Abstract
Epidermal stem cells (SC) are believed to be resistant to environmental damage for the purpose of self renewal. Most promising SC markers include integrin α6 and p63. The aim of our study was to determine whether the integrin α6+p63+ cell fraction representative of the epidermal progenitor or SC is increased after ultraviolet B (UVB) irradiation and to clarify the hypothesis that epidermal SC are resistant to high-dose UVB damage. We irradiated early passage normal human keratinocytes (NHK) with 0, 25, 50, and 100 mJ/cm2 UVB. The percentage of cell death was calculated. Real-time reverse transcriptase-polymerase chain reaction (RT-PCR) and western blotting analyses were performed to identify integrin α6 and p63, and flow cytometry analysis with integrin α6 and p63 antibodies was done. After 50 and 100 mJ/cm2 UVB, integrin α6+p63+ cells were found to be much increased by fluorescence-activated cell sorting. Expression of integrin α6 and p63 was increased in NHK after UVB irradiation, which was shown with real-time RT-PCR and western blotting analyses. We concluded that an increase of integrin α6+p63+ cells after high-dose UVB may suggest that the putative progenitor or SC are resistant to UVB irradiation.
Key words: integrin α6+p63+ cells, stem cells, ultraviolet B.
Introduction
The keratinocytes in human epidermis are turned over constantly and replaced by a population of epidermal progenitor or stem cells (SC) located in the basal layer of the epidermis.1 SC can be defined as undifferentiated cells capable of self-maintenance, and have the ability to produce a large number of differentiated cells and to regenerate tissue after injury.1–3 Increased resistance to oxidative stress from noxious stimuli including radiation has been reported for mesenchymal2 and embryonic stem cells,3 compared with cancer cells and various differentiated cells, respectively. Skin responds to a ultraviolet B (UVB) irradiation in many ways4 but little is known regarding the UVB response of human epidermal SC. Human epidermal SC have been reported to express high levels of integrins, which bind to the ligand collagen type IV,5–9 and the isolation of epidermal SC by rapid attachment to collagen type IV has been reported.5,8 The integrin family of transmembrane receptors, especially integrin α6, seems one of the important putative progenitor or SC markers to date.6–8 These putative SC are enriched in a cell population with elevated integrin α6 expression and low CD71 levels (α6bri CD71dim cells).7,8 Transcription factor p63 is another epithelial SC regulatory protein known to be essential for regenerative proliferation.10–12 In previous reports certain cells maintain p63 during serial cultivation and p63 is abundant in holoclones.11 In one report sixty-five percent of cells rapidly adhering to type IV collagen were p63+.12 However, an in vivo skin biopsy showed that there are too many p63+ cells in the basal and suprabasal area.10 Because there is no single marker to identify epidermal SC, we analyzed integrin α6 and p63 double positive cells.
One of the characteristics of adult progenitor or SC is a relative resistance to noxious environments.2,3,13 UVB irradiation of an acute damaging dose can be insulting to skin4 but epidermal SC may be resistant for the purpose of self-maintenance. Therefore we tried to determine whether the integrin α6+p63+ cell fraction representative of epidermal progenitor or SC is increased after UVB irradiation and to clarify the hypothesis that epidermal SC are resistant to UVB damage.
