Abstract
We are limited in our ability to predict climate-change-induced range shifts by our inadequate understanding of how non-climatic factors contribute to determining range limits along putatively climatic gradients. Here, we present a unique combination of observations and experiments demonstrating that seed predation and soil properties strongly limit regeneration beyond the upper elevational range limit of sugar maple, a tree species of major economic importance. Most strikingly, regeneration beyond the range limit occurred almost exclusively when seeds were experimentally protected from predators. Regeneration from seed was depressed on soil from beyond the range edge when this soil was transplanted to sites within the range, with indirect evidence suggesting that fungal pathogens play a role. Non-climatic factors are clearly in need of careful attention when attempting to predict the biotic consequences of climate change. At minimum, we can expect non-climatic factors to create substantial time lags between the creation of more favourable climatic conditions and range expansion.
Keywords: climate change, range expansion, substrate, biotic interactions, seed predation, Acer saccharum
1. Introduction
Climate change promises to reshuffle species' distributions across the earth [1,2], but predicting the outcome presents a grand scientific challenge given the many spatially correlated factors involved. For plant species, climate, soil properties and biotic interactions combine to determine geographical distributions [3], but the relative importance of these factors in defining range limits is difficult to study given that they often change in concert along the same gradients [4,5], such as elevation or latitude. Based on climatic factors alone, we predict that distributions will move upslope and to higher latitudes as annual temperatures increase. There have been many observed cases of distribution shifts towards higher elevations or latitudes [2], yet often at rates slower than climate change itself [1,6], suggesting that lags or non-climatic factors are slowing down climate-induced species' range shifts. While many studies point to climate and soil as the leading determinants of the distributions of terrestrial plant species, there is a paucity of data addressing how edaphic factors may influence species' range shifts with climate change [7–9].
Biotic interactions are also expected to be altered by climate change, but their potentially important influence on geographical range limits has not received sufficient attention in empirical studies [10–13]. The presence of mutualists, competitors, parasites or predators can influence the occurrence and abundance of a species in a given locale, and the strength of these interactions can also change across environmental gradients (e.g. [14–17]). However, few studies have tested how biotic interactions influence the location of species' range limits (see [12] for review), and repeated calls have been made for greater consideration and empirical study of biotic interactions in the context of species' range expansions with climate change [12,13,17]. The few studies conducted to date suggest that positive biotic interactions may accelerate the rate at which species' distributions respond to climate change while negative interactions may slow them [12,18].
Given the frequent spatial correlation of climate, soil properties and community composition [3], experimentation is clearly needed to test the contributions of non-climatic factors to limiting species' distributions along putatively climatic gradients [5,7]. However, in the case of soil, simple manipulations (e.g. water or nutrient addition) may be of limited value given that soil is characterized by a complex set of abiotic and biotic properties that influence the establishment and growth of plants [19]. One alternative is to transplant bulk soil across sites at different elevations or latitudes, but we are aware of no such studies applied beyond a species's distribution, and very few that manipulate soils within a species's natural range [20,21], perhaps given the massive effort required to transport large quantities of soil at relevant scales.
To test the role of soil properties in defining the upper elevational range limit of sugar maple (Acer saccharum Marshall), a tree species of considerable economic importance in eastern North America [22], we conducted a reciprocal transplant experiment of soil and seeds across elevations. Following suggestive evidence from this first experiment of seed predation at high elevations, we then conducted a second experiment explicitly testing for the potential of seed predation to also constrain upward elevational migration.
Our study area, Parc national du Mont Mégantic, Québec, is representative of many mountainous areas in northeastern North America, where sugar maple is a dominant component of the deciduous forest at low elevations [23] (figure 1a; electronic supplementary material, figure S1), giving way to boreal forest dominated by spruce (Picea spp.) and balsam fir (Abies balsamea (L.) Mill.) at the highest elevations. Within this park, there is evidence that plant distributions are shifting upslope, but at a much slower rate than climate isotherms, suggesting a lag in species' response to warming climatic conditions [24]. At our site and others [25,26], soil in the boreal forest tends to be more acidic, and to have slower decomposition rates (higher C : N ratio) and shallower fine till, than in the deciduous forest. We predicted that soil from higher elevations would have a negative impact on seedling establishment, as would the high-elevation planting site itself (due to unfavourable climate). Based on observations in the first experiment, we also predicted that the magnitude of seed predation would increase with elevation. Our results provide some of the first direct experimental evidence revealing that non-climatic factors, specifically seed predation and soil properties, strongly constrain range expansion along a putatively climatic gradient (i.e. elevation).
Figure 1.
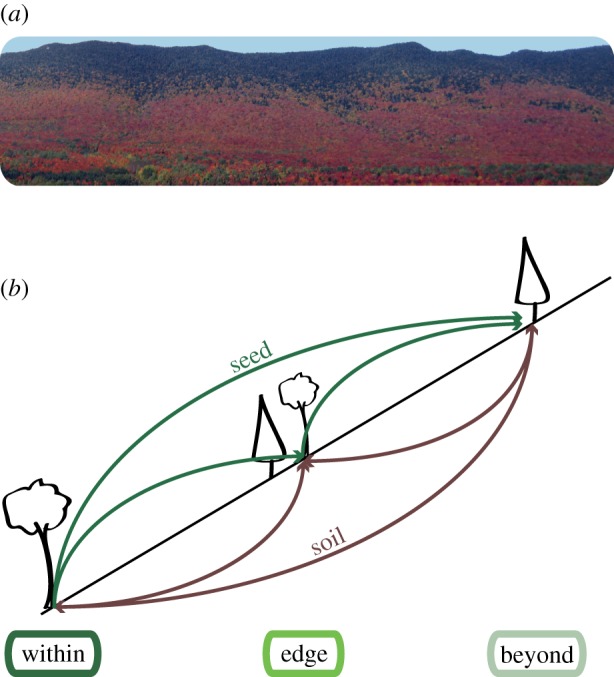
Experimental design along the elevational gradient. (a) East-facing slope of Mont St Joseph and adjacent peaks in Parc national du Mont Mégantic, Québec, where the field experiment was conducted. Sugar maple populations display red autumn foliage downslope from conifer populations. (b) Conceptual diagram showing relocation of soil (brown) and seed (green) along the elevational gradient. Soil and seed were also planted into source locations (i.e. soil from ‘within’ was installed at ‘within’ in addition to at the ‘edge’ and ‘beyond’). This was replicated on two elevational transects, with soil and seed (when possible) moved across transects at a given elevation as well.
