Abstract
Background
Chemotherapy-induced ovarian failure (CIOF) is a frequent side effect of adjuvant chemotherapy that results in rapid bone loss. We hypothesized that zoledronic acid (ZA), a third-generation amino bisphosphonate, would prevent bone loss in premenopausal women who developed CIOF.
Methods
439 women were randomized to intravenous (IV) ZA 4 mg every 3 months for 2 years starting within 1–3 months (mos) after randomization (arm A) or 1 year after randomization (arm B, controls). CIOF was prospectively defined as ≥ 3 months of amenorrhea, follicle-stimulating hormone (FSH) ≥ 30 MIU/ml and non-pregnant at 1 year. The primary endpoint was the percentage change in bone mineral density (BMD) in the lumbar spine (LS) from baseline to 12 months in the ZA and control groups in women who developed CIOF; the secondary endpoint was BMD in LS at 3 years in all randomized women.
Findings
150 (56%) met the definition of CIOF at 1 year. Overall, grade 3 toxicities of ZA were fatigue (1%) arthralgias (1%) and pain (4%). The median percent change (interquartile range, IQR) at 1 year was +1.2% (−0.5% to +2.8%) and −6.7% (−9.7% to −2.9%) p<0.001 and at 3 years was +1.0% (−1.6% to +5.2%) and −0.5% (−3.7% to +3.2%) p=0.019 in arms A and B, respectively.
Interpretation
ZA every 3 months is well tolerated and prevents rapid bone loss in premenopausal women that develop CIOF. Giving ZA with rather than one year after the start adjuvant chemotherapy is the preferred sequence to prevent bone loss.
INTRODUCTION
Chemotherapy-induced ovarian failure (CIOF) occurs in about 50% to 70% of premenopausal women who receive adjuvant chemotherapy for breast cancer.1,2 Chemotherapy, in particular alkylating agents such as cyclophosphamide, decreases primordial follicles and ovarian reserve,3–5 but the precise mechanism of CIOF remains undefined.6 Among the consequences of CIOF is rapid bone loss,7–10 which ranges from 6–8% during the first year and is more similar to that seen after treatment with gonadotropin-releasing hormone agonists or oophorectomy, and up to two to three-fold higher than aromatase inhibitor-induced bone loss.11,12
Some women who develop CIOF are at risk for subsequent osteoporosis13,14 and phase III trials to mitigate bone loss using IV or oral bisphosphonates have been reported.15–19 Cancer and Leukemia Group B (CALGB) trial 79809 is the largest trial designed to test whether IV zoledronic acid (ZA), a third-generation bisphosphonate, can prevent rapid bone loss in premenopausal women receiving adjuvant chemotherapy. The trial was also designed to test the optimal timing of administration of ZA either concurrent with adjuvant chemotherapy or beginning one year after randomization.
PATIENTS AND METHODS
The eligibility criteria and definitions of CIOF utilized in trial 79809 were based on a prior prospective trial.8 In brief, premenopausal, non-pregnant women age 40 years or older with histological evidence of localized (stages I-III) invasive breast cancer were eligible. Premenopausal status was defined as actively menstruating or last menstrual period within 6 months prior to randomization on the trial. Women who had a prior hysterectomy without bilateral oophorectomy were eligible if their serum estradiol (E2) and follicle-stimulating hormone (FSH) were within institutionally-defined premenopausal range prior to starting adjuvant chemotherapy. The adjuvant chemotherapy regimen was not specified and was selected by the treating physician. Subsequent to the completion of adjuvant chemotherapy, women with estrogen and/or progesterone receptor positive tumors received tamoxifen. No prior treatment with a bisphosphonate was permitted and women receiving cardiac glycosides were not eligible because of potential safety concerns if hypocalcaemia or impaired renal function developed consequent to ZA treatment. Each participant signed an IRB-approved, protocol-specific informed consent in accordance with federal and institutional guidelines.