Materials and Methods
Isolation and primary culture of normal human skin keratinocytes
Abdominal skin pieces were obtained from abdominoplasty specimens in female adults. After washing twice with phosphate-buffered saline (PBS), tissues were minced and incubated in 2.0 unit/mL dispase (Gibco BRL, USA) solution for 1 h at 37°C to separate the epidermis from the dermis. Detached epidermal tissues were grasped gently with forceps and transferred to new plates. After incubation in 0.125% trypsin for 20 min at 37°C, dissociated keratinocytes were plated and maintained in monolayer cultures containing Keratinocyte Growth Medium (KGM) (Cambrex, USA). Early passage cells with about 70% confluence were used for our experiments, as previously described.14
Ultraviolet B irradiation, cytotoxicity, flow cytometry
The cultured normal human keratinocytes (NHK) were placed in PBS and exposed to irradiation of 0, 25, 50, and 100 mJ/cm2 UVB. The source of UVB was HandiSol SEC (National Biological Corporation, Twinsburg, OH, USA). For high-dose UVB (50 and 100 mJ/cm2), five 10 cm dishes for collecting live cells for flow cytometry were irradiated together. Lactate dehydrogenase (LDH) release was measured, as previously described.15 Triplicate samples of cell-free medium were taken at 24 h after exposure and 100 µL of the supernatent was placed into a 96-well plate. LDH dye solution (Biovision) was added and allowed to stand for 30 min for color development. By measuring absorbance of the samples at 490 nm, the percentage of cell death was calculated and compared to normal control cells and high control cells treated with 1% Triton-X. For fluorescence-activated cell sorting (FACS), after collecting the multiple 10 cm dishes altogether in one tube, the collected cells were washed twice and floating degraded cells were removed with cell debris. The retained cells were stained with integrin α6 (Santa Cruz, CA, USA) and p63 (Biosciences Pharmingen, USA). The secondary antibodies used were fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (DiNonA, Korea) and phycoerythrin-conjugated goat anti-mouse IgG (DiNonA, Korea). Appropriate isotype controls were used. The stained cells were analyzed on a Becton Dickson FACS caliber (B&D, USA).
Real-time reverse transcriptase-polymerase chain reaction
Total RNA was extracted from control cells and UVB-treated cells, followed by real-time RT-PCR analysis for the integrin α6 and p63 genes. Results of real-time RT-PCR are presented as the mean ± standard deviation, performed in triplicate wells (n=3) with internal normalization using 36B4, with similar results being observed in two or more independent experiments.
Primer sequences for integrin α6, p63, and 36B4 genes are as follows: integrin α6 sense primer 5′-ATAAATTTTGCACCCGAG-3′, and antisense primer 5′-GTTGGAAGGGCT-GTTTGTCACTGT-3′; p63 sense primer 5′-ATGTCCCAGAGCACACAG-3′, and antisense primer 5′-GGGTGATGGAGAGAGAGCATC-3′; 36B4 sense primer 5′-GCAATGTTGCCAGT-GTCTGT-3′, and antisense primer 5′-GCCTTGACCTTTTCAGCAAG-3′. The PCR were performed following the cycling parameters on a Minicycler™ PCR system (MJ Research, Inc.): 10 min at 94°C followed by 25 cycles of 1 min at 94°C, 1 min at 55°C, 1 min at 72°C, and a final cycle at 72°C for 10 min. Quantitation of the PCR products was scanned and performed using a Quantity One program (Bio-Rad, Hercules, CA, USA).
Western blotting analysis of cultured normal human keratinocytes
Cells were harvested by scraping and lysed. A 20 µg sample of total protein was separated on 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to polyvinylidene fluoride (PVDF) filters. Filters were blocked with 5% non-fat milk in PBS for 1 h at room temperature, and incubated with primary antibodies in 5% non-fat milk in TPBS (PBS containing 0.05% Tween 20) overnight at 4°C.
After washing with TPBS three times, filters were incubated for 1 h with horseradish peroxidase (HRP)-conjugated secondary antibody (Zymed, CA, USA), and in 5% non-fat milk in TPBS (1:5000 dilution). The filters were rinsed with TPBS three times, and an enhanced chemiluminescent detection assay was performed using the ECL kit (Amersham Life Science, UK). Primary antibodies against p63 (Biosciences Pharmingen, USA), integrin α6, K1, and tubulin (Santa Cruz, CA, USA) were used.
Results
Ultraviolet B irradiation and lactate dehydrogenase assay
After UVB 50 mJ/cm2 irradiation, viable normal keratinocytes were decreased while large apoptotic and small round keratinocytes were dominant (Figure 1). Cell death was calculated by LDH assay: 11.3%, 8.3%, and 10.7% (mean 10.1%) at 0 mJ/cm2; 52.1%, 60.3%, and 59.9% (mean 57.4%) at 25 mJ/cm2; 67.3%, 71.5%, and 62.1% (mean 66.9%) at 50 mJ/cm2; and 91.8%, 89.1% and 90.3% (mean 90.4%) at 100 mJ/cm2. Cell death was dose-dependent (Figure 2).
Figure 1.
