Abstract
Ellagic is a polyphenolic compound with anti-fibrotic and antioxidant properties, and exhibits antitumor properties against various cancer cells in vitro. There are few studies, however, which examine the effects of ellagic acid on melanoma. In the present study, we observe effects of ellagic acid on melanoma cells in vitro. Three metastatic melanoma cell lines (1205Lu, WM852c and A375) were examined to determine the effects of ellagic acid on melanoma cell viability, cell-cycle, apoptosis, NF-κβ activity, and IL-1β & IL-8 secretion. Cell viability assays demonstrated that ellagic acid possesses an inhibitory effect on cell proliferation at concentrations between 25 and 100 µM. In addition, ellagic acid promoted G1 cell cycle arrest, increased levels of apoptosis and decreased synthesis of IL-1β and IL-8 in melanoma cells. Ellagic acid also decreased NF-κβ activity, suggesting at least one potential mechanism by which ellagic acid may exert its effects in melanoma cells. Our findings support further investigation into prospective roles for ellagic acid as a therapeutic, adjuvant, or preventive agent for melanoma.
Key words: ellagic acid, melanoma, NF-κB, IL-1β, IL-8.
Introduction
Ellagic acid (EA) is a polyphenolic compound found in various types of fruit, including berries, pomegranates, and nuts, and is becoming a popular dietary supplement. It possesses antifibrotic,1,2 antiproliferative,3,4 and antitumorigenic5,6 properties. EA is known to be protective against several types of cancer. EA has been shown to induce apoptosis in human melanoma cells in vitro.7
Few studies have investigated the effects of EA on melanoma, however, and the mechanistic action of EA in this setting is not well defined. In vitro studies have shown that EA decreases tyrosinase activity in mouse melanoma cells by chelating the copper tyrosinase cofactor.8 We are not aware of any other studies that have defined a mechanism by which EA exerts its effects in melanoma.
The exact mechanism by which EA exerts its effects in other types of cancer is also unclear, but several potential mechanisms have been suggested. EA has been shown to modulate a variety of signaling pathways in cancer cells, including NF-κB, iNOS, and Wnt. Alternative mechanisms through which EA exerts anti-cancer effects have also been proposed. One group9 showed that EA prevented copper and catecholamine transmitter-mediated oxidative DNA damage, thus suggesting a protective role for EA in preventing reactive oxygen species production, lipid peroxidation, and DNA strand breaks. EA has also been shown to induce apoptosis via caspase pathways as well as potentiating trans-retinoic acid-mediated cell-differentiation on human leukemia cell lines.10 Another study showed that EA inhibits components of Wnt signaling pathways known to play a pivotal role in human colon carcinogenesis.11 Additionally, EA has been shown to reduce hepatic phase I CYP enzymes responsible for converting estrogen to harmful metabolites implicated in mammary tumorigenesis.12 EA has been shown to downregulate iNOS, COX-2, TNF-α and IL-6 secretion by inhibiting nuclear factor-kappa β (NF-κβ) in colon and pancreatic cancers.13 Pomegranate fruit extracts (including EA) decrease NF-κβ expression in UVB-stimulated keratinocytes, decreasing skin tumorigenesis.14,15 Here we report on the effect of EA on melanoma cells, including inhibition of the NF-κB pathway.
Materials and Methods
Tissue culture
Human metastatic melanoma cell lines (1205LU, WM852c and A375) were cultured in RPMI 1640 medium (GIBCO BRL, Gaithersburg, Maryland, USA) supplemented with 10% fetal bovine serum (FBS, Gemini Bio-Products, Inc., Woodland, CA, USA) and antibiotics (penicillin (10,000 IU/mL), streptomycin (10,000 IU/mL), and amphotericin B (25 microg/mL, Cellgro, Manassas, VA, USA) and were incubated at 37°C and 5% CO2.
Ellagic acid treatment
EA was obtained from (MP Biochemicals, Solon, OH, USA) and dissolved in sterile DMSO (5 mM) and stored at −20°C. Separate, fresh 100 µL aliquots of EA were used for each experiment and excess reagent was disposed of according to protocol.
