is evidence that offspring antipredator behaviour [14], mate preference [15] and morphology [16] can be sensitive to the effects of fathers.
In this study, we manipulated access to paternal care in the three-spined stickleback and assessed the consequences for offspring later in life (approx. six months of age). Offspring without tending fathers (orphans) would rarely survive in nature owing to high levels of embryo and fry predation. However, by comparing father-reared and orphaned siblings, we can examine the influence of paternal care on offspring traits while controlling for the effects of father and mother identity. We measured offspring anxiety in response to different stressors and assessed whether variation in offspring anxiety was related to the quality of paternal care they had received. Thigmotaxis (i.e. remaining close to walls and searching for escape) and erratic movements are measures of anxiety in both rodents and fishes [17,18], thus we used pecking at the tank walls as our measure of offspring anxiety. While related to time spent near the sides of the tank, repeated pecking up and down the tank walls (towards the black outer covering) is an extreme type of ‘wall-following’ that is distinct from foraging, freezing or swimming near the outer edge. As a first step in determining whether the link between paternal care and offspring anxiety might be mediated by epigenetic changes to the offspring genome, we measured the expression in offspring brains of a methyltransferase involved in de novo methylation (Dnmt3a). Methyltransferases responsible for de novo methylation, particularly Dnmt3a, have been implicated in behavioural and neuronal plasticity [19–22] and their expression can be indicative of global methylation patterns in the genome [19,21,23]. Finally, to determine whether the influence of paternal care on offspring anxiety might be adaptive under ecologically relevant conditions, we assessed whether an individual's tendency to react to stressors with anxiety affected their survival in an encounter with a live predator.
2. Results and discussion
(a). Effects of paternal care on offspring anxiety
Offspring deprived of paternal care had heightened anxiety behaviour. Orphaned offspring showed over four times more anxiety than their father-reared siblings in both a novel environment and during an encounter with a predator model (figure 1; repeated-measures-mixed model results: rearing treatment: F1,12 = 5.38, p = 0.038; stressor: F1,96 = 2.41, p = 0.124; sex: F1,96 = 3.54, p = 0.063). There was also substantial variation among fathers in the anxiety behaviour of their offspring (random effect of father identity: χ2 = 15, p = 0.0001).
Figure 1.
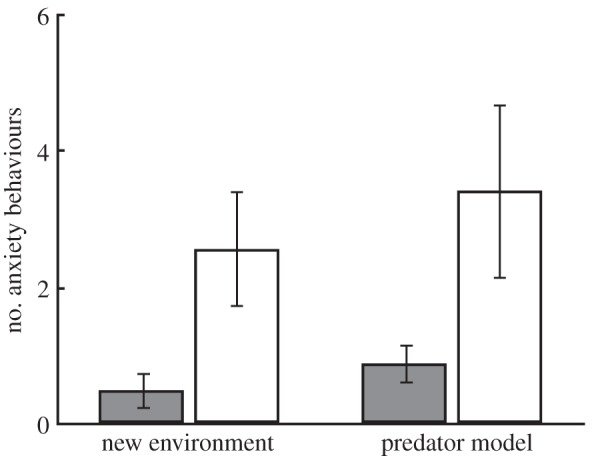
Orphaned offspring (white bars) showed more anxiety behaviours in response to both a new environment and a predator model than offspring reared by their father (grey bars). Shown are means ± s.e. across fathers (n = 56 offspring).
The difference between father-reared and orphaned siblings in their anxiety behaviour was related to the type of care father-reared offspring had received from their father (figure 2a; r = −0.90, p = 0.006). Fathers that provided more direct offspring care in the form of time spent at the nest and nest fanning bouts, had orphaned offspring with elevated anxiety compared with their father-reared siblings. In contrast, fathers that behaved in a defensive manner and performed a greater number of predator inspections at the expense of spending time at the nest with offspring had orphaned and father-reared offspring that showed similarly low levels of anxiety. This pattern was driven by a strong positive relationship between orphaned offspring behaviour and the paternal care they were prevented from receiving while all father-reared offspring showed low levels of anxiety regardless of the paternal care they had received (electronic supplementary material, figure S1A). Thus, the absence of high levels of direct paternal care resulted in particularly anxious offspring, but the absence of low levels of direct care (and high levels of nest defence) did not. It is important to point out that while offspring anxiety was associated with father care at the nest, performing more predator inspections might be an equally important facet of care in terms of fry survival, particularly in the face of a live predator.