Figure 1 describes the randomization and treatment plan. After registration, eligible women were randomized either to IV infusion of ZA 4 mg over 15 minutes every 3 months for a total of 8 treatments either beginning within 1–3 months after starting adjuvant chemotherapy (arm A) or 1 year (12–14 months) after randomization (arm B). Within 4 weeks prior to randomization all trial participants had a baseline dual energy x-ray absorptiometry (DEXA) scan, E2, FSH and non-pregnant β-HCG. The E2 and FSH were not collected on a specified day of the menstrual cycle. These tests were repeated at 1 and 3 years after randomization. Every 3 months all trial participants had a medical history, vital signs, ECOG performance status, physical exam, an assessment of toxicities (for patients randomized to arm B toxicity grading started 1 year after randomization), determination of serum calcium and magnesium and the date of last menstrual period recorded. Serum creatinine was measured prior to each ZA dose. All women were instructed to take at least 1000 mg of supplemental calcium and 400 IU of vitamin D throughout the duration of the trial and compliance was assessed by self-report every 3 months, with the actual doses recorded on the study follow-up forms.
Figure 1.
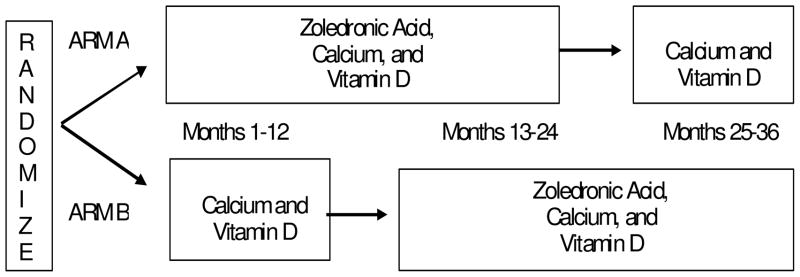
CALGB Trial 79809 Schema
No dose reductions of ZA were permitted. If the baseline serum creatinine was ≤ 1.4 mg/dL at the time of study entry, an increase in creatinine of 0.5 mg/dL or more required delaying the ZA dose until the serum creatinine returned to no higher than 10% above the baseline value. If the baseline serum creatinine was > 1.4 mg/dL at the time of study entry, an increase of 1.0 mg/dL or more required delaying the ZA dose until the serum creatinine returned to no higher than 10% above the baseline value. If the ZA dose was delayed the intervals at which the creatinine was repeated was at the discretion of the treating physician.
Study Design and Data Analysis
The primary trial endpoint was the percentage change in LS bone mineral density (BMD) measured at 1 year post randomization compare to baseline in the women who developed CIOF. CIOF was defined prospectively at the 1 year evaluation point according to the following definition: ≥ 3 months of amenorrhea, FSH ≥ 30 MIU/ml and non-pregnant as established in a prior prospective trial.7 The secondary endpoint was BMD measured at 3 years in all randomized women.
The randomization scheme used was a stratified random permuted blocks design with balanced assignments to each treatment.20,21 Stratification factors were tamoxifen use (no vs. yes) and nodal status (negative, positive, or unknown in the case of neoadjuvant treatment). The patient's institution was not a stratification factor, but partial balancing within institutions was achieved. In a prior study7 a standard deviation of about 0.2 g/cm2 in LS BMD was observed at 1 year. Assuming an equal standard deviation in the two arms of 0.2 g/cm2, 80 patients per arm were needed so that the t-test had at least 80% power (with a two-sided significance level of 0.05) to detect a difference between means of BMD of 1.0 g/cm2 (arm A) and 0.91 g/cm2 (arm B) at 1 year. Assuming the rate of CIOF to be 50% and an attrition rate of 20% at 1 year,7,19 the sample size was increased to 200 per treatment arm or a total of 400 women. Women who did not meet the criteria of CIOF were excluded from the primary endpoint analysis. The secondary endpoint was LS BMD at 3 years post-randomization in all women who had a baseline and 3 year BMD evaluation.
