Abstract
Sexual selection promotes the prevalence of heritable traits that increase an individual's reproductive rate. Despite theoretically strong directional selection, sexually selected traits can show inter-individual variation. Here, we investigate whether red skin ornamentation, a rare example of a male mammalian trait involved in mate attraction, influences fecundity and is heritable in rhesus macaques (Macaca mulatta), and explore the mechanisms that are involved in maintaining trait variation. Interestingly, the trait is expressed by and is attractive to both sexes. We collected facial images of 266 free-ranging individuals and modelled skin redness and darkness to rhesus macaque vision. We used 20 years of genetic parentage data to calculate selection gradients on the trait and perform heritability analyses. Results show that males who were both darkly coloured and high-ranking enjoyed higher fecundity. Female skin redness was positively linked to fecundity, although it remains unclear whether this influences male selectiveness. Heritability explained 10–15% of the variation in redness and darkness, and up to 30% for skin darkness when sexes are considered separately, suggesting sex-influenced inheritance. Our results suggest that inter-individual variation is maintained through condition-dependence, with an added effect of balancing selection on male skin darkness, providing rare evidence for a mammalian trait selected through inter-sexual selection.
Keywords: sexual selection, quantitative genetics, selection gradient, ornaments, signalling
1. Introduction
Sexual selection promotes the prevalence of heritable traits that increase an individual's reproductive rate [1,2]. Any trait that confers an advantage to individuals in reproductive competition should theoretically be under strong directional selection, with extreme phenotypic trait expression rapidly becoming fixed in the population. Despite this, sexually selected traits can exhibit a great deal of inter-individual phenotypic variation, with numerous mechanisms proposed to maintain this variation. First, if the level of trait expression is dependent on the carrier's condition (condition-dependency), there might be low levels of heritability of sexually selected traits despite a strong linear association between phenotypic expression and fecundity (e.g. stalk-eyed flies, Cyrtodiopsis dalmann [3]). Second, variation can also be maintained though balancing, non-directional selection processes, in which there is no linear correlation between phenotype expression and lifetime reproductive success. For instance, disruptive selection can maintain diversity when the extremes in trait variation have reproductive advantages (e.g. lazuli buntings, Passerina amoena [4]), especially in cases where the evolution of a male sexually selected trait is under a complex interaction between different mechanisms of sexual selection (e.g. cockroaches, Nauphoeta cinerea [5]; three-spine sticklebacks, Gasterosteus aculeatus [6]). Another mechanism of balancing selection is overdominance in which variation is maintained because the most successful males are heterozygous (e.g. Soay sheep, Ovis aries [7]). Finally, it remains possible that in some cases, traits have indeed been under strong directional selection and that the inter-individual variation in traits perceived by human observers or detected by our measuring tools is unrelated to variation in reproductive output, such that the perceived variation is not biologically meaningful.
Uniquely among mammals, certain primate species exhibit bright and extravagant red skin colours involved in intraspecific communication [8,9]. One of the best-studied species in this regard is the rhesus macaque (Macaca mulatta), in which the ornament is expressed by and attractive to both sexes. Coloration is exhibited in the face, genital and hindquarter areas (referred to as sex skin), and reaches its maximal expression in reproductive contexts—throughout the mating season for males [10,11], and around the timing of the peri-ovulatory phase for females [12,13]. The trait is under the control of sex steroid hormones and is expressed through an action on oestrogen receptors that influence the degree of epidermal blood flow in the epidermis [14–16]. In females, oestrogen is synthetized primarily by the ovaries, while oestrogen in males is produced through aromatization of testosterone in the sex skin [15]. As oestrogen is produced through different pathways in the two sexes, the trait is likely to be sex-influenced—an autosomal trait in which expression is nonetheless influenced by the carrier's sex.
Experimental and observational studies have shown that rhesus skin coloration is involved in mate selection: both sexes show interest in darker red faces of opposite-sex conspecifics [17,18], females sexually solicit dark red males more frequently [19], and males pay more attention to images of female faces collected during the peri-ovulatory phase [20]. The ornament is hypothesized to be attractive because it provides reliable information about a carrier's general condition (condition-dependency), with expression influenced by various endogenous and exogenous factors influencing blood flow and oxygenation, such as health, stress and activity [9,16,21,22]. Moreover, male coloration might be an indicator of immune strength because colour expression has been linked to testosterone, an immuno-suppressant [23]. Although sex skin coloration influences conspecific behaviours in a sexual context, there is no evidence to date that variation in skin coloration predicts male mating success [11,19]; no comparable study has been conducted for females.
Owing to the underlying physiological mechanisms involved in the expression of the trait, condition-dependency could explain inter-individual variation. This might also be explained by an interaction between different mechanisms of sexual selection. Indeed, morphological and behavioural traits suggest that male rhesus macaques experience both inter- and intra-sexual selection, including both direct and indirect forms of intra-sexual selection. In addition to red skin ornaments that are attractive to females (products of inter-sexual selection), rhesus males exhibit large relative testis volumes (indicating indirect competition at the level of sperm) [24] and some degree of sexual dimorphism in body weight and canine length (indicating direct competition) [25]. At the behavioural level, males organize themselves into a dominance hierarchy that provides priority-of-access to fertile females and the ability to mate-guard [26]. However, the efficiency of this mate-guarding tactic is unclear [27,28], and females are frequently reported to reject mating attempts by high-ranking males while actively soliciting males of variable ranks [29,30], including peripheral and extra-group males [31,32]. As there is no association between dominance rank and male skin colour [11,19], this could lead to complex nonlinear selection on male skin coloration. Sexual selection processes could also influence selection on female skin ornamentation because male rhesus macaques are selective [33]. If skin colour is informative about variation in female condition, males should invest more time and energy towards dark red females, which could potentially lead to directional selection on female coloration.