2. Material and methods
(a). Study sites and species
The studied elevational gradient at Parc national du Mont Mégantic, Québec, Canada (45°26′51″N, 71°06′52″W) is east-facing and transitions from sugar maple-dominated stands at low elevation to balsam fir stands at high elevation (figure 1a), traversing approximately 470 m in elevation (600–1070 m.a.s.l.). Sugar maple is a deciduous tree species native to northeastern North America, spanning a latitudinal gradient from approximately 35–49°N. It occurs at low- to mid-elevation stands throughout its range, notably in the Appalachian Mountains in which our study area occurs, where the upper-elevational species limit is thought to be controlled by climatic factors [25]. It is anticipated that sugar maple will shift its distribution as environmental conditions become more favourable at the northern limit of its range with climate change [27]. Sugar maple is a monoecious species that flowers in early spring prior to leaf flush, and wind-disperses its seed in autumn. Propagules (winged samaras) overwinter on the ground, experiencing several months of cold stratification, and seedlings emerge early the following spring, with germination occurring at approximately 1°C [22]. Establishment of sugar maple is favoured by well-drained loam soils with pH of 5.5–7.3, but the species can tolerate a range of soil textures provided they are neither too dry nor too shallow [22]. Research on nutritional requirements indicates that sugar maple survival, growth and reproduction can be limited by low soil concentrations of calcium and magnesium [28,29], which can become depleted following acid deposition [29,30].
Sites were established in three elevational zones spanning the distribution of sugar maple from within its range to just beyond the upper elevational limit. The sites, replicated along two transects (‘north’ and ‘south’), were located: (i) within its distribution in stands dominated by sugar maple (approx. 726–747 m.a.s.l., hereafter ‘within’); (ii) at the edge of its distribution where sugar maple was less dominant (relative importance of 42% versus 92% at lower elevations) and occurred alongside yellow birch (Betula alleghensiensis Britt.), balsam fir and red spruce (Picea rubens Sarg.; approx. 796–827 m.a.s.l., hereafter ‘edge’); and (iii) beyond sugar maple's elevational limit within a mix of balsam fir, red spruce and yellow birch (approx. 883–884 m.a.s.l., hereafter ‘beyond’). For context, we quantified the density of adult and seedling sugar maples across the elevational gradient, determining that abundance is uniformly high up to approximately 800 m elevation, declining rapidly to zero above 850 m (electronic supplementary material, figure S1 shows the distribution of sugar maple seedlings and trees across the elevational gradient). Based on previous studies of Acer spp. and other species with winged samaras, in which seed dispersal distances of up to 300 m have been estimated [31,32], we selected sites beyond the species's elevational limit that were well within realistic long-distance dispersal distances from edge populations (less than 100 m).
(b). Substrate reciprocal transplant experiment
We reciprocally transplanted soil (as well as leaf litter) among the three sites (within, edge and beyond), and the soil treatment was crossed with two seed populations (within and edge; figure 1). Soil and seed were also reciprocally transplanted laterally between sites along the two transects at their elevation of origin (i.e. between ‘within’ sites on the north and south transect), to test for between-transect differences. Finally, soil was excavated and replanted at its home location as a control. Most treatments consisted of 2 l dug holes refilled with treatment soil in a 2 l peat pot (see below for details). An additional no-pot treatment was included to test the effects of the pot on seedling emergence and survival. This resulted in eight treatment combinations at sites within and at the edge of the species's distribution. Treatment combinations beyond sugar maple's range differed, as there was no possibility of moving seed across transects at the beyond sites (the species is not naturally present at these sites). Beyond the range limit, seed from within and from the edge was planted on soil from beyond, both in pots and with the no-pot treatment. Seed from within and from the edge was also planted on its home soil (i.e. soil from within and the edge) for a total of six treatment combinations.
At each site, each treatment combination was replicated once in each of 10 blocks, in order to control for the effects of environmental heterogeneity. The number of soil samples (2 l) excavated from within each block was equal to the number of treatment combinations to be established in that block. All excavated soil from a single site (e.g. within, north transect) was mixed by hand and sub-samples were transported to appropriate planting locations (figure 1). A composite mix of three soil samples collected from each block was brought back to the laboratory for nutrient analysis (see electronic supplementary material for full methods and results). Leaf litter was also collected at each site and transported to the appropriate planting locations with its associated soil.
We placed a 14 cm diameter, 2 l compressed peat pot into each excavated hole within a block. The peat pot kept the experimental soil contained while still allowing for the natural movement of water through the soil. The rim of the pot was positioned slightly above ground to act as a barrier preventing the loss of seeds to run-off. The pot was filled with the appropriate soil treatment until the experimental soil reached the natural soil surface of the planting site. The ‘no-pot’ treatment was also installed in each block, where only the rim (approx. 3 cm high) of the pot was positioned around the experimental soil. This treatment allowed for the testing of a pot effect, while the presence of the rim prevented the loss of seed from run-off. As no effect of the pot treatment was detected (within: F-value = 3.2, p = 0.08; edge: F-value = 2.4, p = 0.1; beyond: F-value = 1.8, p = 0.2; electronic supplementary material, table S1), henceforth all descriptions of methods referring to pots also include the no-pot treatment.
Sugar maple seed in samara were collected during peak seed dispersal (late September to early October 2011) from sites within and at the edge of sugar maple's distribution. Seed was air-dried and stored at 4°C until the time of planting. In October 2011, after the period of natural sugar maple seed fall, 10 sugar maple seeds were placed on the soil surface in each pot and covered with leaf litter from the same source as the soil treatment in the pot. Leaf litter was carefully examined to remove all naturally occurring seeds, and placed in each experimental pot in roughly the same quantity as occurs naturally at its site of soil origin. Sugar maple germination and emergence was first measured in late April 2012, shortly after snow melt. Seedling emergence, height and survival were monitored throughout the growing season, approximately once every four weeks. Emergence was defined as the splitting of the seed coat and unfurling of the cotyledons.