Patient registration, randomization and data collection were managed by the CALGB Statistical Center. Data quality was ensured by careful review of data by CALGB Statistical Center staff and by the study chairperson. Statistical analyses were performed by CALGB statisticians. The CALGB DSMB provided oversight to the study. This phase III trial was monitored at least twice annually by the DSMB. Planned interim analyses used the Lan-Demets analog of the O’Brien-Fleming sequential boundary to maintain an overall significance level of 0.05.22 The DSMB recommended release of results at the first interim analysis in November 2008 when the observed p-value for the change in LS BMD at 1 year from randomization (0.0004) was below the Lan-DeMets boundary p-value (0.001). This is the final analysis of the study as this report includes 1 the 3 year data.
RESULTS
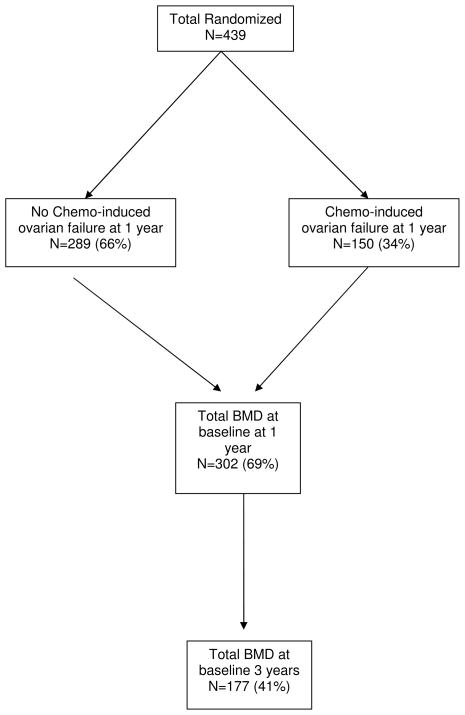
From 12/2001 to 12/2006 439 women were randomized on the trial (See Figure 2). All 439 were eligible for randomization. Of these, 173 (39%) were ineligible for the primary endpoint of CIOF because of the following: they did not have a BMD measured at baseline (n=4); and either they did not have BMD (n=133) or an FSH measured (n=36) at 1 year, or they were measured after 12–14 months. Of the 266 (n=61%) randomized women eligible for assessment of the primary endpoint, 116 (44%) were either actively menstruating or had FSH ≤ 30 MIU/ml at 12 mos and 150 (56%) met the definition of CIOF at 12 mos. The rates of CIOF did not differ among the treatment arms. BMD was available at baseline and 36 months after randomization for 177 (40%) women.
Figure 2.
CALGB Trial 79809 CONSORT Diagram
The baseline demographic characteristics of the randomized women are described in Table 1. The treatment arms were well balanced. The adjuvant chemotherapy regimens were primarily cyclophosphamide and doxorubicin alone or with paclitaxel; tamoxifen was administered to 70% of women. The baseline characteristics of the 116 women that retained menstrual function were similar to those of the 150 women who met the criteria for CIOF (data not shown). Table 1 also describes the baseline characteristics of the 177 randomized women who had their BMD measured at baseline and 36 months. Compliance with recommended daily doses of calcium and vitamin D assessed by self-report was virtually 100% in both treatment arms during the first year.
Table 1.