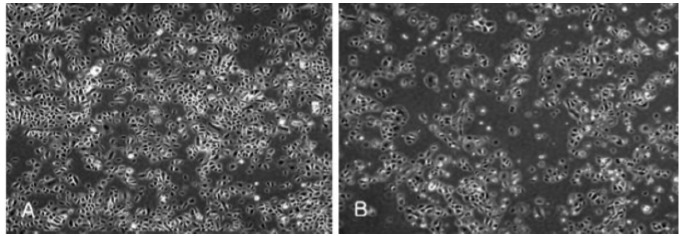
After ultraviolet B 50 mJ/cm2 irradiation, viable normal keratinocytes were decreased while irregular-sized apoptotic and small round keratinocytes were dominant (B) compared to normal human keratinocytes before ultraviolet B (A).
Figure 2.
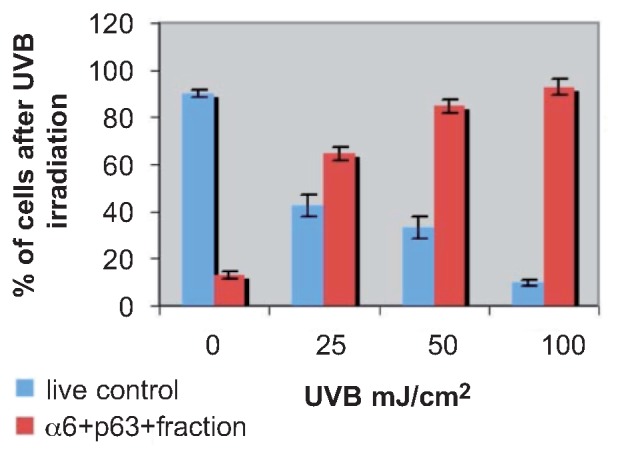
Percentage of live cells after ultraviolet B irradiation of 0, 25, 50, and 100 mJ/cm2. Fluorescence-activated cell sorting results of the integrin α6+p63+ fraction after ultraviolet B irradiation are represented at ultraviolet B irradiation of 0, 25, 50, and 100 mJ/cm2.
Fluorescence-activated cell sorting
On FACS, more integrin α6+p63+ cell fractions were found in UVB-treated cells than in control cells. Our results showed that UVB irradiation enriched integrin α6+p63+ cells from 13.0% at 0 mJ/cm2 to 92.8% at 100 mJ/cm2: 14.6%, 11.3%, and 13.1% (mean 13.0%) at 0 mJ/cm2; 67.9%, 63.2%, and 62.5% (mean 64.5%) at 25 mJ/cm2; 87.8%, 83.3%, and 82.4% (mean 84.7%) at 50 mJ/cm2; and 89.5%, 96.2% and 92.6% (mean 92.8%) at 100 mJ/cm2 (Figure 2).
Real-time reverse transcriptase-polymerase chain reaction and western blotting
Real-time RT-PCR showed that NHK had high levels of p63 and integrin α6 genes transcriptionally after UVB at 50 mJ/cm2 (Figure 3). Western blotting demonstrated that NHK possessed high levels of p63 and integrin α6 after UVB at 50 and 100 mJ/cm2. However, K1 was weakly expressed in control cells only (Figure 4).
Figure 3.
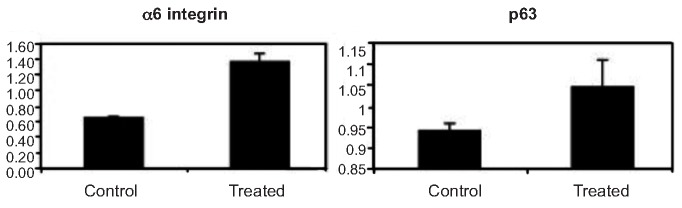
Induction of mRNA of integrin α6 and p63 in normal human keratinocytes with or without ultraviolet B treatment by real-time RT-PCR. Results showed that normal human keratinocytes treated by ultraviolet B at 50 mJ/cm2 had higher levels of these two genes transcriptionally.
Figure 4.

Western blotting results of integrin α6, p63, K1, and tubulin (top to bottom) at ultraviolet B irradiation of 0, 50, and 100 mJ/cm2 (left to right). Results showed that normal human keratinocytes after ultraviolet B at 50 and 100 mJ/cm2 possessed higher levels of p63 and integrin α6. K1 was weakly expressed in control cells only.