(c). Granivore exclusion experiment
Following observational evidence of seed predation at high elevation (remnants of samara with seeds removed), we directly tested the hypothesis that seed predation reduced regeneration by implementing a granivore exclosure experiment in autumn 2012. Three treatments were implemented in each site (within, edge, beyond) along the two transects (north, south): (i) caged, (ii) cage-control and (iii) full control. Cages were 20 × 20 × 20 cm cubes constructed from 1 cm hardware cloth. Prior to cage installation, litter was cleared from a 20 × 20 cm patch of forest floor and approximately 3 cm of substrate was carefully excavated in a single piece. The cage was fastened in the resulting cavity with 2 inch stainless steel nails and the excavated substrate placed inside the cage bottom, preventing granivores from burrowing under the cage sides. Cage-controls were constructed similarly to full cages but with two open sides to allow small mammal access, and were installed in the same manner to test the effects of the cage material itself from deterring predators. The full control consisted of a non-manipulated area equal to the other treatments. Each treatment combination was replicated twice within each block in each site–transect combination, for a total of 120 replicates of each treatment: cages, cage-controls and full controls.
The three treatments were each planted with five maple seeds, verified to be filled, in October 2012. In each case, litter was cleared from the planting area prior to seed placement, and replaced after careful cleaning to remove natural maple seeds. Natural production of sugar maple seed in 2012 was extremely low (C.D.B. & M.V. 2012, unpublished data) and thus there was little risk of natural seed influencing the results of the predator-exclusion experiment. Seeds remaining in the experiment were counted in April 2013 after carefully sorting through the leaf litter at each experimental treatment. The sum of numbers of germinated and non-germinated seeds containing embryos was tallied as the number of intact seeds remaining in each treatment. The presence of an empty fruit shell was not counted as an intact seed, as this indicated that the embryo had been predated upon.
(d). Statistical analyses
We used generalized linear mixed-effects models (GLMMs) to analyse the response of (i) maple recruitment and (ii) seedling growth to planting site, soil source, seed source, and the interaction between planting site and soil source, with ‘transect’ and ‘block’ included as random effects. Maple recruitment models assumed a Poisson distribution of residuals as the raw data are based on counts. We analysed the within-site response of (i) maple recruitment (Poisson distribution) and (ii) maple growth (normal distribution) at each elevation using GLMMs, with soil source and seed source included as fixed factors and ‘transect’ and ‘block’ as random effects. The number of (i) intact seeds and (ii) emerged seedlings following the predator-exclusion experiment were analysed with generalized linear models using the Poisson distribution, with planting site and cage treatment as fixed factors. Raw data were averaged for each treatment within each block. In each case, standard procedures for model diagnostics were conducted [33]. All analyses were conducted using R v. 3.0.1 [34], using the ‘lme4’ package for GLMMs [35] and ‘MASS’ for generalized linear models [36].
3. Results
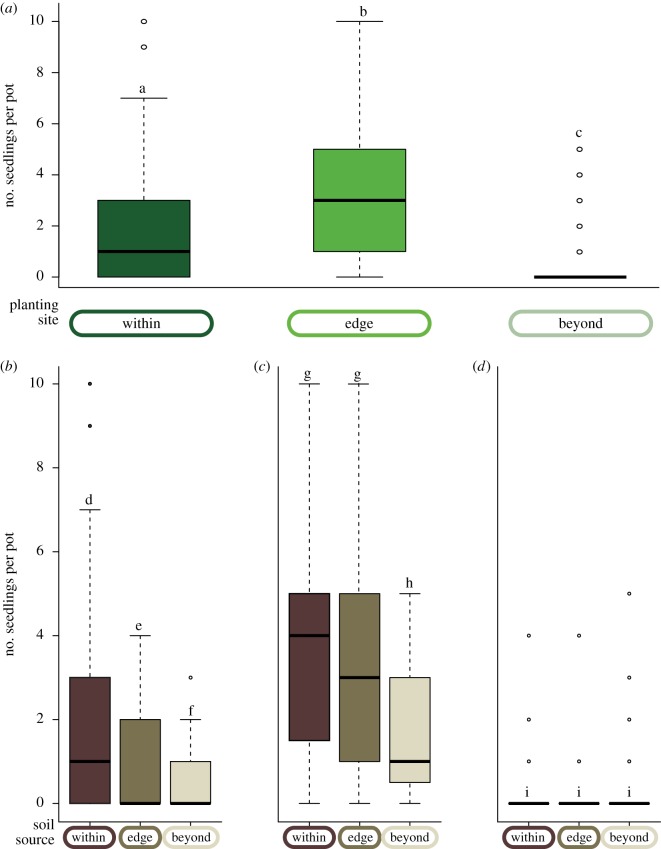
Regardless of soil source, sugar maple emergence and survival in the reciprocal transplant experiment was lowest at high elevation sites, beyond its current distribution, as predicted by the hypothesis that climate controls the elevational distribution of the species (z-value = −4.64, p < 0.0001; figure 2a; electronic supplementary material, figure S2 (results separated by transect), table S2). However, we also found an effect of soil source on sugar maple regeneration: when planted in a favourable climate (within its range), maple regeneration was lower on soil from ‘beyond’ the range than on soil from ‘within’ or the ‘edge’ (z-value = −4.38, p < 0.0001; figure 2b–d; electronic supplementary material, figure S2 and table S3). Regeneration was also lower at sites within the range than at range edge sites, regardless of soil source (z-value = 5.98, p < 0.0001; electronic supplementary material, table S2).
Figure 2.
Seedling regeneration response to elevation and soil source. Number of seedlings per pot by (a) planting site, regardless of soil source, and planted at sites (b) within, (c) at the edge and (d) beyond sugar maple's natural distribution, separated by soil source. The number of seedlings represents those individuals that survived beyond initial germination and persisted into late July during the first year of recruitment. Boxes here, and in subsequent figures, show the 25–75% quartiles of the data, with the median indicated by the line through the centre of the box. Whiskers extending from the box represent the 95% quartiles, and extreme observations are shown as hollow circles. Different letters over boxes within a single panel indicate statistically significant differences between treatments at α = 0.05 level. Note that these graphs summarize raw data, while significant differences were tested after accounting for transect and block effects. (Online version in colour.)