Baseline Patients Characteristics by Arm
| N (%) | ||||
|---|---|---|---|---|
| Arm A- ZA (n=70) | Arm B- Control (n=80) | Total CIOF (n=150) | Total at 3 years (177) | |
| Age (yr)* | 46 (40–57) | 48 (40–59) | 47 (40–59) | 47 (40–59) |
| Race | ||||
| White | 69 (99) | 73 (91) | 142 (95) | 165 (93) |
| Black | 0 (0) | 3 (4) | 3 (2) | 8 (5) |
| Asian | 0 (0) | 1 (1) | 1 (1) | 1 (2) |
| Other | 1 (1) | 3 (4) | 4 (3) | 2 (1) |
| ECOG PS | ||||
| 0 | 69 (93) | 71 (91) | 136 (92) | 165 (93) |
| 1 | 5 (7) | 7 (8) | 14 (8) | 12 (7) |
| Nodal Status | ||||
| negative | 29 (41) | 27 (34) | 56 (37) | 81 (46) |
| positive | 34 (49) | 45 (56) | 79 (53) | 89 (50) |
| unknown | 7 (10) | 8 (10) | 15 (10) | 7 (4) |
| Stage | ||||
| I/II | 55 (79) | 65 (82) | 120 (80) | 145 (82) |
| III | 13(18) | 10 (12) | 23 (15) | 26 (15) |
| unknown | 2 (3) | 5 (6) | 7 (5) | 6 (3) |
| E2 (pg/ml)* | 34 (7–324) | 31 (1–248) | 32 (1–324) | 35 (4–531) |
| FSH (miu/ml)* | 28 (1–103) | 34 (1–92) | 33 (1–109) | 21 (0.6–115) |
| LS BMD (g/cm2)* | 1.1 (0.8–1.6) | 1.1 (0.7–1.9) | 1.1 (0.7–1.9) | 1.1 (0.7–1.9) |
| Tamoxifen (%) | 51 (73) | 56 (70) | 107 (71) | 125 (71) |
Abbreviations: Eastern Cooperative Oncology Group (ECOG); performance status (PS);
estradiol (E2); follicle stimulating hormone (FSH); lumbar spine (LS); and bone mineral density (BMD).
median (range)
As expected, the FSH increased and E2 decreased significantly during the first 12 months in women who developed CIOF. The median (range) FSH was 33 miu/ml (1-109) and 52 (30-142) p<0.0001 and E2 pg/ml was 32 (7-324) and 20 (5-795) p<0.0001 at baseline and 12 months, respectively. Toxicities of ZA in arms A and B are described in Table 3. The majority of ZA-related toxicities were grades 1 and 2. Grade 3 fatigue, arthralgias and pain were experienced in 1%, 2% and 8% of women, respectively. There were no cases of serious renal toxicity or osteonecrosis observed. There were no differences in ZA-related toxicities among women who did or did not develop CIOF (data not shown).
Table 3.
Median (interquartile range) Percent Difference in BMD from Baseline to 1 or 3 years
| ZA-Arm A | Control-Arm B | p | |
|---|---|---|---|
| Total with CIOF at 1 year (n=150) | 1.2 (−0.5 to +2.8) | −6.7 (−2.9 to −9.7) | <0.001 |
| Total women at 1 year (n=302) | 1.4 (−0.7 to +3.9) | −5.5 (−2.3 to −8.8) | <0.001 |
| Total women at 3 years mos (n=177) | 1.0 (−1.6 to +5.20) | −0.5 (−3.7 to +3.2) | 0.019 |
Abbreviations: Bone mineral density (BMD); chemotherapy-induced ovarian failure (CIOF); zoledronic acid (ZA).
BMD results are described in Table 3. Women who developed CIOF and were randomized to arm A had less bone loss compared with those who were randomized to arm B (+1.2% vs. −6.7%, p<0.001). Adjusting on the stratification factors (tamoxifen and nodal status), the results remained statistically significant. Analysis of all women who had baseline and 1 year BMD measured, irrespective of CIOF, yielded similar results (+1.4 vs. −5.5, p<0.001) although the magnitude of differences in bone loss was less. At 3 years less bone loss was observed in the women who received ZA (arm A) during the adjuvant chemotherapy (+1% vs. −0.5%, p=0.019).