Figure 2.
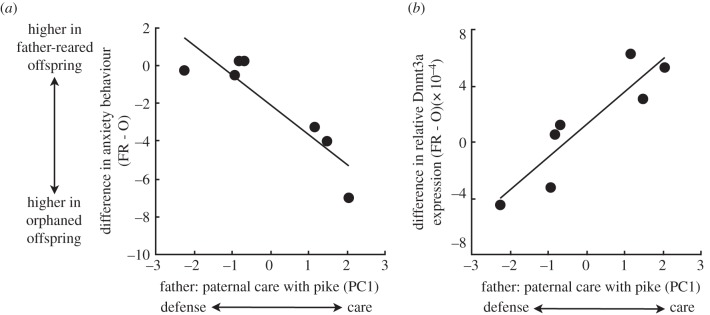
The greater the amount of direct paternal care father-reared offspring received from their father, the greater the difference between father-reared and orphaned siblings in (a) their average anxiety behaviour in a new environment and (b) their average whole brain Dnmt3a gene expression. Each circle indicates the difference (father-reared minus orphaned siblings) between the average anxiety during 3 min in a new environment or average whole brain gene expression of the same four offspring per treatment per father (n = 56 offspring total).
These results show that offspring vulnerability to the absence of paternal care is highly variable across families and is related to the level of paternal care. Fathers that provide high levels of direct care to offspring have offspring that show heightened anxiety when they are deprived of this high level of paternal care (i.e. high caring fathers have highly reactive orphans). In contrast, fathers that show less direct care and more predator defence have offspring that show minimal anxiety when they are deprived of this low level of direct care (i.e. low caring fathers have minimally reactive orphans). The role offspring play in directly manipulating paternal care remains unclear; however there is evidence that paternal care in sticklebacks can be a dynamic back-and-forth process. For example, the speed at which fry evade fathers is matched by the retrieval behaviour of fathers [24]. Thus, active fry might ‘demand’ more care from their fathers by forcing fathers to retrieve them. When fathers are absent, these active fry are not retrieved by their father and this unchecked activity might deplete energy reserves with consequences for future growth, gene expression and behaviour. Whether future offspring anxiety is altered in response to their own early activity, the direct paternal care they receive, or merely the presence of a larger fish (whether the father or a predator) during development remains unclear, although in nature, these factors are clearly intertwined.
(b). Epigenetic effects of paternal care on offspring brain gene expression
Fathers that provided high levels of direct care had father-reared offspring with elevated Dnmt3a expression in their brains compared with orphaned siblings (figure 2b; r = 0.90, p = 0.005). In contrast, fathers that behaved in a defensive manner at the expense of direct offspring care had father-reared offspring with lower Dnmt3a expression compared with orphaned siblings. Paternal care was associated with average gene expression in father-reared offspring, but not orphaned offspring (electronic supplementary material, figure S1B). Furthermore, when we ignore paternal care, Dnmt3a expression and offspring anxiety were not significantly correlated (r = 0.08, p = 0.560, n = 56). Thus, it is not simply the case that showing anxiety increases expression of Dnmt3a within individual offspring. Rather, this pattern is consistent with the hypothesis that father care itself influences the average Dnmt3a expression of offspring.
Dnmt3a expression was highest in father-reared offspring of high caring fathers where paternal care is crucial for preventing heightened offspring anxiety. Although we cannot determine causation, our results are consistent with the hypothesis that high levels of direct offspring care during development increases de novo methylation in offspring leading to changes in gene expression and behaviour. However, our ability to link paternal care to offspring anxiety mechanistically is limited, and we do not know which genes are being influenced nor where they are expressed in the brain. Thus, although paternal care might lead to methylation-induced repression of genes associated with anxiety, this is one of many possibilities. For example, the pattern is also consistent with paternal care altering oxygen availability during development leading to differential methylation of genes associated with respiration and metabolism. As methyltransferase expression is often associated with global methylation patterns [19,21,23], our results suggest that exploring the association between paternal care and genome-wide methylation patterns in offspring would be fruitful and provide insights into the genes affected by paternal care. Our results also indicate that not all fathers are equal in terms of their influence on offspring and that variation in paternal care among fathers is an important contributor to the patterns of offspring behaviour and gene expression.