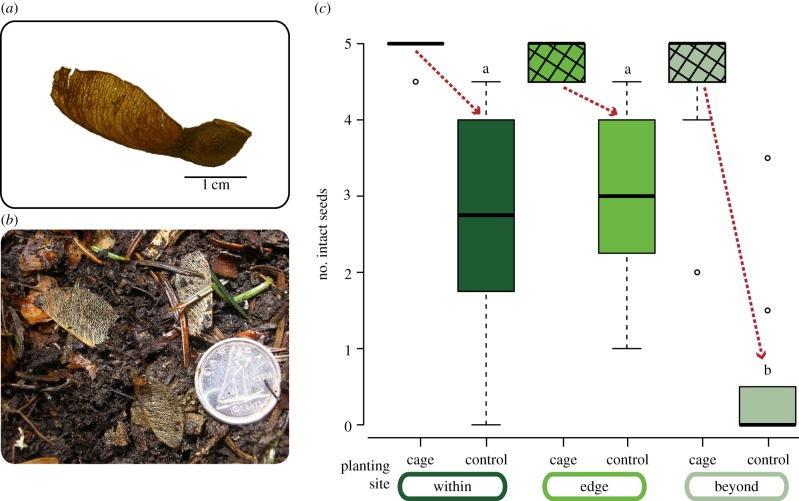
So few seedlings emerged at sites beyond the sugar maple's range that we could not examine the influence of soil from ‘within’ planted beyond sugar maple's distribution (i.e. in a less favourable climate). Field observations indicated that the low regeneration at high-elevation sites in our initial experiment appeared to have been caused to a considerable degree by seed predation. Seeds were planted in autumn 2011, and in spring 2012 we found a high incidence of samara (fruit) wings without the portion of the fruit that contains the seed (figure 3a,b), suggesting likely consumption by small mammals.
Figure 3.
Effects of predation on seed availability. (a) Intact sugar maple fruit with seed and samara wing also intact. (b) Samara wings without seeds, suggesting post-dispersal seed predation by small mammals (the Canadian dime is 18 mm in diameter). (c) Number of seeds escaping predation in cages (cross-hatched) and control plots at sites across the elevational distribution of sugar maple. Letters indicate significant differences as in figure 2.
The subsequent granivore exclusion experiment initiated in 2012 revealed that the effect of granivore exclusion on the number of intact seeds or seedlings was evident across the elevational gradient (z-value = 3.52, p < 0.0001; electronic supplementary material, table S4). Notably, the effect was by far most pronounced beyond sugar maple's current distribution (interaction term for granivore exclusion×sites beyond range limit: z-value = 4.42, p < 0.0001; figure 3c). Most control plots at high elevation contained no seeds or seedlings at all, whereas nearly every exclusion cage had all seeds present and intact. Those seeds that were protected from predation germinated at the same rate as measured within sugar maple's natural range (analysis of variance of germinated seedlings counted in cages within versus beyond: F-value = 0.29, p = 0.75).
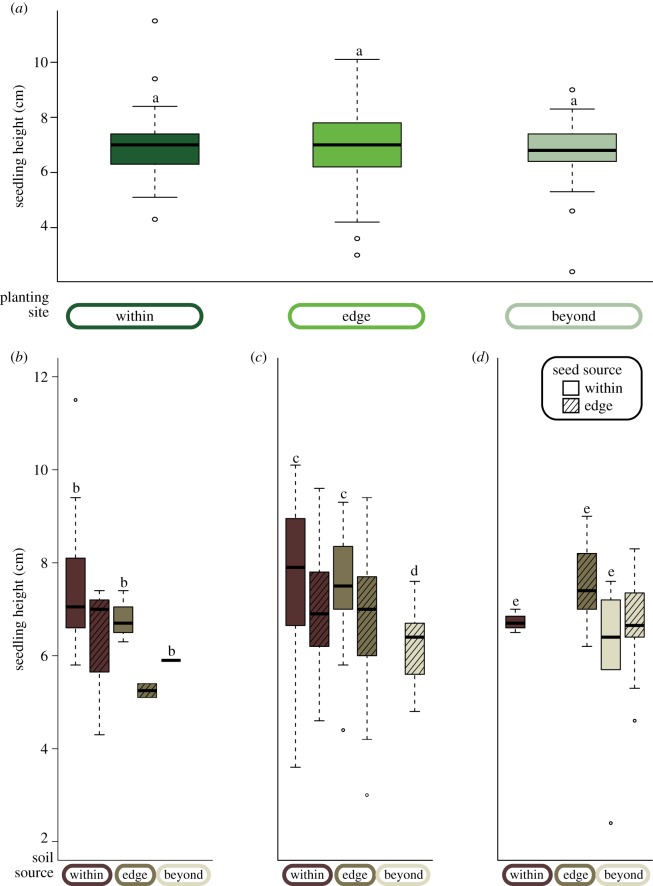
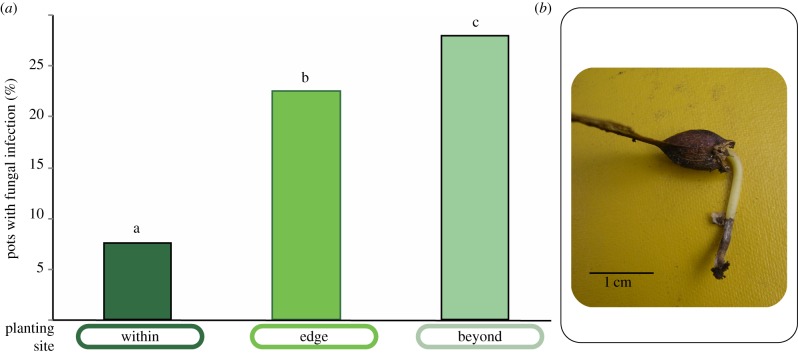
Consistent with the results for establishment, there was a small effect of soil source on seedling height (soil from beyond versus within: t-value = −1.94, p = 0.0054; figure 4b–d); however, there was no difference in growth between those seedlings that established beyond and within sugar maple's current range (figure 4a; electronic supplementary material, table S5). We observed an effect of seed source on seedling growth: seeds from within the range grew into taller seedlings than seeds from the range edge (e.g. within seed: 7.3 ± 1.3 cm versus edge seed: 5.8 ± 1.3 cm at sites within the range (mean ± s.d.); figure 4; electronic supplementary material, table S5). Finally, of those seedlings emerging from seeds that escaped predation, the proportion that was observed to have a fungal infection at the root : shoot interface increased from low-to-high elevation sites (χ2 = 12.78, p = 0.0008; figure 5).