DISCUSSION
In women who developed CIOF, ZA prevented bone loss whereas in the control group a highly statistically significant difference in bone loss from baseline to 1 year was observed. The main toxicities of ZA during and after adjuvant chemotherapy were mild fever, pain and arthralgias; importantly there was no clinically significant renal toxicity or osteonecrosis observed. CALGB 79809 has several features that distinguish it from prior trials;15–19 it is the largest trial, it included a prospective protocol-specified definition of CIOF and it was designed to test the optimal timing of IV ZA administration either administered concurrently with adjuvant chemotherapy or beginning 1 year after the adjuvant chemotherapy started.
ZA and other IV and oral bisphosphonates prevent bone loss in premenopausal women receiving adjuvant chemotherapy (Table 4). The one exception is the randomized trial by Hines et al in which there was no difference in BMD measured at one year in the LS between the oral risedronate and placebo groups.16 Perhaps, the lack of a definition for CIOF and the use of an oral, less potent bisphosphonate may have contributed to the null result. The relevant questions are identifying those women who are most (and least) at risk for bone loss and the optimal timing, schedule and duration of bisphosphonate administration. It is instructive to look at the natural history of CIOF by examining the placebo or control-treated groups in prior trials. Women who retain menstrual function after adjuvant chemotherapy are at little or no risk of bone loss at 1 year, whereas those who develop prolonged amenorrhea or CIOF experience a highly statistically significant bone loss in the LS in the range of 4% to 8%.7,15,17,19 These results are consistent with the 6.7% decrease in LS BMD observed in the control group in this trial.
Table 4.
Randomized Bisphosphonate Trials in Premenopausal Women Treated with Adjuvant Chemotherapy
| Trial | N | Chemo | Dose/Schedule | % Change in LS BMD | P | |
|---|---|---|---|---|---|---|
| CALGB 79809 | ZA vs. CON | 150 177 |
AC, ACT | IV ZA 4 mg Q3 mo × 2 years |
+1.2 vs. −6.7 α
+1.0 vs. −0.5γ |
<0. 001 0.019 |
| Hershman18 2008 | ZA vs. PLAC | 114 | AC, ACT | IV ZA 4 mg Q3 mo × 1 year |
−0.6 vs. −4.4† | <0.05 |
| Hines19 2008 | RISE D vs. PLAC | 170 | AC; ACT | PO RISED 35 mg Q week X 1 year | −4.3 vs. −5.8† | 0.18 |
| Fuleihan20 2005 | PAM vs. PLAC | 87 | CAF | IV PAM 60 mg Q3 mo × 1 year |
1.9 vs. −3.2†
0.9 vs. −4.0α |
0.002 0.03 |
| Powles21 1998 | CLOD vs. PLAC | 118 | CMF, MMC, CEF | PO CLOD 1600 mg Q day × 2 years |
0.3 vs. −4.0†
−4.0 vs. −3.9 |
0.003 0.9 |
| Saarto22 1997 | CLOD vs. CON | 113 | CMF | PO CLOD 1600 mg Q day × 2 years |
−0.7 vs. −3.7†
−3.2 vs. −6.8α |
0.0005 NA |
Abbreviations: Lumbar spine (LS); bone mineral density (BMD); clodronate (CLOD); no treatment control (CON); placebo (PLAC); risedronate (RISED); pamidronate (PAM); zoledronic acid (ZA); cyclophosphamide, methotrexate, fluorouracil (CMF); mitoxantrone, methotrexate, cyclophosphamide (MMC); epirubicin (E); doxorubicin, cyclophosphamide (AC); paclitaxel (T).