The objective of the study was to: (i) assess whether the variation in red ornaments influences fecundity and is heritable in male and female rhesus macaques—two necessary conditions for the trait to be considered under sexual selection; (ii) examine what types of selection the trait is under by performing selection gradient analyses; and (iii) explore how inter-individual variation in expression is maintained. In addition to being expressed and used by both sexes, this trait is of particular interest because it is one of the rare examples of a mammalian ornament for which clear involvement in mate selection at the behavioural level has been demonstrated (see [34]). We hypothesized that maintenance of inter-individual variation in this trait could be due to two non-mutually exclusive processes. First, if the trait is condition-dependent, it would show lower levels of heritability, despite being a predictor of reproductive success. Second, variation could be maintained through nonlinear selection owing to a complex interaction between different mechanisms of sexual selection acting on both males and females.
We explored these questions in the large free-ranging population of rhesus macaques on the island of Cayo Santiago, Puerto Rico. This field site offers the most comprehensive pedigree of any free-ranging population of non-human primate, providing a rare opportunity to probe the evolutionary basis of traits in a long-lived mammal in a naturalistic setting [35–38]. Because we are interested in variation in visual signal expression, we explore skin colour and darkness variation as perceived by conspecifics by transforming colour measurements into rhesus macaque colour space. Intra- and inter-individual variation in sex skin varies in both chromatic (redness) and achromatic (darkness) components [13,39]. These two components covary [19], but are likely to be influenced by different underlying physiological processes: while variation in darkness reflects variation in blood flow, variation in redness might also be influenced by variation in blood oxygenation, such that redder skin contains more oxygenated blood [22,40]. As blood flow and oxygenation can vary separately, this may lead to different patterns of selection and inheritance for these two components. To our knowledge, this is the first study to assess the heritability of non-human mammalian skin coloration, and the first to investigate the strength of different modes of selection on skin colour evolution in any mammal species.
2. Material and methods
(a). Field site and subjects
The free-ranging population of rhesus macaques on Cayo Santiago Island is managed by the Caribbean Primate Research Center (CPRC) and was established in 1938 based on ≈400 monkeys [41]. The population is now composed of ≈1000 individuals divided into naturally-formed groups, allowing male dispersal and gene flow. The population shows no significant effect of inbreeding [35,42], and the variance in lifetime reproductive success between individuals is sufficient to create opportunities for selection [43]. Date of birth, natal and current group, maternal ID, and matriline membership are provided by the CPRC.
(b). Image collection and subject sampling
We collected images of faces as colour characteristics of sex skin covary between areas [11]. In order to minimize the effect of intra-individual variation, we collected images only from non-moving subjects to limit any activity effects on skin colour. We also collected images at the end of the six-month mating season and onset of the six-month birth season (June 2012) when male skin coloration reaches baseline [10], and before most females reach the third trimester of pregnancy when they develop a dark red pregnancy mask [44,45]. In order to consider differences in female reproductive state as a potential confounding factor in the analyses, we took note of any indicators of sexual activity around the time pictures were collected (see details below). We sampled individuals from all groups but focused on those born in or currently residing in groups R and V, two groups: (i) descended from different founding females; and (ii) exhibiting no core home range overlap. We also included roaming males (not in a social group). We collected 1.79 ± 0.87 images per individual (range: 1–4) of 127 adult males (87.6% of all males of 5 years old or older) and 139 adult females (44.2% of all females of 3.5 years old or older). Multiple images of subjects and a colour standard (X-rite ColorChecker passport) were captured in RAW format from 1 to 3 m away from subjects using a calibrated Canon EOS Rebel T2i camera with a 18 megapixel CMOS APS-sensor and an EFS55-250 mm f/4–5.6 IS lens [19]. Facial skin colour was quantified by measuring colour values from images converted to 16-bit TIFF files using DCRAW (Dave Coffin) [19].
(c). Assessment of skin redness and darkness
Details on the methods used to assess skin redness and darkness can be found elsewhere [19]. In short, we first took average red (R), green (G) and blue (B) measurements from a fixed portion of the face and the neutral grey patches of the colour standard RGB. Values for the faces and standards were then transformed from the camera's colour space to rhesus colour space [39]. Based on the processing of colours early in the primate visual pathway, we calculated redness as the Red–Green Opponency Channel, which was measured as (LW − MW)/(LW + MW), and darkness as the Luminance Channel (achromatic) as (LW + MW)/2 [46]—where greater saturation of blood is darker, and thus less luminous. The average intra-individual coefficient of variation between sets of images used in the analyses was low (redness: 6.1%, darkness: 5.3%) showing that trait measurement was highly consistent within individuals, and thus repeatable. We averaged all sets of images when more than one was available.
(d). Genetic parentage assignment and pedigree information
The CPRC genetic parentage database spans a period of 20 years (1992–2011) and was established using a combination of exclusions and likelihood calculations [27,28,43,47]. Sires were identified for 264 out of 266 (99.2%) of the subjects, with all our male subjects excluded as potential sires in the remaining cases. Maternal grandfathers were identified for 206 subjects (77.4%) and paternal grandfathers for 216 (81.2%). The sample of subjects for which facial images were collected included 64 mother–offspring and 82 father–offspring pairs, including 34 cases in which the offspring and both parents were sampled. A total of 135 subjects had at least one maternal half-sibling sampled and 196 had a paternal half-sibling, with a total of three pairs of full-siblings. The sample also included 19 grandparent–grandoffspring pairs.
(e). Measures of reproductive success
Lifetime reproductive success could not be used because subjects differed in age, and only a fraction of the subjects (n = 27) had reached reproductive senescence (set at more than or equal to 17 years old [43,48]). Instead, we calculated fecundity (reproductive rate) as the number of offspring produced divided by the number of years since first reproduction. In order to minimize the risks of overestimating the variance in fecundity owing to cross-sectional sampling, only individuals that produced at least one offspring were included in selection gradient analyses (n = 81 males, 91 females). Note that most members of the population that survived to maturity reproduce at some point in their lifetime [43] and that male age at first reproduction greatly varies among males but has no effect on lifetime reproductive success [49].
(f). Demographic variables and other confounding factors
Age, mother ID, household and current group were extracted from the CPRC monthly census. Household, defined as females that share a common ancestor in the founding population, was broken into sub-categories based on common ancestors when these categories matched dominance rank categories too closely (see below).