Discussion
The epidermis has a self-renewing capacity throughout life and this continuous turnover is mediated by progenitor or stem cells.1 The effort to identify epidermal SC has gained momentum recently. SC and transit-amplifying cells (TAC) were first identified according to their different proliferative characteristics.1 In the normal epidermis stem cells are quiescent and tend not to divide, except in response to tissue damage or to being placed in culture when they are capable of sustained self-renewal. By contrast, TAC are dividing actively in vivo, but in culture they undergo terminal differentiation within a few rounds of division. SC are believed to be resistant to environmental conditions.2,3 Some authors have shown that human epidermal SC can survive in a dehydrated state in sodium chloride for months and after transplantation give rise to keratinocyte progenies.13
Molecular markers of epidermal SC have been identified through a candidate approach and, more recently, by global gene expression profiling. These makers enable cells to be compared entirely on the basis of their spatial location rather than relying on existing markers to FACS-positive and -negative populations. Until now sorting based on the α6bri CD71dim population has been reliable in isolating epidermal SC.15,16 Kaur and Li reported comparative expression and function of the β1 versus α6 β4 integrins in keratinocyte SC, TAC, and post-mitotic differentiating cells of epidermis.16 They showed that the α6bri fraction is a purer population of keratinocyte SC than the β1bri fraction. Our previous work showed the β1-positive K1-negative fraction included TAC as well as SC.17 Previously p63 was found to be critical for maintaining the proliferative potential of epidermal SC and progenitor cells.10–12 Therefore, in this study, we used the integrin α6+p63+ fraction for confirming the purer fraction of progenitor or SC by FACS.
Very little is known regarding the response to UVB of human epidermal SC. We report here that the integrin α6+p63+ double positive NHKs are resistant to an acute damaging dose of UVB. Recent reports have shown that label-retaining epidermal SC in murine epidermis accumulate ultraviolet (UV) damage. These results are from chronic low-level UV exposure.18 From a previous report high-dose UVB of more than 80 mJ/cm2 is known to induce necrosis instead of apoptosis.19 Exposure of keratinocytes to UVB induced a corresponding dose-dependent decrease in cell viability. In a human keratinocyte cell line (CCD-1106), more than 60% of cells were shown to have died by crystal violet staining, and they detached from culture plates 24 h after exposure to 200 mJ/cm2 UVB.19 In addition UVB markedly decreased viable cell numbers in normal epidermal keratinocytes.19,20 Consequently this high dose of UVB made selection of epidermal SC represented by α6+p63+ cells possible.
In our study p63+ cells were less affected by UVB irradiation. UVB-treated cells had a higher fraction of p63+ cells among all the viable cells compared with control cells. However, this high dose of UVB is not relevant in everyday life. Therefore we chose a dose of 50 mJ/cm2 for real-time RT-PCR. Our previous work on ionizing radiation led us to hypothesize that an extremely high dose of UVB can be used for a survival test of possible epidermal SC.21 The oral mucosal progenitor or SC were resistant to ionizing radiation, indicating that oral mucosal progenitor or SC survived radiotherapy and might contribute to recovery from radiation mucositis. To maintain tissue homeostasis, progenitor or SC may have strict regulatory mechanisms to prevent apoptosis.22 These resistant characteristics may be one of the most important and basic features of adult SC, including skin SC. The resistance to UVB can be applied further to confirm the usefulness of developing epidermal SC markers and isolation techniques.
Acknowledgments:
this work was supported by the ASAN Institute of Life Sciences (2006-415).