result in women with prolonged amenorrhea or chemotherapy-induced ovarian failure (CIOF)
At 1 year
At 2 years
At 3 years
Fracture data are not available in trial 79809 or any of the other trials described in Table 4. This is because in premenopausal or newly postmenopausal women the fracture risk is very low;23 even the largest trials are too small and the follow-up duration too short to detect any differences in fracture rates that may occur decades later. However, several studies report that breast cancer survivors have a higher incidence of vertebral factures, osteoporosis and undiagnosed osteoporosis relative to women without breast cancer.24,25 In addition, very limited data are available on the relationship between BMD, T scores and fracture risk in premenopausal women or women with CIOF,26,27 in contrast to postmenopausal women where the relationship between BMD, T-scores and fractures is well established. The International Society for Clinical Densitometry advocates using Z-scores (the standard deviations above or below an individual’s BMD relative to the mean BMD of an age, gender and ethnicity matched population) instead of T-scores in premenopausal women. A Z-score of lower and higher than -2 is classified as below and in the expected range for age, respectively.28 Individual Z-scores were not collected in this trial so it is unknown how many women randomized to control (Arm B) were below the expected range for age. Nonetheless, it seems prudent to mitigate this rapid bone loss as it may lessen the risk of osteoporosis developing decades later.
Bone loss appears to be greatest in the first 6 to 12 months after initiating adjuvant chemotherapy in women who develop CIOF or prolonged amenorrhea due to rapid estrogen deprivation. Although CIOF is a frequent side effect of adjuvant chemotherapy, the term lacks a standard and precise definition.1 Prolonged amenorrhea [i.e. 6 or 12 months] after adjuvant chemotherapy using self-reported menstrual diaries or standardized questionnaires is reported in clinical trials. However, up to 27% of women with amenorrhea after adjuvant chemotherapy will resume menses after a median duration of 12 months.29 The definition of CIOF used in this trial [i.e., 3 or months of amenorrhea, FSH of 30 MIU/ml or more higher and non-pregnant] was the same definition used in a prior prospective trial.7 A subset of those women were followed for 24 months and had sustained elevations of FSH in the postmenopausal range without resumption of menses. Thus, distinguishing between CIOF that is permanent and amenorrhea that may reverse is an important issue not only for bone loss but for the selection of optimal anti-estrogen treatment, fertility considerations, overall quality of life and informed consent.
In postmenopausal women tamoxifen preserves BMD whereas in menstruating women tamoxifen causes bone loss.30 Less is known about women who develop CIOF and receive tamoxifen. For this reason, the BMD results in women who did not receive ZA but were treated with tamoxifen are of interest. These tamoxifen-treated women had less bone loss at 1 year then those that did not receive the drug (median percent change in LS BMD of −4.4% versus −8.1%). Although these results should be interpreted with caution as this was an unplanned subset analysis, they are consistent with the results of another trial in premenopausal women experiencing prolonged amenorrhea after adjuvant chemotherapy and also received tamoxifen.31 A bone-sparing effect of tamoxifen may occur in women who develop CIOF but larger studies are required to confirm this. These data also suggest that women with estrogen receptor negative tumors who develop CIOF may benefit most from early interventions to mitigate bone loss.
At 3 years after randomization there was statistically significant higher BMD in arm A although the magnitude of difference in bone loss in the treatment arms is much smaller at 3 as opposed to 1 year (Table 3). As ZA does not appear to increase the toxicities of adjuvant chemotherapy (Table 2), for practical purpose the preferred sequence ZA administered starting with the adjuvant chemotherapy. The optimal schedule of ZA for bone loss in women with CIOF remains unknown. A 5 mg ZA dose annually improved BMD, reduced fractures and is approved for use in postmenopausal women with osteoporosis.32 An annual 4 mg dose of ZA was studied in a small phase II trial in 44 postmenopausal women with breast cancer and osteopenia.33 Statistically significant increases in BMD and decreases in bone resorption markers were sustained for up to 3 years following the single dose. Whether an annual dose of ZA will be as effective in the setting of the rapid bone loss associated with CIOF remains to be tested.
Table 2.