Rhesus females form highly despotic, nepotistic, linear and stable hierarchies in which rank is inherited from the mother [50,51]. Natal and current rank are similar for females, but not for males because they disperse at puberty. We assessed natal dominance rank by combining demographic data with behavioural data obtained from studies conducted in 2007–2012 by ourselves and colleagues, allowing us to extrapolate the natal rank of young females and dispersed males. Dominance hierarchies were divided into three categories: high-, mid- and low-ranking, including all closely related individuals (mother/offspring, grandmother/grandchildren and half-siblings) in the same category [52].
Male current dominance rank does not influence skin redness or darkness [11,19] and was thus not included in the heritability analyses. However, it is known to influence reproductive success [27,31] and was thus included in the selection gradient analyses. We did not have detailed dominance rank from behavioural observations available for sampled males throughout their reproductive lives. However, rhesus males reach dominance through queuing [53], leading to a strong association between dominance rank and residency length [53–55]. As such, we used male dispersal rate as a proxy for each male's average dominance rank over time, which we calculated as the number of times each male has changed social group divided by the number of years since first reproduction. Males who reach high dominance ranks have a lower dispersal rate, and vice versa.
We included estimated female reproductive status in all analyses. As females become significantly darker at mid-cycle [12,13], we classified as ‘sexually active’: (i) females who were seen copulating or in consort [28] when the picture was taken; and/or (ii) females for which pictures were collected within 10 days of the estimated time of conception (based on the date of the subsequent birth and a mean 165-day pregnancy length [56]) (n = 39 females; 27 included in selection gradient analyses). Females whose pictures were taken more than 10 days after the estimated date of conception were classified as ‘pregnant’ (n = 103 females; 83 included in selection gradient analyses). We classified in a separate category females estimated to be in their third trimester of pregnancy (images taken more than or equal to 100 days after the estimated date of conception), as a coloured ‘pregnancy mask’ can develop at this time (n = 12 females; five included in selection gradient analyses) [44,45]. Females for which reproductive state could not be estimated were classified as ‘unknown’ (n = 8 females; seven included in selection gradient analyses).
Finally, the productivity (i.e. the number of offspring produced yearly) of the groups in which males reside can influence their overall reproductive success independently of their dominance rank, age, skin colour or other traits. As such, we calculated the average number of offspring born in the groups in which subject males lived during their reproductive life.
(g). Data analyses
(i). Fecundity and selection gradient
To calculate the effect of the level of trait expression on fecundity, we used the regression coefficient (or estimate) of general linear models (GLMs) testing for the effect of skin redness and darkness (predictors) on fecundity (response) [57,58]. We ran three models to assess different modes of selection on the trait. First, in order to estimate the strength of linear (i.e. directional) selection (β) on skin measures, redness and darkness were standardized, while relative measures of fecundity were calculated for fecundity by dividing by the sample average. Second, we estimated quadratic selection (γii) by squaring the standardized measures of skin redness and darkness. We included the linear terms in the model to correct for the effect of directional selection on the variance, and doubled the regression coefficient and standard errors in order to correctly estimate selection gradients [59]. Negative quadratic estimates are suggestive of stabilizing selection (which maintains intermediate expression), whereas positive estimates are suggestive of disruptive selection (which pushes the distribution of trait values towards the two extremes). Finally, in order to examine whether and how skin ornaments and dominance rank interact in influencing fecundity, we calculated the correlational selection gradient (γij, i ≠ j) by multiplying both standardized measures of darkness and of redness with standardized dominance rank. Here, positive estimates indicate that highest reproductive output is reached when both traits are at maximal value, whereas negative estimates suggest that the highest reproductive output is reached when either one of the two traits is maximized.
For fixed effects, we included natal rank, age and reproductive status for females, and age, dispersal rate (i.e. estimated average dominance rank) and average group productivity for males. All continuous demographic variables were square-root-transformed in male models to achieve normal distributions; no transformations were required in female models. Moreover, in order to fulfil model assumptions concerning the distribution of the residuals, fecundity measures were log-transformed in male models. However, as the assessment of selection gradients should be undertaken with non-transformed measures of fecundity [57], we provide the estimates of models using untransformed measures of fecundity (i.e. β, γii, γij). GLMs were performed in R using the glm function, with significance level set at α < 0.05.
(ii). Heritability analyses
We analysed the heritability of rhesus facial skin colour using animal models, which combine pedigree information in linear mixed effects models to estimate the additive genetic variance of the trait of interest in the population under study [36,60]. For both redness and darkness, we fit a univariate general linear mixed model using the R package MCMCglmm [61,62]. Animal ID, maternal ID, household and current group were modelled as random effects, whereas age, sex, natal rank and reproductive status (females only) were modelled as fixed effects. Sex and natal rank were subsequently excluded because they were not significant factors in the models. As the trait is likely to be sex-influenced (i.e. show greater inheritance in expression from same-sex parents), we also ran models using data from only males and females separately, removing information about the opposite sex parent by creating a unique dummy ID for each subject.
Each model was run for 2 550 000 iterations with the first 50 000 iterations dropped as a burn-in and the process sampled every 1000 iterations thereafter. Each model was verified to have converged on a stationary distribution by visually assessing plots of the Markov chain Monte Carlo (MCMC) chain. Estimates of additive genetic variance were obtained by calculating the mode of the posterior distribution of the animal effect. Estimates of the narrow-sense heritability of each visual channel were then calculated as the proportion of total phenotypic variance explained by additive genetic variance (h2 = VA/VP). We ran the models using different priors: (i) flat improper prior; (ii) parameter expanded; (iii) inverse-Wishart; and (iv) inverse-Gamma [38,60,62,63]. Models with flat priors would not compute, while those with parameter expanded priors did not respect MCMC diagnostics related to auto-correlation and the distribution of the posteriors (trace and density estimates) [64]. Of the two remaining prior types, we selected inverse-Gamma priors based on consistent production of the lowest deviance information criteria values.