References
- 1.Jones PH, Harper S, Watt FM. Stem cell patterning and fate in human epidermis. Cell. 1995;80:83–93. doi: 10.1016/0092-8674(95)90453-0. [DOI] [PubMed] [Google Scholar]
- 2.Chen MF, Lin CT, Chen WC, et al. The sensitivity of human mesenchymal stem cells to ionizing radiation. Int J Rad Oncol Biol Phys. 2006;66:244–53. doi: 10.1016/j.ijrobp.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 3.Saretzki G, Armstrong L, Leake A, et al. Stress defense in murine embryonic stem cells is superior to that of various differentiated murine cells. Stem Cells. 2004;22:962–71. doi: 10.1634/stemcells.22-6-962. [DOI] [PubMed] [Google Scholar]
- 4.Yoo HC, Lee HW, Lee JE, et al. Change of inducible nitric oxide synthase expression by ultraviolet B irradiation on the skin of a rat. Ann Dermatol. 2001;13:16–21. [Google Scholar]
- 5.Bickenbach JR, Chism E. Selection and extended growth of murine epidermal stem cells in culture. Exp Cell Res. 1998;244:184–95. doi: 10.1006/excr.1998.4163. [DOI] [PubMed] [Google Scholar]
- 6.Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–24. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- 7.Kaur P. Interfollicular epidermal stem cells: identification, challenges, potential. J Invest Dermatol. 2006;126:1450–8. doi: 10.1038/sj.jid.5700184. [DOI] [PubMed] [Google Scholar]
- 8.Kim DS, Cho HJ, Choi HR, et al. Isolation of human epidermal stem cells by adherence and the reconstruction of skin equivalents. Cell Mol Life Sci. 2004;61:2774–81. doi: 10.1007/s00018-004-4288-4. [DOI] [PubMed] [Google Scholar]
- 9.Potten CS, Booth C. Keratinocyte stem cells: a commentary. J Invest Dermatol. 2002;119:888–99. doi: 10.1046/j.1523-1747.2002.00020.x. [DOI] [PubMed] [Google Scholar]
- 10.Nylander K, Vojtesek B, Nenutil R, et al. Differential expression of p63 isoforms in normal tissues and neoplastic cells. J Pathol. 2002;198:417–27. doi: 10.1002/path.1231. [DOI] [PubMed] [Google Scholar]
- 11.Pellegrini G, Dellambra E, Golisano O, et al. p63 identifies keratinocyte stem cells. Proc Nat Acad Sci USA. 2001;98:3156–61. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radu E, Simionescu O, Regalia T, et al. Stem cells (p63+) in keratinocyte cultures from human adult skin. J Cell Mol Med. 2002;6:593–8. doi: 10.1111/j.1582-4934.2002.tb00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olszewski WL, Moscicka M, Zolich D, et al. Human keratinocyte stem cells survive for months in sodium chloride and can be successfully transplanted. Transplant Proc. 2005;37:525–6. doi: 10.1016/j.transproceed.2004.12.174. [DOI] [PubMed] [Google Scholar]
- 14.Hong KK, Lew BL, Kim YI, et al. The effect of TNF-alpha and INF-gamma on the telomerase activity of cultured human keratinocyte. Ann Dermatol. 2007;19:147–52. [Google Scholar]
- 15.Korzeniewski C, Callewaert DM. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 1983;64:313–20. doi: 10.1016/0022-1759(83)90438-6. [DOI] [PubMed] [Google Scholar]
- 16.Kaur P, Li A. Adhesive properties of human basal epidermal cells: an analysis of keratinocyte stem cells, transit amplifying cells, and postmitotic differentiating cells. J Invest Dermatol. 2000;114:413–20. doi: 10.1046/j.1523-1747.2000.00884.x. [DOI] [PubMed] [Google Scholar]
- 17.Choi JH. Epidermal stem cells. Korean J Invest Dermatol. 2003;10:137–42. [Google Scholar]
- 18.Nijhof JG, van Pelt C, Mulder AA, et al. Epidermal stem and progenitor cells in murine epidermis accumulate UV damage despite NER proficiency. Carcinogenesis. 2007;28:792–800. doi: 10.1093/carcin/bgl213. [DOI] [PubMed] [Google Scholar]
- 19.Caricchio R, McPhie L, Cohen PL. Ultraviolet B radiation-induced cell death: critical role of ultraviolet dose in inflammation and lupus autoantigen redistribution. J Immunol. 2003;171:5778–86. doi: 10.4049/jimmunol.171.11.5778. [DOI] [PubMed] [Google Scholar]
- 20.Kim PK, Weller R, Hua Y, et al. Ultraviolet irradiation increases FADD protein in apoptotic human keratinocytes. Biochem Biophys Res Comm. 2003;302:290–5. doi: 10.1016/s0006-291x(03)00186-4. [DOI] [PubMed] [Google Scholar]
- 21.Jeong EJ, Choi SH, Chang SE, et al. Putative progenitor/stem cells isolated from human oral mucosa are resistant to ionizing radiation. J Dermatol Sci. 2008;50:65–8. doi: 10.1016/j.jdermsci.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]