(c). Effects of anxiety on survival
Having shown that paternal care reduces offspring anxiety, potentially via altered offspring genome methylation patterns, we tested how an individual's tendency to show anxiety (i.e. pecking at the walls) in response to stressors was related to survival under predation risk. We found that juveniles showing heightened anxiety when encountering novel stimuli in the absence of a live predator were attacked and captured more quickly by a live Northern pike (Esox luscius) compared with juveniles that did not show any anxiety (figure 3; effect of anxiety on attack latency: F1,74.6 = 6.41, p = 0.013, random effect of family: χ2 = 3.0, p = 0.083; effect of anxiety on survival: F1,74.4 = 4.73, p = 0.033, random effect of family: χ2 = 3.7, p = 0.054). Thus, being anxious has negative consequences for offspring when they encounter a predator. This suggests that by reducing offspring anxiety, paternal care can enhance offspring survival.
Figure 3.
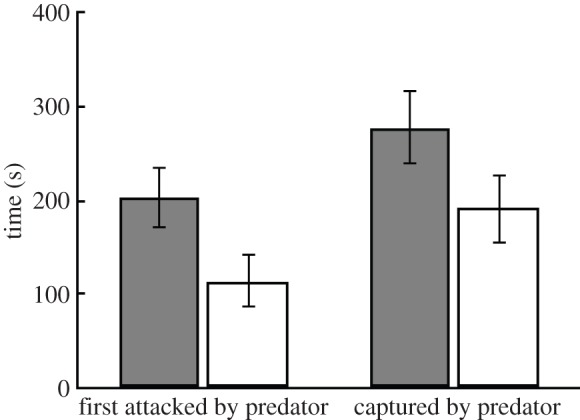
Orphans who pecked at the wall when encountering novel stimuli (white bars, n = 40 individuals) were attacked and captured more quickly by a live Northern pike than orphans who did not show any anxiety behaviour when encountering novel stimuli (grey bars, n = 37 individuals). Shown are means ± s.e.
Taken together, our results provide a unique example of adaptive parent–offspring behavioural matching. Whether offspring show heightened anxiety later in life depends on the level of care they received from their father early in development, with mismatches between parent and offspring traits (i.e. offspring from high caring fathers receiving no care) having negative consequences for offspring interactions with predators. A number of traits in both fathers (e.g. parental care behaviours, sensitivity to offspring movement) and offspring (e.g. early swimming activity, reaction to stressors) can potentially play an important role in these parent–offspring interactions. Determining the degree to which these father and offspring traits are heritable could shed light on the importance of these paternal effects for evolution.
If parental care (or sensitivity to offspring demand) and offspring behaviour are heritable, parent–offspring coadaptation can give rise to genetic correlations between them, with selection favouring particular combinations of parent and offspring traits [9,25]. It is also possible that this parent–offspring matching is a result of an indirect genetic effect with the genes for parental care (i.e. the extended parental phenotype) influencing offspring behavioural development without offspring anxiety being heritable per se [25]. In sticklebacks, there is evidence that fathers tend to be consistent across breeding attempts [26]. However, an encounter with a model predator while caring for offspring can have consequences for offspring behaviour and morphology suggesting that offspring development might be altered by subtle shifts in father behaviour [16]. The covariation between parent and offspring behaviour could also be environmentally induced (i.e. owing to transgenerational plasticity). Parents might adjust their investment in offspring care based on the environment they have experienced [27], or even the environment their partner had experienced [28]. In sticklebacks, maternal predator-exposure has a number of negative consequences for offspring [29,30] and thus it is possible that differences among families in anxiety are driven by maternal identity or experiences. Fathers might adjust their care to compensate for these negative maternal effects on offspring [12] by responding to offspring cues tied to maternal condition [27,31].