Cell Titer 96 aqueous one solution cell proliferation assay (MTS assay) for the quantification of cell viability
Experiments were performed according to the manufacturer's instructions (Promega, Madison, WI, USA). Approximately 2.5×103 cells were seeded in 96-well plates and incubated at 37C° and 5% CO2 for 1 day. EA was added to cells in 0, 25, 50 and 100 µM concentrations and cells were incubated for 24, 48 and 72 h under previously described conditions. MTS reagent was added to the cells and incubated for 1 h, followed by spectophotometric analysis using an ELX808 Ultra Microplate Reader (Bio-Tek Instruments, Inc., Winooski, VT, USA) and KCjunior v. 1.10 software (BioTek Instruments).
Annexin V apoptosis detection assay
An annexin-FITC apoptosis detection kit (BD Biosciences Pharmingen, San Diego, CA, USA) was used, following the manufacturer's instructions; 4×105 cells were seeded in 10 cm plates and incubated for 24 h. Cells were treated with ellagic acid in DMSO at 0, 25, 50 and 100 µM concentrations. Cells were incubated as previously described for 72 h and stained with Annexin V antibodies and PI. Samples were analyzed by the FACS core using a Beckman Coulter FC500 flow cytometer (Beckman Coulter). Apoptotic cells were defined as being positive for Annexin V.
Cell cycle analysis
4×105 cells were seeded on 10 cm tissue culture dish and incubated overnight prior to treatment with EA. Cells were treated with 0 or 50 µM EA and incubated at 37°C for 48 h. Cells were then detached from the plate, stained with propidium iodide (PI) and allowed to incubate overnight at 4°C. Cells were then analyzed with by the University of Colorado Denver Fluorescent Activated Cell Sorting (FACS) core using a Beckman Coulter FC500 flow cytometer (Beckman Coulter Inc, Brea, CA, USA).
NF-κβ luciferase reporter assay
2.5×103 cells were seeded into 24-well plates and were transfected after 12 h of incubation. Transfection with pNFκβ-MetLuc2 Reporter Vector (Clontech, Mountain View, CA, USA) was performed according to protocol described by the Lipofectamine 2000 kit (Invitrogen, Carlsbad, CA, USA). Cells were then treated with 0, 25, 50 and 100 µM EA and incubated for 24 or 48 h. Luminescence was then measured with a luminometer (Promega, Madison, WI, USA).
Measurement of gene expression (IL-1β, IL-8)
1205Lu metastatic melanoma cells were seeded and incubated for 24 h under previously described conditions. Cells were then treated with 0 or 50 µM EA for 48 h. Cells were detached from culture dishes and RNA was extracted from treated and untreated cells using the RNAqueous-Micro kit (Ambion, Austin, TX, USA), and subsequently reverse transcribed using random primers and MMLV reverse transcriptase (Promega, Madison, WI, USA). Real-time quantitative reserve transcription-PCR (qRT-PCR) was performed with Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) on the MX3000P PCR system (Strategene, La Jolla, CA, USA). Primers were designed to generate a PCR product of 50 to 150 bp. Thermal cycling conditions were 95°C for 10 min followed by 42 cycles of 15 s at 95°C, 1 min at 60°C. GAPDH was used as a control to normalize the results.
Statistical analysis
The viabilities of each treated cell line were compared to control was evaluated using a Student's t-test. Values of P <0.05 were considered to be statistically significant.
Results
Melanoma cell viability is inhibited by Ellagic acid
Cells were incubated with different concentrations of EA for 24, 48, and 72 h and cell viability was measured using the MTS assay (Figure 1A, B). Inhibition of cell growth was detected as early as 24 h at 50 and 100 µM concentrations EA for 1205Lu cells (P<0.01 and P<0.05, respectively) and at 25, 50, and 100 µM concentrations EA for WM852c cells (P <0.05, P<0.05 and P <0.01, respectively). EA continued to inhibit cell growth for a 72 h period at 25, 50 and 100 µM concentrations (P <0.05, P<0.01 or P<0.001 for all data points measured).
Figure 1.