3. Results
Males showed no evidence of fecundity-related differences linked to sex skin redness or darkness in the linear or quadratic models, but a significant positive relationship was found for darkness (low luminance) in the correlational model (figure 1 and table 1). This suggests that optimal fecundity is observed when darkness and high social status are combined. As for females, redness was positively associated with fecundity, such that redder females reproduced at higher rates (figure 2), but no significant effect was found in the quadratic or correlational model (table 1).
Figure 1.
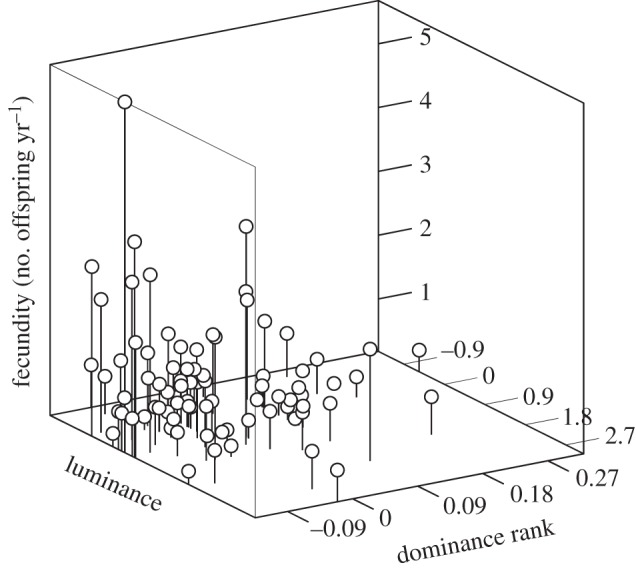
Three-dimensional plot illustrating the effect of male luminance and dominance rank on fecundity. Note that greater saturation of blood leads to darker, and thus less luminous, sex skin. The plot was created with ‘Excel 3D Scatter Plot’ macros available for free at http://www.doka.ch/Excel3Dscatterplot.htm.
Table 1.
Selection gradients of skin redness and darkness with standard errors, and corresponding GLM results. (For males, demographic variables were square-root-transformed and fecundity log-transformed. Estimates of selection gradients provided for males were assessed based on models using non-transformed measures of fecundity, however (see Material and methods for details). Note that regression coefficients and standard errors of the quadratic selection gradients were doubled (see Material and methods for more details). Significant selection gradient results are given in bold. AIC, Akaike information criteria.)
| linear | quadratic | correlational | |
|---|---|---|---|
| males | |||
| redness | β = 0.149 ± 0.110 | γii = 0.080 ± 0.173 | γij = −0.001 ± 0.134 |
| t = 1.329, p = 0.187 | t = 1.167, p = 0.247 | t =−1.224, p = 0.225 | |
| darkness | β = 0.162 ± 0.120 | γii =−0.229 ± 0.165 | γij = 1.229 ± 1.337 |
| t = 1.649, p = 0.103 | t =−0.092, p = 0.362 | t = 2.041, p = 0.044 | |
| age | t = 0.732, p = 0.466 | t = 0.840, p = 0.403 | t = 0.807, p = 0.422 |
| dominance ranka | t =−3.208, p = 0.002 | t =−2.961, p = 0.004 | t =− 2.531, p = 0.014 |
| group productivityb | t = 2.494, p = 0.015 | t = 2.640, p = 0.010 | t = 2.490, p = 0.015 |
| AIC | 169.82 | 171.67 | 168.88 |
| females | |||
| redness | β = 0.118 ± 0.030 | γii = 0.024 ± 0.030 | γij =−0.318 ± 0.272 |
| t = 3.897, p < 0.001 | t = 0.805, p = 0.423 | t =−1.169, p = 0.246 | |
| darkness | β = 0.013 ± 0.035 | γii =−0.000 ± 0.024 | γij =−0.407 ± 0.230 |
| t = 0.357, p = 0.722 | t =−0.002, p = 0.998 | t = 1.769, p = 0.081 | |
| age | t = 18.767, p < 0.001 | t = 18.526, p < 0.001 | t = 18.418, p < 0.001 |
| rank | t =−1.847, p = 0.069 | t =−1.702, p = 0.093 | t = 2.676, p = 0.351 |
| reproductive statusc | t = 2.122, p = 0.037 | t = 2.141, p = 0.035 | t = 2.676, p = 0.009 |
| AIC | 28.793 | 31.999 | 40.613 |
aFor males, dispersal rate is used as a proxy for dominance rank through a subject's life.
bFor males only. Group productivity is calculated based on the average number of offspring produced yearly in the groups in which each male resided.
cEstimates based on behavioural observation and date of birth during the following birth season (see Material and methods for details).
Figure 2.
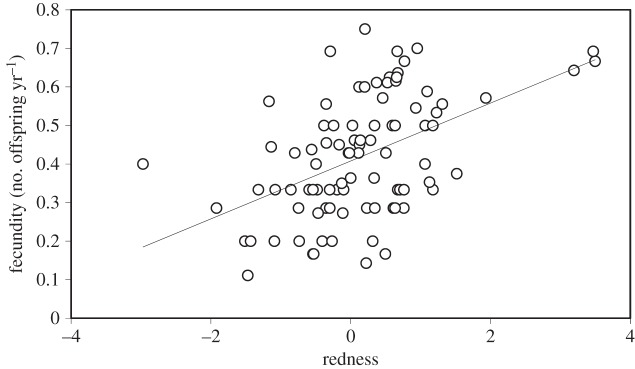
Linear relationships between female redness and fecundity.
Facial skin redness and darkness were heritable, with between 10% and 15% of the variation among subjects explained by additive genetic variance (h2; table 2). When the two sexes were considered separately, slightly different patterns emerged between redness and darkness: heritability of skin darkness was 2–2.5 times higher for both sexes and explained 25–30% of the variation, while heritability of redness levels remained roughly the same. It should be noted that the confidence intervals for skin darkness are very wide, and overlap with those of skin redness. Environmental effects were less important for darkness than for redness, in both the general and sex-linked analyses (table 2).
Table 2.