Here, we show that patenal care reduces offspring anxiety in a potentially adaptive way, with the behavioural costs of care deprivation being the greatest for offspring of fathers that provide high levels of direct offspring care. Furthermore, paternal care alters offspring whole brain Dnmt3a expression, which has the potential to modify the expression of many genes in offspring, some of which might influence both their anxiety and future parental care [4]. The challenge of future work is to determine the extent to which these paternal effects can be transmitted to future generations, for example by altering offspring's paternal care [13], the mechanisms underlying such transgenerational plasticity, and whether similar behavioural matching between parents and offspring can help explain the differential sensitivity to environmental influences evident among individuals of so many species, including humans [1,3,8].
3. Material and methods
(a). Paternal effects on offspring anxiety and gene expression
Adult sticklebacks were caught from a high-predation freshwater population (Eliburn, Scotland) [14] and mated at University of Illinois. We split fertilized clutches into ‘orphaned offspring’ which were reared without father care and ‘father-reared offspring’ which were returned to their genetic father. We measured paternal care directed towards father-reared offspring three days after hatching under predator threat. With a Northern pike model in their tank for 15 min, we recorded the father's (i) predator inspections (approaching the model within a body length), (ii) nest-fanning bouts, and (iii) total time spent at the nest. We combined paternal behaviours into a metric of paternal care using principal components analysis (PC1 explained 82% of variation; electronic supplementary material, table S1), with positive values associated with direct offspring care (high levels of nest fanning and time spent at the nest) and negative values associated with defence (high levels of predator inspections).
Six months later, we measured offspring anxiety in response to two novel and potentially stressful stimuli (3 min each): (i) a new environment and (ii) a Northern pike model. Using a repeated-measures analysis and accounting for the effect of father identity, we examined how receiving paternal care affected anxiety and general swimming activity in response to these stimuli. One hour after the behaviour assay, individuals were euthanized, whole brains dissected, and using qRT-PCR, we measured expression of Dnmt3a relative to an endogeneous control gene (Gapdh; as in [32]). We measured individual anxiety and gene expression for father-reared and orphaned siblings (four each) from seven fathers (n = 56 offspring total). Using ANCOVAs, we examined how receiving paternal care, as well as the quality of this care (PC1), affected average offspring anxiety in response to a novel environment and average offspring Dnmt3a expression (electronic supplementary material, table S2). We followed up on significant interactions using correlation analyses between paternal care (PC1) and the difference between father-reared and orphaned siblings in average behaviour and Dnmt3a expression. Note that anxiety differences between father-reared and orphaned siblings were not due to differences in general swimming activity throughout the tank (electronic supplementary material, table S3).
(b). Effects of anxiety on survival
We measured the anxiety (i.e. pecking at the tank walls) of single orphaned juveniles (approx. six months old) in response to several novel and potentially stressful stimuli (e.g. new environment, a Northern pike model, an unfamiliar object and after removal of a conspecific shoal). Two hours later, juveniles were moved to a tank containing a single Northern pike where we recorded attacks and captures [29,33]. Orphaned juveniles (n = 77 from [29,33]) were unrelated to those in the paternal care manipulation.
Allowing predators to interact freely with their prey was essential to quantify survival; however, we provided numerous refuges in the predator tanks in order to give the sticklebacks an opportunity to hide or escape from the pike. In planning the original maternal effects study [29], we minimized the number of subjects used and results from the predation experiment have contributed to multiple papers [29,33] maximizing the insights gained from a single survival assay.
Additional methods and results for both the paternal care experiment and predation experiment can be found in the electronic supplementary material.
Supplementary Material
Acknowledgements
We thank T. Ceau, D. Ernst, M. Grobis, J. Hamilton, S. Khoo, L. Pintor, D. Roche, V. Sefton, L. Stein, E. Suhr, T. Tufte and J. Wang, as well as the Spirit Lake Fish Hatchery (Iowa) and the Jake Wolf Fish Hatchery (Illinois). We thank O. Sanogo, M. Schrader and T. Newman for help with molecular methods and G. Robinson for access to the qPCR machine. We thank R. Kilner, M. Schrader and R. Thorogood for comments on the manuscript. Both authors contributed extensively to the work presented in this paper.
Ethics statement
These experiments were approved by the Animal Care and Use Committee of University of Illinois (protocol no. 09204).
Data accessibility
Data associated with this paper have been deposited in Dryad (doi:10.5061/dryad.gn577).