Figure 4.
Seedling growth response to elevation, soil source and seed source. Effect of (a) planting site, regardless of soil source, and (b–d) soil and seed source on seedling height. Soil source effects are separated by planting sites (b) within, (c) at the edge and (d) beyond sugar maple's natural distribution, thus the effects of elevation are negligible within each of the three lower panels. Seed came from populations within and at the edge of sugar maple's distribution within the study area. Missing boxes indicate that no seedlings emerged and/or survived in that experimental treatment. Letters indicate significant differences as in figure 2, with letters in (b–d) indicating differences between soil source treatments. Seed source differences are not indicated in the figure but were detected where seedlings germinated from seed collected within sugar maple's distribution were significantly taller than those using seed from the range edge. (Online version in colour.)
Figure 5.
Observations of pathogens on sugar maple seedlings. (a) Percentage of all experimental pots with observations of fungal pathogen on seedling stems and radicles as seen in (b). Percentage was calculated by counting the number of pots with infected seedlings present and dividing by the total number of pots containing seedlings in each site. Infection was present on living and dead seedlings. Letters indicate significant differences as in figure 2. (Online version in colour.)
4. Discussion
Here, we present direct evidence from two integrated experiments that non-climatic factors, particularly biotic interactions, strongly control the range limit of sugar maple along a gradient typically thought of as primarily climatic. Until now, we have had very little empirically supported understanding of how correlated factors such as soil and species' interactions might modify or even dominate direct climate effects, due to the difficulty in untangling the influence of abiotic and biotic factors occurring along the same gradient. While cold temperatures and short growing seasons almost certainly contribute to reduced abundance of many species at high elevations or latitudes [37,38], in this case proximate, non-climatic factors appear to exert dominant control over the distributional range limit along what is generally considered a ‘climatic’ gradient. These findings thus indicate that non-climatic factors are likely to constrain the rate of climate-induced range expansion, and therefore require far greater consideration in predictions of future species' distributions.
The granivore exclosure experiment clearly demonstrated that seed predation greatly reduced sugar maple regeneration beyond its current upper elevational range limit, as observational data from the previous year had suggested. While post-dispersal seed predation of hardwood species has been documented [39,40], the magnitude of predation pressure beyond the range limit was an unexpected result, pointing to a fundamental constraint on potential range expansion. Evidence at the planting sites, where intact sugar maple samara wings remained with small bite marks removed at the point of seed attachment, evident immediately upon snowmelt, indicated that seeds were consumed over the autumn or winter by a rodent (E. J. Vander Wal, Memorial University 2012, personal communication). Based on the small mammals previously documented in Parc National du Mont-Mégantic [41], the most likely seed predator in our study was the southern red-backed vole (Myodes gapperi Vigors, 1830), which is active year round and incorporates tree seeds, including maples, into its winter diet [42]. Whether the main seed predator is more abundant at high elevations or simply consumes a higher proportion of maple seeds when present is unknown. While it is possible that the amount of food available to a seed predator would influence the magnitude of its effect on sugar maple seeds [43], it is notable that considerable seed predation was evident beyond sugar maple's elevational distribution in both 2011 and 2012, despite considerable differences in seed production. Based on the density of first-year seedlings in 2012 (electronic supplementary material, figure S1), there were at least six viable seeds m−2 (mean; range = 1–42 viable seeds m−2) dispersed in 2011, known regionally as a sugar maple mast year [27], and exceedingly few in 2012 (C.D.B. & M.V. 2012, unpublished data). Further investigations into resource availability, small mammal population size and seed predation pressure at range limits are warranted.
Our finding contrasts with that of some tropical alpine systems, where dispersal to higher elevations can facilitate an escape from establishment-limiting seed predation [44]. Interestingly, in our system, those seedlings that escaped predation and established beyond sugar maple's current range survived and grew as well as seedlings within and at the edge of its range. This suggests that seedling herbivory or other factors potentially limiting seedlings do not play major roles in controlling maple establishment beyond its current distribution, unlike herbivory-controlled deciduous tree species' limits common to the southern Scandes of Europe [45]. While we did not address the factors that determine growth and fitness of saplings or adult trees, at which stage climate may well play a role [46,47], the seed and seedling stages in trees are of profound demographic importance given very high average mortality [48]. In combination, the results from our two field experiments point to seed predation as a critical bottleneck constraining establishment beyond sugar maple's current range limit, and more generally to the importance of the seed-to-seedling transition in limiting potential range expansion [10,49]. This implies that, assuming species' distributions are at equilibrium with current climate to begin with, climate change alone is insufficient to allow expansion of a species's distribution when limiting factors such as seed predation are still present.
Our results do not allow us to make strong inferences concerning the specific soil properties underlying our observed effects of soil source on sugar maple regeneration, but the data do provide some clues. Interestingly, regeneration was actually slightly lower at sites within the range than at range edge sites, and differences in regeneration across ‘within’ versus ‘edge’ sites mirror differences in the availability of nutrients, suggesting the possibility of nutrient limitation at the lowest-elevation sites. The availability of the major soil nutrients—nitrogen, phosphorus and potassium (as well as soil water content)—show, if anything, a tendency to increase with elevation (soil characteristics shown in the electronic supplementary material, figure S3). This suggests that our studied system probably does not suffer from acid deposition-induced nutrient limitations (specifically, calcium, magnesium and manganese) that have caused sugar maple declines in some other northeastern North American forests [28,30]. As reduced regeneration on high elevation soils is probably not a result of nutrient or moisture deficiency, it follows that biotic factors may play an important role, and indeed we observed an increasing incidence of fungal infection on the roots of emerging seedlings at higher elevations, consistent with previous evidence of fungal pathogens as a mortality agent of sugar maple [48]. Little is known about the role soil pathogens may play in species' range expansions with climate change [11]; however, there is evidence for an increase in root pathogen activity with climate warming in some tree species [50], and a general pattern of an increase in pathogens with climate warming across terrestrial systems [51], indicating a need for further studies to test the role of fungal pathogens along the elevation gradient.