Heritability estimates of skin redness and darkness, with confidence intervals in parentheses. Analyses are presented for both the general model (where both sexes are considered; ‘all’) and for the sex-influenced analyses (where only same-sex parent–offspring dyads are considered; ‘females’ and ‘males’).
| skin redness |
skin darkness |
|||||
|---|---|---|---|---|---|---|
| all | males | females | all | males | females | |
| n = 266 | n = 127 | n = 139 | n = 266 | n = 127 | n = 139 | |
| heritability | 11.80 (5.28–18.03) | 12.78 (5.89–18.94) | 7.37 (2.10–15.87) | 12.51 (5.68–35.96) | 30.70 (8.12–56.74) | 24.52 (3.61–43.72) |
| natal effects | ||||||
| maternal effect | 10.90 (5.85–19.47) | 9.47 (4.67–15.68) | 7.00 (1.73–13.66) | 9.01 (3.51–27.50) | 8.63 (3.09–16.87) | 7.77 (2.00–20.94) |
| household effect | 24.88 (9.68–39.11) | 22.67 (8.41–36.55) | 20.85 (4.21–44.97) | 10.24 (3.27–26.84) | 8.58 (3.61–24.02) | 13.44 (3.09–37.29) |
| group effect | 35.48 (19.84–64.94) | 47.59 (22.51–68.76) | 44.90 (22.19–83.98) | 9.76 (3.48–31.05) | 9.32 (3.63–26.24) | 19.88 (3.48–59.34) |
| fixed effects | ||||||
| age | significant | significant | n.s. | significant | significant | significant |
| reproductive status | — | significant | n.s. | — | significant | significant |
| DIC | −763.4321 | −1648.227 | −820.4348 | −444.871 | −791.3593 | −472.4431 |
Age had strong effects on skin darkness, with older individuals being lighter than younger individuals, and older males (but not females) being redder (table 2). We also found an effect of reproductive status, mainly owing to pregnant females being redder, and sexually active females being darker, than other females (table 2). Facial skin redness or darkness was not influenced by sex.
4. Discussion
Our results show that inter-individual variation in red skin coloration influences fecundity and is heritable in Cayo Santiago rhesus macaques. Based solely on studies showing female attraction towards dark males [17,19], we would have expected directional selection on the ornament. Our results rather indicate that skin darkness provided significant reproductive advantages to males when combined with high dominance rank—i.e. in a context where males are being potentially successful at both inter-sexual attraction and intra-sexual competition. As the two traits do not covary, this suggests that red skin ornaments are under balancing selection, a mechanism that would contribute to the maintenance of intraspecific variation. A similar phenomenon has been reported for relative gonad mass and body size in three-spine stickleback [6], and male pheromones and dominance rank in cockroaches [5]. In Cayo Santiago rhesus macaques, male dominance rank is mainly reached through queuing with little direct fighting [53–55]. While high dominance rank increases access to a primary mating tactic, mate-guarding [26,31], the efficiency of this tactic has been shown to depend greatly on female cooperation [65]. It is possible that females are more likely to cooperate in contexts where mate-guarding males are more attractive to them, such as males who exhibit darker skin colour, though this is currently untested.
For females, our results show that fecundity was positively related to skin redness, the aspect of the ornament that is most likely to be influenced by blood oxygenation, and in turn by health and condition. As rhesus males are selective, they may gain by selecting redder females as preferred mates [18]. However, our perceptual modelling studies suggest that rhesus macaque coloration varies much more achromatically than chromatically [11,13], and it thus remains unclear whether inter-individual variation in redness alone is perceived by males and thus could be used in mate selection. As rhesus macaques form stable heterosexual groups, males may have numerous opportunities to examine female skin redness in good lightning conditions, which would allow trait perception. Alternatively, it is possible that the link between skin redness and fecundity is an indirect consequence of healthier females producing more offspring, without a role for sexual selection. Future studies should examine directly whether inter-female variation in skin colour influences male mate choice.
When sexes are combined for analyses, skin redness and darkness showed heritability, but at rather low levels (h2 ≈ 12%), suggesting that they might be highly influenced by the context in which they are expressed. Based on the underlying physiology of the trait, this suggests that the trait is condition-dependent, although this remains to be tested directly. Interestingly, the two aspects of the ornament revealed different patterns of heritability when the two sexes are considered separately. While skin redness heritability remained around 10%, levels of heritability for skin darkness increased by up to 2.5-fold. This suggests that skin darkness might be less condition-dependent than redness, and under some level of sex-influenced inheritance. At 25–30%, skin darkness reaches levels of heritability similar to those reported for male body size and weaponry more commonly encountered in mammals (e.g. white-tailed deer, Odocoileus virginianus [66]; red deer, Cervus elaphus [67]; Soay sheep [7]).
The difference between skin redness and darkness in patterns of selection and heritability is likely to be linked to the underlying mechanisms involved in expression of the signal. Both blood oxygenation (skin redness) and blood flow (skin darkness) are influenced by various factors affecting health (see Introduction). However, blood flow in sex skin is further influenced by the number, sensitivity and activation of oestrogen receptors in the facial epidermis capillaries, as well as by testosterone concentrations in males and oestrogen concentrations in females [15]. These are under genetic control and are likely to be sex-influenced, with genes related to steroid hormone control particularly influenced by the same-sex parent. As such, skin darkness created by blood flow is likely to be the aspect of the ornament that is under sexual selection through mate choice. We speculate that this may have evolved to make information about the signaller's condition conveyed by skin redness more conspicuous. Despite this, it is important to note that our analyses reveal large confidence intervals for both chromatic and achromatic aspects of the ornament, leading to an overlap in their ranges. This opens the question of whether the two components actually exhibit significantly different levels of heritability. Alternatively, large intervals could emerge from analyses if unknown factors not included here also influence trait expression [68], or if condition-dependence leads to inter-individual variation in the extent to which genetic background predicts trait expression at any one time.
In summary, our study reveals that the red skin ornamentation exhibited by rhesus macaques influences fecundity and is heritable. Collectively, our results suggest that condition-dependence contributes to inter-individual variation in sexual skin redness and darkness, with variation in darkness further maintained by balancing selection owing to an interaction between inter- and intra-sexual selection on males. When combined with prior observational and experimental evidence, these results confirm that this ornament is under sexual selection and create arguably the most complete and thorough evidence for a trait selected through inter- rather than intra-sexual selection in a large mammal [34]. This work highlights the relevance of using an integrated approach combining behavioural observations with quantitative genetics as a way of understanding the mechanisms by which traits are selected and maintained.
