Abstract
Tumor responses in advanced basal cell carcinoma (BCC) have been observed in clinical trials with vismodegib, a SMO antagonist. The result of SMO antagonism is inhibition Hedgehog Signaling Pathway (HHSP) downstream target genes. HHSP inhibition has been shown to affect stem cells responsible for blood, mammary, and neural development. We report on our experience of treating two patients with advanced BCC participating. These two patients have had no new BCCs develop for at least 2.25 years. Both patients have been receiving ongoing daily treatment with vismodegib for greater than 2.75 years without experiencing any significant side effects. After prolonged continuous daily dosing with a SMO antagonist, we have not observed a significant alteration in hematologic parameters or physical abnormalities of the pectoral regions of two patients with advanced BCC.
Key words: basal cell carcinoma, vismodegib, basal cell nevus syndrome, hedgehog signaling pathway.
Introduction
The hedgehog signaling pathway (HHSP) is an important pathway for growth and development, including promotion of primitive hematopoietic,1 neural,2 and mammary3 stem cells. It is also required to bring the hair follicle from the resting to the growth phase.4 Loss of heterozygosity and inactivation mutations in PTCH1 and SMO have been implicated in the development of the majority of basal cell carcinomas (BCCs)5–7 and in patients with basal cell nevus syndrome (BCNS).7,8 Vismodegib (GDC-0449) is a synthetic small molecule inhibitor of SMO that blocks downstream HHSP target genes; it has favorable pharmaceutical properties and greater potency than cyclopamine.9,10 A phase I dose-finding, safety, and tolerability study of vismodegib in patients with advanced BCC and solid tumors was conducted, demonstrating primarily mild to moderate side effects.6,7 Here we report on our experience of treating two patients with advanced BCC participating in the phase I study6,7 who have received ongoing daily treatment for 2.75–>3 years without experiencing any significant side effects that might be anticipated with chronic HHSP inhibition.
Case Report #1
A 49-year-old Caucasian man presented to our clinic with BCC metastatic to the lung and lymph nodes of the left neck. Eight years earlier he had been treated with cryotherapy and imiquimod cream for BCCs on the neck. Approximately 6.5 years later, he underwent his first surgical excision of cutaneous BCC of the neck and margins were reportedly positive. Subsequently, he was found to have multifocal metastatic BCC in the lungs, confirmed by video-assisted thoracic surgery (VATS) removal of a 1.5 cm left lower lung mass 1 month before evaluation at our clinic. His medical history was otherwise unremarkable except for approximately 2 years of heavy drinking; he had since abstained for over 2 years.
Physical examination was significant for healed left flank scar from VATS, several hard, fixed palpable lymph nodes in the left posterior cervical chain measuring 1–3 cm, and a 3.1 cm hard fixed left supraclavicular lymph node. There were small cutaneous BCCs in the left supraclavicular region, left neck (Figure 1A), and left forearm (one each). Computed tomography (CT) imaging revealed multiple pulmonary nodules (∼20, the largest was 1.4 cm) (Figure 1B) and multiple left neck (Figure 1C) and supraclavicular lymph node involvement (size ranging from 1.4 to 3.1 cm). Fluorine-18-2-fluoro-2-deoxy-D-glucose positron-emission tomography (PET)/CT imaging identified 3–5 hypermetabolic foci in the right and left lung (peak standardized uptake value [SUV] 12.3) and 10–15 hypermetabolic foci in the left neck and supraclavicular fossa (peak SUV 14.8).
Figure 1.

Baseline photograph and computed tomography (CT) images of Patient 1: A) photograph of left neck area at baseline; B) CT scan at baseline showing multifocal pulmonary nodules several of which were >1 cm; C) CT scan at baseline showing left neck adenopathy >1 cm.