It is also possible that the occurrence of mutualistic soil organisms changes along the elevational gradient. Some species may require the presence of mutualists to tolerate the environment beyond their current range limits or to create a competitive advantage over other species [12,18]. Recent experimental evidence has demonstrated that Bromus spp. show more rapid range expansion when their mutualist fungal endophytes are present to alleviate environmental stresses [18]. Sugar maple is typically found in association with arbuscular mycorrhizal (AM) fungi [52], probably requiring the symbiotic relationship for efficient nutrient acquisition in order to thrive. Low soil pH has been associated with reduced AM fungus development [53], suggesting the possibility that higher-elevation sites, where conifers are more prevalent, may not have appropriate AM fungus communities for sugar maple. While the absence of any growth reduction in seedlings established at high elevation (figure 4) is not what one would expect if AM fungi were lacking at high-elevation sites, it is possible that insufficient AM colonization upon germination contributed to seedling mortality by other causes. The relationships between AM fungi, nutrient acquisition, edaphic factors and sugar maple growth are not clear [28,52], and additional research is needed to discern the precise role of antagonistic versus beneficial fungi in controlling sugar maple's elevational distribution.
Overall, we have both direct (the exclosure experiment) and indirect (the soil transplants) experimental evidence that non-climatic interactions play an important role in defining elevational range limits. It is well known that biotic interactions contribute to determining species' distributions [3,14], but their importance is often thought to be greatest at more abiotically benign range edges (e.g. low elevation or latitude), with climatic or other abiotic factors most important at physically stressful range edges (e.g. high elevation or latitude) [15,38,54]. Observational studies often provide suggestive evidence of a role for biotic interactions (most often competition and facilitation) in controlling species' distributional limits [44,55], but without direct experimentation, the mechanisms often can only be speculated upon [10,12,54]. Here, we have conducted one of the few experiments to date (see also [14,18]) demonstrating that a dominant species is constrained by biotic factors at what is typically presumed to be climatically determined range limit [25], in a region that is currently experiencing climate warming [24]. These biotic effects appear large enough in magnitude to impede the species's establishment in novel habitats (seed predation) and to override the effects of favourable climatic conditions (as tested by transplanting soil from beyond to within the range).
We found some evidence that seedling success depends on source population, with seeds from within the range growing into taller seedlings than seeds from the range edge. While we cannot ascribe this difference to either genetic differences among populations or to maternal effects (we did not measure seed weights), the result on its own nonetheless has important implications for incorporating results of empirical studies into models of range expansion [56]. In particular, using life-history traits from the centre of species' ranges to estimate establishment success beyond the range edge [57] could result in an overestimate of establishment success during natural range expansion given that seeds from the range edge itself are those most likely to initiate new populations [7,58].
In sum, while the importance of non-climatic factors in limiting species' ranges is well known, little experimental research has directly tested for the role of these non-climatic factors across putatively climatic gradients such as elevation or latitude. Here, we present experimental evidence demonstrating the substantial role non-climatic factors play in determining potential range expansion under climate change. Our data emphasize the clear need for non-climatic factors to be integrated into predictions of range shifts under climate change, and indeed the great difficulty in anticipating important factors such as seed predation. Soil conditions and seed predation clearly present constraints on the ability of sugar maple to colonize higher elevations as climatic conditions become suitable. Over the long term, climate change may lead to changes in these non-climatic factors themselves. For example, colonization of broad-leaved trees can raise soil pH and increase soil microbe densities [59], possibly relieving certain constraints on sugar maple establishment. Nonetheless, even in systems where climate may be the ultimate determinate of the range limit, at a bare minimum these non-climatic factors are likely to introduce a substantial time lag into the process of range expansion [60]. Only further empirical studies and incorporation of such complexities into models of range expansion will allow us to move towards more accurate predictions of species' distributions under global environmental change.
Supplementary Material
Acknowledgements
We thank C.-A. Ouimet, Parc national du Mont-Mégantic, Société des établissements de plein air du Québec, R. Beauséjour, V. Demers, L. Garcia, A.-S. Goyette, G. Lajoie, I. Myers-Smith, W. Parsons, J. Savage, I. Teasedale and E. Vander Wal, who helped execute this study, and A. Angert for comments on an earlier draft of the manuscript.
Data accessibility
Complete raw data from this study are available in the Dryad data repository (doi:10.5061/dryad.d5j34).
Funding statement
Funding was provided by the Natural Sciences and Engineering Research Council of Canada.