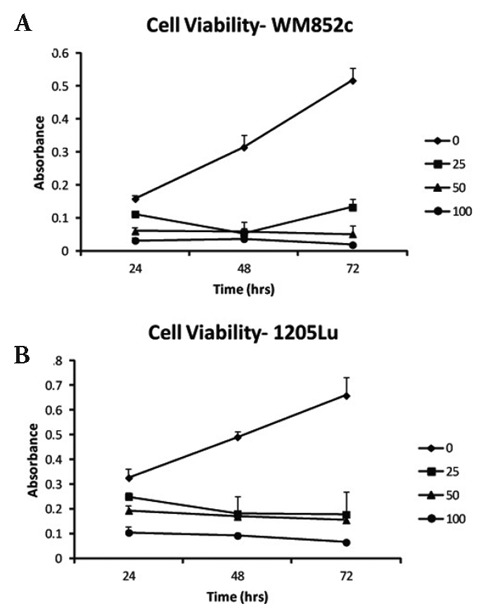
Cell growth over time with different concentrations of EA. A) WM852c and B) 1205Lu cells were incubated with different concentrations of EA for 24, 48, and 72 h and cell viability was measured using MTS assay.
Ellagic acid induces apoptosis in melanoma cell lines
Decreased viability is caused by cell death and/or decreased cell growth. In order to determine if EA exerted a proapoptotic effect in metastatic melanoma, three cell lines (1205LU, WM852c, A375) were incubated in 25, 50 and 100 µM concentrations of EA for 72 h (Figure 2A). After staining for the apoptotic marker Annexin V, cell lines were sorted with flow cytometry. 1205LU cells demonstrated increased apoptosis when compared to controls at 50 and 100 µM (P<0.01 and 0<0.001, respectively). Furthermore, both WM852c and A375 cell lines had increased levels of apoptotic cells at 100 µM concentrations EA (P <0.05). In general, there was a trend toward increased apoptosis with increasing concentrations of EA.
Figure 2.
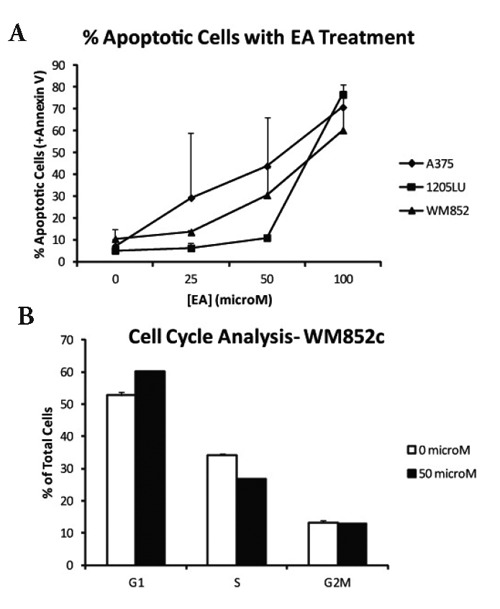
A) Apoptotic cells at 72 h. Three cell lines (1205Lu, WM852c, A375) were incubated in 25, 50 and 100 µM concentrations of EA for 72 h. After staining for the apoptotic marker Annexin V, cell lines were then sorted with flow cytometry. B) Cell cycle analysis. WM852c cells were incubated for 48 h in 50 µM EA. After staining, cells were sorted according to the phase of their cell cycle.
Ellagic acid induced G1 cell cycle arrest
In order to determine if EA inhibited cell proliferation via cell cycle arrest, WM852c cells were incubated for 48 h in 50 µM EA. After staining, cells were sorted according to the phase of their cell cycle (Figure 2B). Cell cycle analysis of WM852c cells in G1 phase suggested disruption of cellular replication (P<0.01), suggesting that EA inhibited melanoma cell proliferation via G1 cell cycle arrest.
Ellagic acid downregulates NF-κβ activity in metastatic melanoma cells
Several studies reported that EA modulates the NF-κβ pathway in cancer cells, therefore NF-κβ activity and expression in EA-treated cells was compared to untreated controls (Figure 3A, B). At 24 h, NF-κβ expression was decreased in both cell lines for all concentrations tested except for 25 µM in 1205Lu (for 1205Lu, P<0.001 for 50 and 100 µM concentrations; for WM852, P <0.01 for 50 µM, P <0.001 for 25 and 100 µM EA). At 48 h, there was decreased expression of NF-κβ with EA treatment at 25, 50 and 100 µM (for 1205Lu, P <0.01 for all concentrations; for WM852, P <0.05, P<0.01 and P<0.01 for 25, 50 and 100 µM EA, respectively).
Figure 3.