Funding statement
Funding was provided by NIH R01 GM082937 to A.M.B. and M. Band. K.E.M. was supported by NIH/NICHD T32 HD007333 and NSF IOS 1121980 to A.M.B. and K.E.M.
References
- 1.Daskalakis NP, Bagot RC, Parker KJ, Vinkers CH, de Kloet ER. 2013. The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology 38, 1858–1873. ( 10.1016/j.psyneuen.2013.06.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y, Labonte B, Wen XL, Turecki G, Meaney MJ. 2013. Epigenetic mechanisms for the early environmental regulation of hippocampal glucocorticoid receptor gene expression in rodents and humans. Neuropsychopharmacology 38, 111–123. ( 10.1038/npp.2012.149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parker KJ, Maestripieri D. 2011. Identifying key features of early stressful experiences that produce stress vulnerability and resilience in primates. Neurosci. Biobehav. Rev. 35, 1466–1483. ( 10.1016/j.neubiorev.2010.09.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Champagne FA, Curley JM. 2012. Genetics and epigenetics of parental care. In The evolution of parental care (eds Royle NJ, Smiseth PT, Kolliker M.), pp. 304–324. Oxford, UK: Oxford University Press. [Google Scholar]
- 5.Birnie AK, Taylor JH, Cavanaugh J, French JA. 2013. Quality of maternal and paternal care predicts later stress reactivity in the cooperatively-breeding marmoset (Callithrix geoffroyi). Psychoneuroendocrinology 38, 3003–3014. ( 10.1016/j.psyneuen.2013.08.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia R, Tai FD, An SC, Zhang X, Broders H. 2009. Effects of neonatal paternal deprivation or early deprivation on anxiety and social behaviors of the adults in mandarin voles. Behav. Process. 82, 271–278. ( 10.1016/j.beproc.2009.07.006) [DOI] [PubMed] [Google Scholar]
- 7.Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. 2011. Differential susceptibility to the environment: an evolutionary-neurodevelopmental theory. Dev. Psychopathol. 23, 7–28. ( 10.1017/S0954579410000611) [DOI] [PubMed] [Google Scholar]
- 8.Yehuda R, Flory JD, Pratchett LC, Buxbaum J, Ising M, Holsboer F. 2010. Putative biological mechanisms for the association between early life adversity and the subsequent development of PTSD. Psychopharmacology 212, 405–417. ( 10.1007/s00213-010-1969-6) [DOI] [PubMed] [Google Scholar]
- 9.Kilner RM, Hinde CA. 2012. Parent–offspring conflict. In The evolution of parental care (eds Royle NJ, Smiseth PT, Kolliker M.), pp. 119–132. Oxford, UK: Oxford University Press. [Google Scholar]
- 10.Hinde CA, Johnstone RA, Kilner RM. 2010. Parent–offspring conflict and coadaptation. Science 327, 1373–1376. ( 10.1126/science.1186056) [DOI] [PubMed] [Google Scholar]
- 11.Love OP, Williams TD. 2008. The adaptive value of stress-induced phenotypes: effects of maternally derived corticosterone on sex-biased investment, cost of reproduction, and maternal fitness. Am. Nat. 172, E135–E149. ( 10.1086/590959) [DOI] [PubMed] [Google Scholar]
- 12.Curley JP, Mashoodh R, Champagne FA. 2011. Epigenetics and the origins of paternal effects. Horm. Behav. 59, 306–314. ( 10.1016/j.yhbeh.2010.06.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gleason ED, Marler CA. 2013. Non-genomic transmission of paternal behaviour between fathers and sons in the monogamous and biparental California mouse. Proc. R. Soc. B 280, 20130824 ( 10.1098/rspb.2013.0824) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tulley JJ, Huntingford FA. 1987. Paternal care and the development of adaptive variation in antipredator responses in sticklebacks. Anim. Behav. 35, 1570–1572. ( 10.1016/S0003-3472(87)80034-9) [DOI] [Google Scholar]