The patient started receiving oral vismodegib 270 mg daily in October 2008. By January 2009, the patient had a confirmed partial response on CT by Response Evaluation Criteria in Solid Tumors (RECIST11) and a complete response on PET/CT (absence of hypermetabolic foci). By April 2009, only a 0.8 cm pulmonary nodule could be measured on CT scan. In December 2009, his vismodegib dose was increased to 300 mg daily when he transitioned from the phase I protocol to the extension study; with the transition to the extension study, the 270 mg dose was no longer available. He maintained a continuing partial response until January 2011 (Figures 2A and 2B), when a left axilla metastasis (non-target progression by RECIST11) was identified and excised. As of October 2011, he continues on vismodegib 300 mg daily without evidence of progression or disease recurrence elsewhere with continued resolution of cutaneous BCC (Figure 2C). His only drug-associated adverse events (AEs) according to the National Cancer Institute Common Toxicity Criteria (version 3.0) have been grade 2 dysgeusia, intermittent grade 1 muscle cramps and fatigue (diminished in frequency by calcium and magnesium supplementation), and grade 2 alopecia. During the phase I study this patient also reported drug-related grade 1 intermittent heartburn and grade 1 weight loss.
Figure 2.
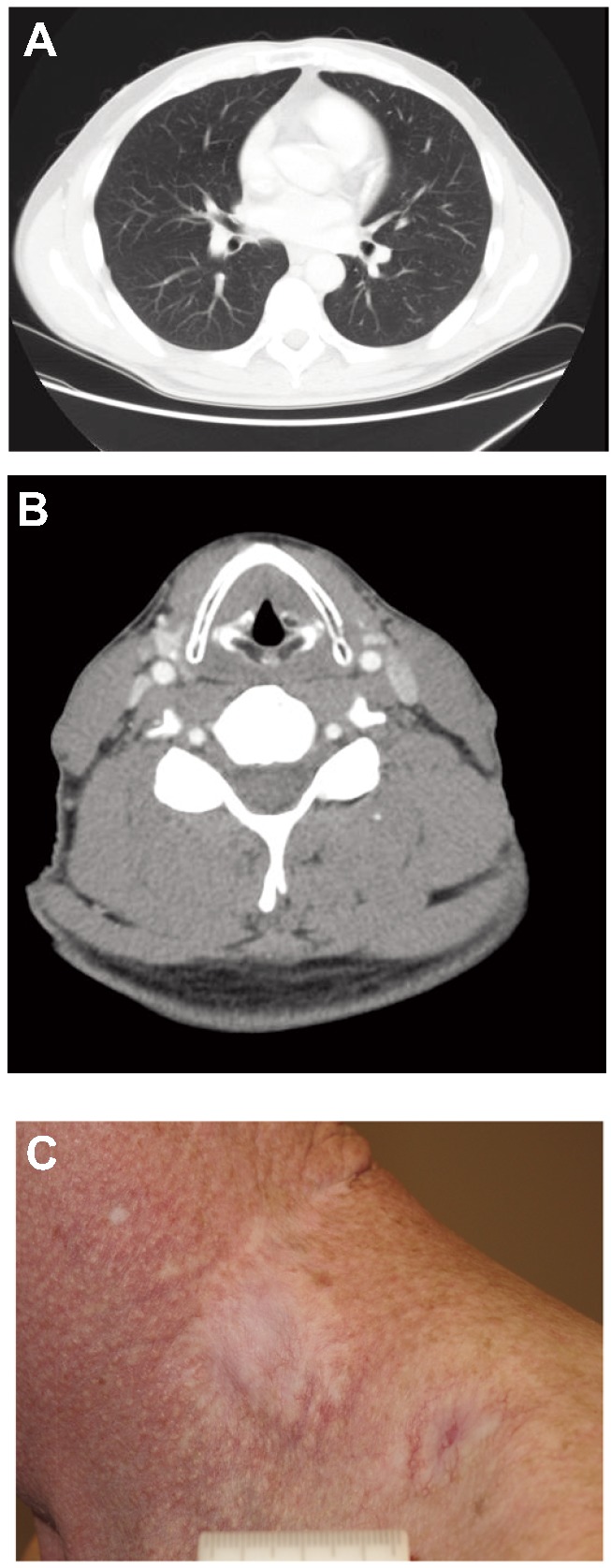
Response photograph and computed tomography (CT) images of Patient 1: A) CT scan after approximately 28 months of treatment with vismodegib showing near complete resolution of all pulmonary nodules; B) CT scan after approximately 28 months of treatment with vismodegib showing resolution of left neck adenopathy.; C) recent photograph of left neck area following 31 months of treatment with vismodegib.