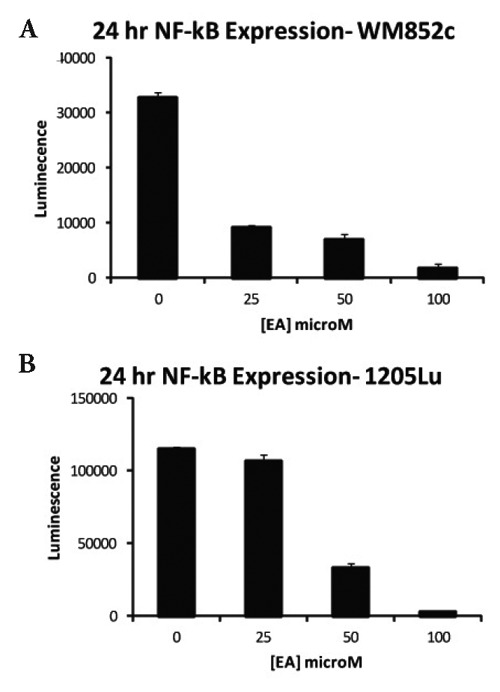
Expression of NF-κβ after EA treatment. A plasmid containing a luciferase reporter gene under the control of NF-κβ was transfected into WM852c (A) and 1205LU cells (B) and measured after 24 hours incubation.
Ellagic acid decreases synthesis of IL-1β and IL-8 in metastatic melanoma cells
Downstream effectors of the NF-κβ pathway which control cell proliferation and apoptosis were measured using quantitative RT-PCR. As indicated in Figure 4, mRNA coding for IL-1β and IL-8 8 were significantly decreased (P<0.05) by treatment with EA.
Figure 4.
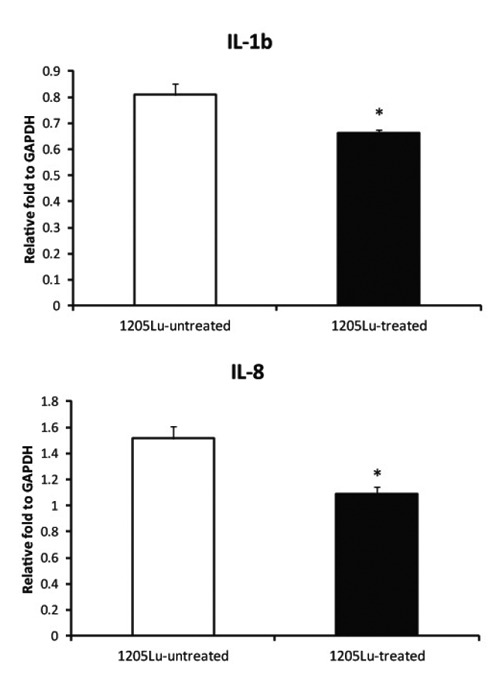
Expression of IL-1β and IL-8 cytokines after EA treatment. Metastatic melanoma cells were seeded and incubated for 24 h. Cells were then treated with 0 or 50 µM EA for 48 h. RNA was extracted from treated and untreated cells and measured by qRT-PCR. GAPDH was used as a control. *P <0.05
Discussion
This study suggests that EA induces apoptosis and G1 cell cycle arrest in melanoma cells at in vitro concentrations between 25 and 100 µM. Inhibition of the NF-κβ pathway was also shown to be a potential mechanism by which EA exerts its antiproliferative effects. The NF-κβ pathway frequently plays a central role in the pathogenesis of many types of cancer, and therapeutic modalities which target the NF-κβ pathway and its downstream intermediates are under investigation.