References
- 1.Chen I-C, Hill JK, Ohlemüller R, Roy DB, Thomas CD. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026. ( 10.1126/science.1206432) [DOI] [PubMed] [Google Scholar]
- 2.Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42. ( 10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 3.Whittaker RH. 1975. Communities and ecosystems. New York, NY: Macmillan. [Google Scholar]
- 4.Fisichelli NA, Frelich LE, Reich PB. 2013. Climate and interrelated tree regeneration drivers in mixed temperate–boreal forests. Landsc. Ecol. 28, 149–159. ( 10.1007/s10980-012-9827-z) [DOI] [Google Scholar]
- 5.Sundqvist MK, Sanders NJ, Wardle DA. 2013. Community and ecosystem responses to elevational gradients: processes, mechanisms, and insights for global change. Annu. Rev. Ecol. Evol. Syst. 44, 261–280. ( 10.1146/annurev-ecolsys-110512-135750) [DOI] [Google Scholar]
- 6.Loarie SR, Duffy PB, Hamilton H, Asner GP, Field CB, Ackerly DD. 2009. The velocity of climate change. Nature 462, 1052–1055. ( 10.1038/nature08649) [DOI] [PubMed] [Google Scholar]
- 7.Sexton JP, McIntyre PJ, Angert AL, Rice KJ. 2009. Evolution and ecology of species range limits. Annu. Rev. Ecol. Evol. Syst. 40, 415–436. ( 10.1146/annurev.ecolsys.110308.120317) [DOI] [Google Scholar]
- 8.Lafleur B, Pare D, Munson AD, Bergeron Y. 2010. Response of northeastern North American forests to climate change: will soil conditions constrain tree species migration? Environ. Rev. 18, 279–289. ( 10.1139/A10-013) [DOI] [Google Scholar]
- 9.Thuiller W. 2013. On the importance of edaphic variables to predict plant species distributions—limits and prospects. J. Veg. Sci. 24, 591–592. ( 10.1111/jvs.12076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaston KJ. 2009. Geographic range limits: achieving synthesis. Proc. R. Soc. B 276, 1395–1406. ( 10.1098/rspb.2008.1480) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Der Putten WH, Macel M, Visser ME. 2010. Predicting species distribution and abundance responses to climate change: why it is essential to include biotic interactions across trophic levels. Phil. Trans. R. Soc. B 365, 2025–2034. ( 10.1098/rstb.2010.0037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.HilleRisLambers J, Harsch MA, Ettinger AK, Ford KR, Theobald EJ. 2013. How will biotic interactions influence climate change-induced range shifts? Ann. N. Y. Acad. Sci. 1297, 112–125. ( 10.1111/nyas.12182) [DOI] [PubMed] [Google Scholar]
- 13.Hargreaves AL, Samis KE, Eckert CG. 2014. Are species’ range limits simply niche limits writ large? A review of transplant experiments beyond the range. Am. Nat. 182, 157–173. ( 10.1086/674525) [DOI] [PubMed] [Google Scholar]
- 14.Connell JH. 1961. The influence of interspecific competition and other factors on the distribution of the barnacle Chthamalus stellatus. Ecology 42, 710–723. ( 10.2307/1933500) [DOI] [Google Scholar]
- 15.Connell JH. 1983. On the prevalence and relative importance of interspecific competition: evidence from field experiments. Am. Nat. 122, 661–696. [Google Scholar]
- 16.Stanton-Geddes J, Tiffin P, Shaw RG. 2012. Role of climate and competitors in limiting fitness across range edges of an annual plant. Ecology 93, 1604–1613. ( 10.1890/11-1701.1) [DOI] [PubMed] [Google Scholar]
- 17.Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD. 2010. A framework for community interactions under climate change. Trends Ecol. Evol. 25, 325–331. ( 10.1016/j.tree.2010.03.002) [DOI] [PubMed] [Google Scholar]
- 18.Afkhami ME, McIntyre PJ, Strauss SY. 2014. Mutualist-mediated effects on species’ range limits across large geographic scales. Ecol. Lett. ( 10.1111/ele.12332) [DOI] [PubMed] [Google Scholar]
- 19.Knoepp JD, Coleman DC, Crossley D, Jr, Clark JS. 2000. Biological indices of soil quality: an ecosystem case study of their use. For. Ecol. Manage. 138, 357–368. ( 10.1016/S0378-1127(00)00424-2) [DOI] [Google Scholar]
- 20.Germino MJ, Hasselquist NJ, McGonigle T, Smith WK, Sheridan PP. 2006. Landscape- and age-based factors affecting fungal colonization of conifer seedling roots at the alpine tree line. Can. J. For. Res. 36, 901–909. ( 10.1139/x05-303) [DOI] [Google Scholar]
- 21.Link SO, Smith JL, Halvorson JJ, Bolton H. 2003. A reciprocal transplant experiment within a climatic gradient in a semiarid shrub-steppe ecosystem: effects on bunchgrass growth and reproduction, soil carbon, and soil nitrogen. Glob. Change Biol. 9, 1097–1105. ( 10.1046/j.1365-2486.2003.00647.x) [DOI] [Google Scholar]
- 22.Burns RM, Honkala BH. 1990. Silvics of North America. Washington, DC: USDA Forest Service. [Google Scholar]
- 23.Marcotte G, Grandtner MM. 1974. Étude écologique de la végétation forestière du Mont Mégantic. Québec, Canada: Service de la recherche, Direction générale des forêts, Ministère des terres et forêts.
- 24.Savage J, Vellend M. In press. Elevational shifts, biotic homogenization and time lags in vegetation change during 40 years of climate warming. Ecography. [Google Scholar]
- 25.Siccama TG. 1974. Vegetation, soil, and climate on the Green Mountains of Vermont. Ecol. Monogr. 44, 325–349. ( 10.2307/2937033) [DOI] [Google Scholar]
- 26.Demers JD, Lee TD, Barrett JP. 1998. Substrate type and the distribution of sugar maple at its elevational limit in the White Mountains, New Hampshire. Can. J. For. Res. 28, 494–498. ( 10.1139/x98-008) [DOI] [Google Scholar]
- 27.Graignic N, Tremblay F, Bergeron Y. 2014. Geographical variation in reproductive capacity of sugar maple (Acer saccharum Marshall) northern peripheral populations. J. Biogeogr. 41, 145–157. ( 10.1111/jbi.12187) [DOI] [Google Scholar]
- 28.Juice SM, Fahey TJ, Siccama TG, Driscoll CT, Denny EG, Eagar C, Cleavitt NL, Minocha R, Richardson AD. 2006. Response of sugar maple to calcium addition to northern hardwood forest. Ecology 87, 1267–1280. ( 10.1890/0012-9658(2006)87[1267:ROSMTC]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 29.Long RP, Horsley SB, Hallett RA, Bailey SW. 2009. Sugar maple growth in relation to nutrition and stress in the northeastern United States. Ecol. Appl. 19, 1454–1466. ( 10.1890/08-1535.1) [DOI] [PubMed] [Google Scholar]