Ethics statement
The investigation was approved by the IACUC of the University of Puerto Rico, Medical Sciences Campus (protocol no. A0100108).
Acknowledgements
We thank the CPRC for permission to conduct this study, and appreciate the support of the staff for collecting demographic and DNA samples, and help in subject identification, in particular E. Davila, J. Resto, G. Caraballo Cruz and N. Rivera Barreto. We thank S. Coyne, E. Dunayer, A. Georgiev and T. Mandalaywala for sharing information; and L. Kulik and S. Bley for help in generating and managing the genetic parentage database. We thank Gregory Blomquist for advice on heritability analyses, and Brian Mautz for discussion on selection gradients; any remaining mistakes are our own. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of NCRR, ORIP or NIH.
Funding statement
This project was made possible by intramural funds from New York University to J.P.H., FQRSC to C.D., DFG (grant no. Wi 1808/3-1) to A.W., as well as NCRR and ORIP of NIH (grant no. 2P40RR03640-25) to C.P.R.C.
References
- 1.Darwin C. 1871. The descent of man and selection in relation to sex. London, UK: John Murray. [Google Scholar]
- 2.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 3.Cotton S, Fowler K, Pomiankowski A. 2004. Condition dependence of sexual ornament size and variation in the stalk-eyed fly Cyrtodiopsis dalmanni (Diptera: Diopsidae). Evolution 58, 1038–1046. ( 10.1111/j.0014-3820.2004.tb00437.x) [DOI] [PubMed] [Google Scholar]
- 4.Greene E, Lyon BE, Muehter VR, Ratcliffe L, Oliver SJ, Boag PT. 2000. Disruptive sexual selection for plumage coloration in a passerine bird. Nature 407, 1000–1003. ( 10.1038/35039500) [DOI] [PubMed] [Google Scholar]
- 5.Moore AJ, Moore PJ. 1999. Balancing sexual selection through opposing mate choice and male competition. Proc. R. Soc. Lond. B 266, 711–716. ( 10.1098/rspb.1999.0694) [DOI] [Google Scholar]
- 6.Bolnick DI. 2004. Can intraspecific competition drive disruptive selection? An experimental test in natural populations of sticklebacks. Evolution 58, 608–618. ( 10.1111/j.0014-3820.2004.tb01683.x) [DOI] [PubMed] [Google Scholar]
- 7.Johnston SE, Gratten J, Berenos C, Pilkington JG, Clutton-Brock TH, Pemberton JM, Slate J. 2013. Life history trade-offs at a single locus maintain sexually selected genetic variation. Nature 502, 93–95. ( 10.1038/nature12489). [DOI] [PubMed] [Google Scholar]
- 8.Caro T. 2005. The adaptive significance of coloration in mammals. Bioscience 55, 125–136. ( 10.1641/0006-3568(2005)055[0125:TASOCI]2.0.CO;2) [DOI] [Google Scholar]
- 9.Bradley BJ, Mundy NI. 2008. The primate palette: the evolution of primate coloration. Evol. Anthropol. 17, 97–111. ( 10.1002/evan.20164) [DOI] [Google Scholar]
- 10.Baulu J. 1976. Seasonal sex skin coloration and hormonal fluctuations in free-ranging and captive monkeys. Horm. Behav. 7, 481–494. ( 10.1016/0018-506X(76)90019-2) [DOI] [PubMed] [Google Scholar]
- 11.Higham JP, Heistermann M, Maestripieri D, Stevens M. 2013. Signaling in multiple modalities in male rhesus macaques: sex skin coloration and barks in relation to androgen levels, social status, and mating behavior. Behav. Ecol. Sociobiol. 67, 1457–1469. ( 10.1007/s00265-013-1521-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubuc C, Brent LJN, Accamando AK, Gerald MS, MacLarnon A, Semple S, Heistermann M, Engelhardt A. 2009. Sexual skin color contains information about the timing of the fertile phase in free-ranging rhesus macaques. Int. J. Primatol. 30, 777–789. ( 10.1007/s10764-009-9369-7) [DOI] [Google Scholar]
- 13.Higham JP, Brent LJN, Dubuc C, Accamando AK, Engelhardt A, Gerald MS, Heistermann M, Stevens M. 2010. Color signal information content and the eye of the beholder: a case study in the rhesus macaque. Behav. Ecol. 21, 739–746. ( 10.1093/beheco/arq047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandenbergh JG. 1965. Hormonal basis of sex skin in male rhesus monkeys. Gen. Comp. Endoc. 5, 31–34. ( 10.1016/0016-6480(65)90065-1) [DOI] [PubMed] [Google Scholar]
- 15.Rhodes L, Argersinger LT, Gantert LT, Friscino BH, Hom G, Pikounis B, Hess DL, Rhodes WL. 1997. Effects of administration of testosterone, dihydrotestosterone, oestrogen and fadrozole, an aromatase inhibitor, on sex skin colour in intact male rhesus macaques. J. Reprod. Fert. 111, 51–57. ( 10.1530/jrf.0.1110051) [DOI] [PubMed] [Google Scholar]
- 16.Dixson AF. 2012. Primate sexuality, 2nd edn Oxford, UK: Oxford University Press. [Google Scholar]
- 17.Waitt C, Little AC, Wolfensohn S, Honess P, Brown AP, Buchanan-Smith HM, Perrett DI. 2003. Evidence from rhesus macaques suggests that male coloration plays a role in female primate mate choice. Proc. R. Soc. Lond. B 270, S144–S146. ( 10.1098/rsbl.2003.0065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waitt C, Gerald MS, Little AC, Krebs JR. 2006. Selective attention toward female secondary sexual color in male rhesus macaques. Am. J. Primatol. 68, 738–744. ( 10.1002/ajp.20264) [DOI] [PubMed] [Google Scholar]