- 15.Kozak GM, Head ML, Boughman JW. 2011. Sexual imprinting on ecologically divergent traits leads to sexual isolation in sticklebacks. Proc. R. Soc. B 278, 2604–2610. ( 10.1098/rspb.2010.2466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein LR, Bell AM. 2014. Paternal programming in sticklebacks. Anim. Behav. ( 10.1016/j.anbehav.2014.07.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart A, Gaikwad S, Kyzar E, Green J, Roth A, Kalueff AV. 2012. Modeling anxiety using adult zebrafish: a conceptual review. Neuropharmacology 62, 35–143. ( 10.1016/j.neuropharm.2011.07.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maximino C, de Brito TM, Batista AWD, Herculano AM, Morato S, Gouveia A. 2010. Measuring anxiety in zebrafish: a critical review. Behav. Brain Res. 214, 157–171. ( 10.1016/j.bbr.2010.05.031) [DOI] [PubMed] [Google Scholar]
- 19.Wu Z, et al. 2012. Dnmt3a regulates both proliferation and differentiation of mouse neural stem cells. J. Neurosci. Res. 90, 1883–1891. ( 10.1002/jnr.23077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng J, Zhou Y, Campbell SL, Le T, Li E, Sweatt JD, Silva AJ, Fan G. 2010. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat. Neurosci. 13, 423–437. ( 10.1038/nn.2514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaPlant Q, et al. 2010. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat. Neurosci. 13, 1137–1141. ( 10.1038/nn.2619) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monsey MS, Ota KT, Akingbade IF, Hong ES, Schafe GE. 2011. Epigenetic alterations are critical for fear memory consolidation and synaptic plasticity in the lateral amygdala. PLoS ONE 6, e19958 ( 10.1371/journal.pone.0019958) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown SE, Weaver ICG, Meaney MJ, Szyf M. 2008. Regional-specific global cytosine methylation and DNA methyltransferase expression in the adult rat hippocampus. Neurosci. Lett. 440, 49–53. ( 10.1016/j.neulet.2008.05.028) [DOI] [PubMed] [Google Scholar]
- 24.Huntingford FA, Wright PJ, Tierney JF. 1994. Adaptive variation in antipredator behaviour in threespine stickleback. In The evolutionary biology of the threespine stickleback (eds Bell MA, Foster SA.), pp. 277–296. Oxford, UK: Oxford University Press. [Google Scholar]
- 25.Kölliker M, Royle NJ, Smiseth PT. 2012. Parent–offspring co-adaptation. In The evolution of parental care (eds Royle NJ, Smiseth PT, Kolliker M.), pp. 285–303. Oxford, UK: Oxford University Press. [Google Scholar]
- 26.Stein LR, Bell AM. 2012. Consistent individual differences in fathering in threepsined stickleback, Gasterosteus aculeatus. Curr. Zool. 58, 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinde CA, Buchanan KL, Kilner RM. 2009. Prenatal environmental effects match offspring begging to parental provisioning. Proc. R. Soc. B 276, 2787–2794. ( 10.1098/rspb.2009.0375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mashoodh R, Franks B, Curley JP, Champagne FA. 2012. Paternal social enrichment effects on maternal behavior and offspring growth. Proc. Natl Acad. Sci. USA 109, 17 232–17 238. ( 10.1073/pnas.1121083109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGhee KE, Pintor LM, Suhr EL, Bell AM. 2012. Maternal exposure to predation risk decreases offspring antipredator behaviour and survival in threespined stickleback. Funct. Ecol. 26, 932–940. ( 10.1111/j.1365-2435.2012.02008.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roche DP, McGhee KE, Bell AM. 2012. Maternal predator-exposure has lifelong consequences for offspring learning in threespined sticklebacks. Biol. Lett. 8, 932–935. ( 10.1098/rsbl.2012.0685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noguera JC, Kim SY, Velando A. 2013. Maternal testosterone influences a begging component that makes fathers work harder in chick provisioning. Horm. Behav. 64, 19–25. ( 10.1016/j.yhbeh.2013.04.008) [DOI] [PubMed] [Google Scholar]
- 32.Sanogo YO, Hankison S, Band M, Obregon A, Bell AM. 2011. Brain transcriptomic response of threespine sticklebacks to cues of a predator. Brain Behav. Evol. 77, 270–285. ( 10.1159/000328221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGhee KE, Pintor LM, Bell AM. 2013. Reciprocal behavioral plasticity and behavioral types during predator–prey interactions. Am. Nat. 182, 704–717. ( 10.1086/673526) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with this paper have been deposited in Dryad (doi:10.5061/dryad.gn577).