- 30.Likens GE, Driscoll CT, Buso DC. 1996. Long-term effects of acid rain: response and recovery of a forest ecosystem. Science 272, 244–246. ( 10.1126/science.272.5259.244) [DOI] [Google Scholar]
- 31.Nathan R, Katul GG, Horn HS, Thomas SM, Oren R, Avissar R, Pacala SW, Levin SA. 2002. Mechanisms of long-distance dispersal of seeds by wind. Nature 418, 409–413. ( 10.1038/nature00844) [DOI] [PubMed] [Google Scholar]
- 32.Clark JS, Silman M, Kern R, Macklin E, HilleRisLambers J. 1999. Seed dispersal near and far: patterns across temperate and tropical forests. Ecology 80, 1475–1494. ( 10.1890/0012-9658(1999)080[1475:SDNAFP]2.0.CO;2) [DOI] [Google Scholar]
- 33.Zuur A, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer. [Google Scholar]
- 34.R Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 35.Bates D, Maechler M, Bolker B. 2011. lme4: Linear mixed-effects models using S4 classes. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 36.Venables WN, Ripley BD. 2002. Modern applied statistics with S, 4th edn New York, NY: Springer. [Google Scholar]
- 37.Woodward FI, Williams BG. 1987. Climate and plant distribution at global and local scales. Vegetatio 69, 189–197. [Google Scholar]
- 38.Körner C, Paulsen J. 2004. A world-wide study of high altitude treeline temperatures. J. Biogeogr. 31, 713–732. [Google Scholar]
- 39.Hsia JF, Francl KE. 2009. Postdispersal sugar maple (Acer saccharum) seed predation by small mammals in a northern hardwood forest. Am. Midl. Nat. 162, 213–223. ( 10.1674/0003-0031-162.2.213) [DOI] [Google Scholar]
- 40.Kellman M. 2004. Sugar maple (Acer saccharum Marsh.) establishment in boreal forest: results of a transplantation experiment. J. Biogeogr. 31, 1515–1522. ( 10.1111/j.1365-2699.2004.01128.x) [DOI] [Google Scholar]
- 41.Graillon P, Giguère S, Philibert H. 2007 Le Parc national du Mont Mégantic: synthèse des connaissances. Québec, Canada: Société des établissements de plein air du Québec. [Google Scholar]
- 42.Martell AM. 1981. Food habits of southern red-backed voles (Clethrionomys gapperi) in Northern Ontario. Can. Field-Nat. 95, 325–328. [Google Scholar]
- 43.Janzen DH. 1971. Seed predation by animals. Annu. Rev. Ecol. Syst. 2, 465–492. [Google Scholar]
- 44.Hillyer R, Silman MR. 2010. Changes in species interactions across a 2.5 km elevation gradient: effects on plant migration in response to climate change. Glob. Change Biol. 16, 3205–3214. ( 10.1111/j.1365-2486.2010.02268.x) [DOI] [Google Scholar]
- 45.Cairns DM, Moen J. 2004. Herbivory influences tree lines. J. Ecol. 92, 1019–1024. ( 10.2307/3599745) [DOI] [Google Scholar]
- 46.Beckage B, Osborne B, Gavin DG, Pucko C, Siccama T, Perkins T. 2008. A rapid upward shift of a forest ecotone during 40 years of warming in the Green Mountains of Vermont. Proc. Natl Acad. Sci. USA 105, 4197–4202. ( 10.1073/pnas.0708921105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fisichelli NA, Frelich LE, Reich PB. 2014. Temperate tree expansion into adjacent boreal forest patches facilitated by warmer temperatures. Ecography 37, 152–161. ( 10.1111/j.1600-0587.2013.00197.x) [DOI] [Google Scholar]
- 48.Cleavitt NL, Fahey TJ, Battles JJ. 2011. Regeneration ecology of sugar maple (Acer saccharum): seedling survival in relation to nutrition, site factors, and damage by insects and pathogens. Can. J. For. Res. 41, 235–244. ( 10.1139/X10-210) [DOI] [Google Scholar]
- 49.Walck JL, Hidayati SN, Dixon KW, Thompson K, Poschlod P. 2011. Climate change and plant regeneration from seed. Glob. Change Biol. 17, 2145–2161. ( 10.1111/j.1365-2486.2010.02368.x) [DOI] [Google Scholar]
- 50.Brasier C. 1996. Phytophthora cinnamomi and oak decline in southern Europe: environmental constraints including climate change. Ann. Sci. For. 53, 347–358. ( 10.1051/forest:19960217) [DOI] [Google Scholar]
- 51.Tylianakis JM, Didham RK, Bascompte J, Wardle DA. 2008. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 11, 1351–1363. ( 10.1111/j.1461-0248.2008.01250.x) [DOI] [PubMed] [Google Scholar]
- 52.Klironomos JN. 1995. Arbuscular mycorrhizae of Acer saccharum in different soil types. Can. J. Bot. 73, 1824–1830. ( 10.1139/b95-193) [DOI] [Google Scholar]
- 53.Clark RB. 1997. Arbuscular mycorrhizal adaptation, spore germination, root colonization, and host plant growth and mineral acquisition at low pH. Plant Soil 192, 15–22. ( 10.1023/A:1004218915413) [DOI] [Google Scholar]
- 54.Brown JH, Stevens GC, Kaufman DM. 1996. The geographic range: size, shape, boundaries, and internal structure. Annu. Rev. Ecol. Syst. 27, 597–623. ( 10.1146/annurev.ecolsys.27.1.597) [DOI] [Google Scholar]
- 55.Ettinger AK, Ford KR, HilleRisLambers J. 2011. Climate determines upper, but not lower, altitudinal range limits of Pacific Northwest conifers. Ecology 92, 1323–1331. ( 10.1890/10-1639.1) [DOI] [PubMed] [Google Scholar]
- 56.Colautti RI, Barrett SCH. 2013. Rapid adaptation to climate facilitates range expansion of an invasive plant. Science 342, 364–366. ( 10.1126/science.1242121) [DOI] [PubMed] [Google Scholar]
- 57.Ibáñez I, Clark JS, Dietze MC. 2008. Evaluating the sources of potential migrant species: implications under climate change. Ecol. Appl. 18, 1664–1678. ( 10.1890/07-1594.1) [DOI] [PubMed] [Google Scholar]
- 58.Murphy HT, VanDerWal J, Lovett-Doust J. 2010. Signatures of range expansion and erosion in eastern North American trees. Ecol. Lett. 13, 1233–1244. ( 10.1111/j.1461-0248.2010.01526.x) [DOI] [PubMed] [Google Scholar]
- 59.Bauhus J, Paré D, Cot̂é L. 1998. Effects of tree species, stand age and soil type on soil microbial biomass and its activity in a southern boreal forest. Soil Biol. Biochem. 30, 1077–1089. ( 10.1016/S0038-0717(97)00213-7) [DOI] [Google Scholar]
- 60.Pennington W. 1986. Lags in adjustment of vegetation to climate caused by the pace of soil development. Evidence from Britain. Vegetatio 67, 105–118. ( 10.1007/BF00037361) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Complete raw data from this study are available in the Dryad data repository (doi:10.5061/dryad.d5j34).