Case Report #2
A 49-year-old Caucasian man with BCNS and active multifocal cutaneous BCCs was referred to our clinic. He underwent multiple therapies for BCC beginning in his 30s including Mohs surgeries, some requiring skin grafting, and cryosurgeries. He had recently averaged 5–10 surgeries for BCC annually. Pertinent medical history included several family members with BCNS.
Physical examination was significant for healed surgical scars, and several BCCs of approximately 0.5 cm on the left cheek and the right and periorbital region. On high-resolution ultrasound (HRU), six sonographic abnormalities were identified, the largest measuring 0.5 cm in the longest dimension.
The patient started receiving oral vismodegib 270 mg daily in March 2008. By December 2008, the patient had complete resolution of all six skin lesions on his face. In November 2009, his vismodegib dose was increased to 300 mg daily when he transitioned from the phase I protocol to the extension study. After more than 3 years of continuous daily dosing with vismodegib, his only drug-associated AEs are grade 1 dysgeusia, intermittent grade 1 muscle cramps (diminished with exercise), and grade 1 alopecia. Grade 1 fatigue was also reported during the phase I study. The patient continues to receive vismodegib 300 mg daily.
Discussion
Inhibition of the HHSP has been shown to affect stem cells responsible for blood, mammary, and neural development. After at least 3 years of continuous daily dosing, these two patients have not experienced significant alteration in hematologic parameters, nor physical abnormalities of their pectoral regions. The drug-related AEs observed in these two patients are all grade 1 or 2, with dysgeusia, possibly due to an effect on taste bud papillae.12 Since our initial report on results treating advanced BCC >18 months ago,6 these two patients have had no new BCCs develop for at least 2.25 and 3+ years, respectively, and are tolerating therapy well. Based on the pharmacokinetic and pharmacodynamic profile of vismodegib, a dose of 150 mg daily is under evaluation in further studies.7 Overall, based on data from these two patients, it appears that long-term vismodegib use in adults with advanced BCC can be an effective therapy with manageable AEs.
Acknowledgments:
we thank the patients and their families, Jody Emery, Vickie Marsh, and Lisa Rumble, and clinical staff for their assistance. Support for third-party writing assistance for this manuscript was provided by Genentech Inc. Through the Genentech-Curis collaboration, vismodegib was discovered by Genentech and was jointly validated by the parties through a series of preclinical studies. Genentech and Roche collaborate on the clinical development and commercialization of vismodegib.
References
- 1.Bhardwaj G, Murdoch B, Wu D, et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol. 2001;2:172–80. doi: 10.1038/84282. [DOI] [PubMed] [Google Scholar]
- 2.Liu S, Dontu G, Mantle ID, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–71. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–7. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- 4.Paladini RD, Saleh J, Qian C, et al. Modulation of hair growth with small molecule agonists of the hedgehog signaling pathway. J Invest Dermatol. 2005;125:638–46. doi: 10.1111/j.0022-202X.2005.23867.x. [DOI] [PubMed] [Google Scholar]
- 5.Weiss GJ, Von Hoff DD. Hunting the hedgehog pathway. Clin Pharmacol Ther. 2010;87:743–7. doi: 10.1038/clpt.2010.34. [DOI] [PubMed] [Google Scholar]
- 6.Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164–72. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 7.LoRusso PM, Rudin CM, Reddy JC, et al. Phase I trial of hedgehog pathway inhibitor GDC-0449 in patients with refractory, locally-advanced or metastatic solid tumors. Clin Cancer Res. 2011;17:2502–11. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hahn H, Wicking C, Zaphiropoulous PG, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–51. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 9.Binns A, James LF, Shupe JL, Everett G. Congenital cyclopian-type malformation in lambs induced by maternal ingestion of a range plant, Veratrum californicum. Am J Vet Res. 1963;24:1164–75. [PubMed] [Google Scholar]
- 10.Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16:2743–8. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 12.Mistretta CM, Liu HX, Gaffield W, MacCallum DK. Cyclopamine and jervine in embryonic rat tongue cultures demonstrate a role for Shh signaling in taste papilla development and patterning: fungiform papillae double in number and form in novel locations in dorsal lingual epithelium. Dev Biol. 2003;254:1–18. doi: 10.1016/s0012-1606(02)00014-3. [DOI] [PubMed] [Google Scholar]