Dysregulation of the NF-κβ pathway in the setting of melanoma, a particularly treatment-resistant malignancy, is very common. Inhibition of NF-κβ has been explored as a potential therapeutic strategy against melanoma with positive results.16 For example, NF-κβ upregulation has been shown to promote angiogenesis in melanoma,17 a key mechanism in tumor growth and maintenance. NF-κβ also increases the rate of melanoma metastasis.18 Conversely, suppression of NF-κβ attenuates the invasive potential of tumors.19 Further evidence of the importance of the NF-κβ pathway in melanoma is that ablation of an upstream effector of the NF-κβ pathway, Ikappaβ kinase beta (IKΚβ), inhibits melanoma tumorigenesis20 and increased susceptibility to chemotherapy.21 Furthermore, p53 is inversely related to NF-κβ expression,22 and p53-mediated G1 cell cycle arrest may result from downregulation of NF-κβ in cancer cell.4
These findings support a potential role for EA as a therapeutic, adjuvant, or preventive agent for melanoma. Future work, however, should address the limitations of this study. While our data shows some evidence that EA treatment is associated with increased cell-cycle arrest and apoptosis, as well as decreased melanoma cell viability and NF-κβ activity, these results were not obtained in the presence of a vehicle control. Follow up work is therefore indicated to determine if EA specifically inhibits melanoma through the pathways observed in this study. Furthermore, the concentrations of EA used in our study are higher than levels that are generally sustainable by normal consumption of EA-containing foods (typically ranging from 5–15 µM.23,24 While the use of higher doses of EA as an adjuvant to other therapies may prove to be of some benefit to patients it is not clear from these results if EA may exert therapeutic benefits at practical dietary doses.
References
- 1.Thresiamma KC, Kuttan R. Inhibition of liver fibrosis by ellagic acid. Indian J Physiol Pharmacol. 1996;40:363–6. [PubMed] [Google Scholar]
- 2.Devipriya N, Sudheer AR, Srinivasan M, Menon VP. Effect of Ellagic acid, a plant polyphenol, on fibrotic markers (MMPs and TIMPs) during alcohol-induced hepatotoxicity. Toxicol Mech Methods. 2007;17:349–56. doi: 10.1080/15376510601077003. [DOI] [PubMed] [Google Scholar]
- 3.Losso JN, Bansode RR, Trappey A, 2nd, et al. In vitro anti-proliferative activities of ellagic acid. J Nutr Biochem. 2004;15:672–8. doi: 10.1016/j.jnutbio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Narayanan BA, Geoffroy O, Willingham MC, et al. p53/p21(WAF1/CIP1) expression and its possible role in G1 arrest and apoptosis in ellagic acid treated cancer cells. Cancer Lett. 1999;136:215–21. doi: 10.1016/s0304-3835(98)00323-1. [DOI] [PubMed] [Google Scholar]
- 5.Mukhtar H, Das M, Khan WA, et al. Exceptional activity of tannic acid among naturally occurring plant phenols in protecting against 7,12 dimethylbenz(a)-anthracene-, benzo(a)pyrene-, 3-methyl-cholanthrene-, and N-methyl-N-nitro-sourea-induced skin tumorigenesis in mice. Cancer Res. 1988;48:2361–5. [PubMed] [Google Scholar]
- 6.Kowalczyk MC, Kowalczyk P, Tolstykh O, et al. Synergistic effects of combined phytochemicals and skin cancer prevention in SENCAR mice. Cancer Prev Res (Phila) 2010;3:170–8. doi: 10.1158/1940-6207.CAPR-09-0196. [DOI] [PubMed] [Google Scholar]
- 7.Kim S, Liu Y, Gaber MW, et al. Development of chitosan-ellagic acid films as a local drug delivery system to induce apoptotic death of human melanoma cells. J Biomed Mater Res B Appl Biomater. 2009;90:145–55. doi: 10.1002/jbm.b.31266. [DOI] [PubMed] [Google Scholar]
- 8.Shimogaki H, Tanaka Y, Tamai H, Masuda M. In vitro and in vivo evaluation of ellagic acid on melanogenesis inhibition. Int J Cosmet Sci. 2000;22:291–303. doi: 10.1046/j.1467-2494.2000.00023.x. [DOI] [PubMed] [Google Scholar]