- 19.Dubuc C, Allen WL, Maestripieri D, Higham JP. 2014. Is male rhesus macaque red colour ornamentation attractive to females? Behav. Ecol. Sociobiol. 68, 1215–1224. ( 10.1007/s00265-014-1732-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higham JP, Hughes KD, Brent LJN, Dubuc C, Engelhardt A, Heistermann M, Maestripieri D, Santos LR, Stevens M. 2011. Familiarity affects assessment of facial signals of female fertility by free-ranging male rhesus macaques. Proc. R. Soc. B 278, 3452–3458. ( 10.1098/rspb.2011.0052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darwin C. 1872. The expression of the emotion in man and animals. London, UK: John Murray. [Google Scholar]
- 22.Changizi MA, Zhang Q, Shimojo S. 2006. Bare skin, blood and the evolution of primate colour vision. Biol. Lett. 2, 217–221. ( 10.1098/rsbl.2006.0440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folstad I, Karter AJ. 1992. Parasites, bright males and the immunocompetence handicap. Am. Nat. 2, 603–622. ( 10.1086/285346) [DOI] [Google Scholar]
- 24.Harcourt AH, Harvey PH, Larson SG, Short RV. 1981. Testis weight, body weight and breeding system in primates. Nature 293, 55–57. ( 10.1038/293055a0) [DOI] [PubMed] [Google Scholar]
- 25.Plavcan JM. 2004. Sexual selection measures of sexual selection, and sexual dimorphism in primates. In Sexual selection in primates: new and comparative perspectives (eds Kappeler PM, van Schaik CP.), pp. 230–252. New York, NY: Cambridge University Press. [Google Scholar]
- 26.Altmann SA. 1962. A field study of the sociobiology of rhesus monkeys, Macaca mulatta. Ann. NY Acad. Sci. 102, 338–435. ( 10.1111/j.1749-6632.1962.tb13650.x) [DOI] [PubMed] [Google Scholar]
- 27.Dubuc C, Muniz L, Heistermann M, Engelhardt A, Widdig A. 2011. Testing the priority-of-access model in a seasonally breeding primate species. Behav. Ecol. Sociobiol. 65, 1615–1627. ( 10.1007/s00265-011-1172-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubuc C, Muniz L, Heistermann M, Widdig A, Engelhardt A. 2012. Do males time their mate-guarding effort with the fertile phase in order to secure fertilisation in Cayo Santiago rhesus macaques? Horm. Behav. 61, 696–705. ( 10.1016/j.yhbeh.2012.03.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manson JH. 1995. Do female rhesus macaques choose novel males? Am. J. Primatol. 37, 285–296. ( 10.1002/ajp.1350370403) [DOI] [PubMed] [Google Scholar]
- 30.Chapais B. 1983. Reproductive activity in relation to male dominance and the likelihood of ovulation in rhesus monkeys . Behav. Ecol. Sociobiol. 12, 215–228. ( 10.1007/BF00290774) [DOI] [Google Scholar]
- 31.Berard JD, Nuernberg P, Epplen JT, Schmidtke J. 1994. Alternative reproductive tactics and reproductive success in male rhesus in male rhesus macaques. Behaviour 129, 177–201. ( 10.1163/156853994X00604) [DOI] [Google Scholar]
- 32.Widdig A, Bercovitch FB, Streich WJ, Sauermann U, Nürnberg P, Krawczak M. 2004. A longitudinal analysis of reproductive skew in male rhesus macaques. Proc. R. Soc. Lond. B 271, 819–826. ( 10.1098/rspb.2003.2666) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soltis J. 2004. Mating systems. In Macaque societies: a model for the study of social organization (eds Thierry B, Singh M, Kaumanns W.), pp. 135–151. New York, NY: Cambridge University Press. [Google Scholar]
- 34.Clutton-Brock TH, McAuliffe K. 2009. Female mate choice in mammals. Q. Rev. Biol. 84, 3–27. ( 10.1086/596461) [DOI] [PubMed] [Google Scholar]
- 35.Blomquist GE. 2009. Fitness-related patterns of genetic variation in rhesus macaques. Genetica 135, 209–219. ( 10.1007/s10709-008-9270-x) [DOI] [PubMed] [Google Scholar]
- 36.Blomquist GE, Brent LJN. 2014. Applying quantitative genetic methods to primate social behavior. Int. J. Primatol. 35, 108–128. ( 10.1007/s10764-013-9709-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brent LJN, Heilbronner SR, Horvath JE, Gonzalez-Martinez J, Ruiz-Lambides A, Robinson AG, Skene JHP, Platt ML. 2013. Genetic origins of social networks in rhesus macaques. Sci. Rep. 3, 1042 ( 10.1038/srep01042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brent LJN, Semple S, MacLarnon A, Gonzalez-Martinez J, Ruiz-Lambides AV, Platt ML. 2014. Personality traits in rhesus macaques (Macaca mulatta) are heritable but do not predict reproductive output. Int. J. Primatol. 35, 188–209. ( 10.1007/s10764-013-9724-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevens M, Stoddard MC, Higham JP. 2009. Studying primate color: towards visual system-dependent methods. Int. J. Primatol. 30, 893–917. ( 10.1007/s10764-009-9356-z) [DOI] [Google Scholar]
- 40.Stephen ID, Coetzee V, Law Smith MJ, Perrett DI. 2009. Skin blood perfusion and oxygenation color affect perceived human health. PLoS ONE 4, e5083 ( 10.1371/journal.pone.0005083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rawlins RG, Kessler MJ. 1986. The history of the Cayo Santiago colony. In The Cayo Santiago Macaques (eds Rawlins RG, Kessler MJ.), pp. 13–45. Albany, NY: State University of New York Press. [Google Scholar]
- 42.Duggleby CR, Haseley PA, Rawlins RG, Kessler MJ. 1986. An overview of blood group genetic studies on the Cayo Santiago rhesus monkeys. In The Cayo Santiago Macaques (eds Rawlins RG, Kessler MJ.), pp. 269–282. Albany, NY: State University of New York Press. [Google Scholar]