- 9.Spencer WA, Jeyabalan J, Kichambre S, Gupta RC. Oxidatively generated DNA damage after Cu(II) catalysis of dopamine and related catecholamine neurotransmitters and neurotoxins: Role of reactive oxygen species. Free Radic Biol Med. 2010;50:139–47. doi: 10.1016/j.freeradbiomed.2010.10.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagiwara Y, Kasukabe T, Kaneko Y, et al. Ellagic acid, a natural polyphenolic compound, induces apoptosis and potentiates retinoic acid-induced differentiation of human leukemia HL-60 cells. Int J Hematol. 2010;92:136–43. doi: 10.1007/s12185-010-0627-4. [DOI] [PubMed] [Google Scholar]
- 11.Sharma M, Li L, Celver J, et al. Effects of fruit ellagitannin extracts, ellagic acid, and their colonic metabolite, urolithin A, on Wnt signaling. J Agric Food Chem. 2009;58:3965–9. doi: 10.1021/jf902857v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aiyer H, Gupta RC. Berries and ellagic acid prevent estrogen-induced mammary tumorigenesis by modulating enzymes of estrogen metabolism. Cancer Prev Res (Phila) 2010;3:727–37. doi: 10.1158/1940-6207.CAPR-09-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umesalma S, Sudhandiran G. Differential inhibitory effects of the polyphenol ellagic acid on inflammatory mediators NF-kappaB, iNOS, COX-2, TNF-alpha, and IL-6 in 1,2-dimethylhydrazine-induced rat colon carcinogenesis. Basic Clin Pharmacol Toxicol. 2010;107:650–5. doi: 10.1111/j.1742-7843.2010.00565.x. [DOI] [PubMed] [Google Scholar]
- 14.Afaq F, Malik A, Syed D, et al. Pomegranate fruit extract modulates UV-B-mediated phosphorylation of mitogen-activated protein kinases and activation of nuclear factor kappa B in normal human epidermal keratinocytes paragraph sign. Photochem Photobiol. 2005;81:38–45. doi: 10.1562/2004-08-06-RA-264. [DOI] [PubMed] [Google Scholar]
- 15.Afaq F, Saleem M, Krueger CG, et al. Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-kappaB pathways and inhibits skin tumorigenesis in CD-1 mice. Int J Cancer. 2005;113:423–33. doi: 10.1002/ijc.20587. [DOI] [PubMed] [Google Scholar]
- 16.Czyz M, Lesiak-Mieczkowska K, Koprowska K, et al. Cell context-dependent activities of parthenolide in primary and metastatic melanoma cells. Br J Pharmacol. 2010;160:1144–57. doi: 10.1111/j.1476-5381.2010.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karst AM, Gao K, Nelson CC, Li G. Nuclear factor kappa B subunit p50 promotes melanoma angiogenesis by upregulating interleukin-6 expression. Int J Cancer. 2009;124:494–501. doi: 10.1002/ijc.23973. [DOI] [PubMed] [Google Scholar]
- 18.Wu FH, Yuan Y, Li D, et al. Endothelial cell-expressed Tim-3 facilitates metastasis of melanoma cells by activating the NF-kappaB pathway. Oncol Rep. 2010;24:693–9. [PubMed] [Google Scholar]
- 19.Kim A, Kim MJ, Yang Y, et al. Suppression of NF-kappaB activity by NDRG2 expression attenuates the invasive potential of highly malignant tumor cells. Carcinogenesis. 2009;30:927–36. doi: 10.1093/carcin/bgp072. [DOI] [PubMed] [Google Scholar]
- 20.Yang J, Splittgerber R, Yull FE, et al. Conditional ablation of Ikkb inhibits melanoma tumor development in mice. J Clin Invest. 2010;120:2563–74. doi: 10.1172/JCI42358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amschler K, Schon MP, Pletz N, et al. NF-kappaB inhibition through proteasome inhibition or IKKbeta blockade increases the susceptibility of melanoma cells to cytostatic treatment through distinct pathways. J Invest Dermatol. 2010;130:1073–86. doi: 10.1038/jid.2009.365. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen MK, Iversen L, Johansen C, et al. IL-8 and p53 are inversely regulated through JNK, p38 and NF-kappaB p65 in HepG2 cells during an inflammatory response. Inflamm Res. 2008;57:329–39. doi: 10.1007/s00011-007-7220-1. [DOI] [PubMed] [Google Scholar]
- 23.Mertens-Talcott SU, Jilma-Stohlawetz P, Rios J, et al. Absorption, metabolism, and antioxidant effects of pomegranate (Punica granatum l.) polyphenols after ingestion of a standardized extract in healthy human volunteers. J Agric Food Chem. 2006;54:8956–61. doi: 10.1021/jf061674h. [DOI] [PubMed] [Google Scholar]
- 24.Seeram NP, Lee R, Heber D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin Chim Acta. 2004;348:63–8. doi: 10.1016/j.cccn.2004.04.029. [DOI] [PubMed] [Google Scholar]