- 43.Dubuc C, Ruiz-Lambides A, Widdig A. 2014. Variance in male lifetime reproductive success and estimation of the degree of polygyny in a primate. Behav. Ecol. 25, 878–889. ( 10.1093/beheco/aru052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rowell TE. 1972. Female reproduction cycles and social behavior in primates. Adv. Stud. Behav. 4, 69–105. ( 10.1016/S0065-3454(08)60007-8) [DOI] [Google Scholar]
- 45.Gerald MS, Waitt C, Little C. 2009. Pregnancy coloration in macaques may act as a warning signal to reduce antagonism by conspecifics. Behav. Process. 80, 7–11. ( 10.1016/j.beproc.2008.08.001) [DOI] [PubMed] [Google Scholar]
- 46.Osorio D, Vorobyev M. 2005. Photoreceptor spectral sensitivities in terrestrial animals: adaptations for luminance and colour vision. Proc. R. Soc. B 272, 1745–1752. ( 10.1098/rspb.2005.3156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kulik L, Muniz L, Mundry R, Widdig A. 2012. Patterns of interventions and the effect of coalitions and sociality on male fitness. Mol. Ecol. 21, 699–714. ( 10.1111/j.1365-294X.2011.05250.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bercovitch FB. 1997. Reproductive strategies of rhesus macaques. Primates 38, 247–263. ( 10.1007/BF02381613) [DOI] [Google Scholar]
- 49.Krawczak M, et al. 2005. Male reproductive timing in rhesus macaques is influenced by the 5HTTLPR promoter polymorphism of the serotonin transporter gene. Biol. Reprod. 72, 1109–1113. ( 10.1095/biolreprod.104.038059) [DOI] [PubMed] [Google Scholar]
- 50.Maestripieri D. 2007. Macachiavellian intelligence. Chicago, IL: University of Chicago Press. [Google Scholar]
- 51.Melnick DJ, Pearl MC. 1987. Cercopithecines in multi-male groups: genetic diversity and population structure. In Primate societies (eds Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT.), pp. 121–134. Chicago, IL: University of Chicago Press. [Google Scholar]
- 52.Belisle P, Chapais B. 2001. Tolerated co-feeding in relation to degree of kinship in Japanese macaques. Behaviour 138, 487–509. ( 10.1163/156853901750382124) [DOI] [Google Scholar]
- 53.Berard JD. 1999. A four-year study of the association between male dominance rank, residency status, and reproductive activity in rhesus macaques (Macaca mulatta). Primates 40, 159–175. ( 10.1007/BF02557708) [DOI] [PubMed] [Google Scholar]
- 54.van Noordwijk MA, van Schaik CP. 2004. Sexual selection and the careers of primate males: paternity concentration, dominance acquisition tactics and transfer decisions. In Sexual selection in primates (eds Kappeler PM, van Schaik CP.), pp. 208–229. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 55.Higham JP, Heistermann M, Maestripieri D. 2013. The endocrinology of male rhesus macaque social and reproductive status: a test of the challenge and social stress hypotheses. Behav. Ecol. Sociobiol. 67, 19–30. ( 10.1007/s00265-012-1420-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Silk J, Short J, Roberts J, Kusnitz J. 1993. Gestation length in rhesus macaques (Macaca mulatta). Int. J. Primatol. 14, 95–104. ( 10.1007/BF02196505) [DOI] [Google Scholar]
- 57.Lande R, Arnold SJ. 1983. Measuring selection on correlated characters. Evolution 37, 1210–1226. ( 10.2307/2408842) [DOI] [PubMed] [Google Scholar]
- 58.Klug H, Lindstrom K, Kokko H. 2010. Who to include in studies of sexual selection is no trivial matter . Ecol. Lett. 13, 1094–1102. ( 10.1111/j.1461-0248.2010.01495.x) [DOI] [PubMed] [Google Scholar]
- 59.Stinchcombe JR, Agrawal AF, Hohenlohe PA, Arnold SJ, Blows MW. 2008. Estimating nonlinear selection gradients using quadratic regression coefficients: double or nothing? Evolution 62, 2435–2440. ( 10.1111/j.1558-5646.2008.00449.x) [DOI] [PubMed] [Google Scholar]
- 60.Wilson AJ, Réale D, Clements MN, Morrissey MM, Postma E, Walling CA, Kruuk LEB, Nussey DH. 2010. An ecologist's guide to the animal model. J. Anim. Ecol. 79, 13–26. ( 10.1111/j.1365-2656.2009.01639.x) [DOI] [PubMed] [Google Scholar]
- 61.R Core Team. 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 62.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22.20808728 [Google Scholar]
- 63.Teplitsky C, Mouawad NG, Balbontín J, de Lope F, Møller AP. 2011. Quantitative genetics of migration syndromes: a study of two barn swallow populations . J. Evol. Biol. 24, 2025–2038. ( 10.1111/j.1420-9101.2011.02342.x) [DOI] [PubMed] [Google Scholar]
- 64.Hadfield JD. 2012. MCMCglmm course notes See http://cran.r-project.org/web/packages/MCMCglmm/vignettes/CourseNotes.pdf.
- 65.Manson JH. 1997. Primate consortships: a critical review. Curr. Anthropol. 38, 353–374. ( 10.1086/204623) [DOI] [Google Scholar]
- 66.Lukefahr SD, Jacobson HA. 1998. Variance component analyses and heritability of antler traits in white-tailed deer. J. Wild. Manage. 62, 262–268. ( 10.2307/3802287) [DOI] [Google Scholar]
- 67.Kruuk LEB, Slate J, Pemberton JM, Brotherstone S, Guiness F, Clutton-Brock T. 2002. Antler size in red deer: heritability and selection but no evolution. Evolution 56, 1653–1695. ( 10.1111/j.0014-3820.2002.tb01480.x) [DOI] [PubMed] [Google Scholar]
- 68.Letcher BH, Coombs JA, Nislow KH. 2011. Maintenance of phenotypic variation repeatability, heritability and size-dependent processes in a wild trout population. Evol. Appl. 4, 602–615. ( 10.1111/j.1752-4571.2011.00184.x) [DOI] [PMC free article] [PubMed] [Google Scholar]