ZA-related Toxicities in Treatment Arm A and B during Year 1
| N (%) | ||||||
|---|---|---|---|---|---|---|
| Grade | Arm | 1 | 2 | 3 | 4 | Total |
| Fever | A | 6 (8) | 0 | 0 | 0 | 6 (8) |
| B | 6 (8) | 0 | 0 | 0 | 6 (8) | |
| Fatigue | A | 16 (23) | 7 (10) | 0 | 0 | 23 (33) |
| B | 10 (13) | 4 (5) | 1 (1) | 1 (1) | 16 (20) | |
| Pain | A | 15 (21) | 9 (13) | 2 (3) | 0 | 26 (37) |
| B | 17 (21) | 8 (10) | 4 (5) | 0 | 29 (36) | |
| Arthralgias | A | 11 (16) | 6 (8) | 1 (1) | 0 | 18 (25) |
| B | 4 (5) | 5 (6) | 1 (1) | 0 | 10 (13) | |
| Creatinine | A | 2 (3) | 0 | 0 | 0 | 2 (3) |
| B | 2 (3) | 0 | 0 | 0 | 2 (3) | |
Abbreviations: zoledronic acid (ZA)
The strengths of trial 79809 are that it included a prospective definition of CIOF and it addressed the optimal timing of ZA. There is a need to develop risk factors for CIOF, independent of age,34 as these are women in whom the benefits of ZA are greatest as opposed to those who retain menstrual function or are transiently amenorrheic. Importantly, after 3 years the BMD was higher when the ZA was administered beginning with the start of adjuvant chemotherapy as opposed to starting the drug 1 year later. Finally, the results of trial 79809 can be considered representative of women treated in the community, where the majority of cancer care occurs, as more than 50% of accrual was from the CALGB Community Clinical Oncology Program (CCOP) institutions. Weaknesses of this trial include the lack of compliance with the protocol specified DEXA scans especially at 1 year. Thirty percent of the randomized women did not did have a DEXA scan at 1 year or the scan was outside the protocol-specified window of 12 to 14 months. Detailed information on reasons for non-compliance was not collected, however, this is in the range of 20–25% reported in other randomized trials.15–18
Recently, 4 mg of ZA every 6 months for three years in premenopausal women receiving adjuvant endocrine therapy prevented bone loss and reduced the risks of systemic recurrences.35,36 If the results of larger trials in which women received adjuvant chemotherapy with or without endocrine therapy confirm these findings then it is likely that ZA will become incorporated into adjuvant therapy regimens because it reduces the risk of recurrence and lessens the amount of bone loss.
Acknowledgments
The authors would like to acknowledge the contributions of the CALGB Central Office, the clinical research associates and physicians of the participating institutions, and especially to women who participated in CALGB 79809 and other clinical trials.
Footnotes
Presented in part at the American Society of Clinical Oncology Meeting May 2008
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Molina JR, Barton DL, Loprinzi CL. Chemotherapy-induced ovarian failure: manifestations and management. Drug Saf. 2005;28:401–16. doi: 10.2165/00002018-200528050-00004. [DOI] [PubMed] [Google Scholar]
- 2.Knobf MT. The Influence of Endocrine Effects of Adjuvant Therapy on Quality of Life Outcomes in Younger Breast Cancer Survivors. Oncologist. 2006;11:96–110. doi: 10.1634/theoncologist.11-2-96. [DOI] [PubMed] [Google Scholar]
- 3.Oktem O, Oktay K. Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer. 2007;110:2222–9. doi: 10.1002/cncr.23071. [DOI] [PubMed] [Google Scholar]
- 4.Schover LR. Premature Ovarian Failure and Its Consequences: Vasomotor Symptoms, Sexuality, and Fertility. J Clin Oncol. 2008;26:753–758. doi: 10.1200/JCO.2007.14.1655. [DOI] [PubMed] [Google Scholar]
- 5.Meskhi A, Seif MW. Premature ovarian failure. Curr Opin Obstet Gynecol. 2006;18:418–26. doi: 10.1097/01.gco.0000233937.36554.d3. [DOI] [PubMed] [Google Scholar]
- 6.Cohen LE. Cancer treatment and the ovary: the effects of chemotherapy and radiation. Ann N Y Acad Sci. 2008;1135:123–5. doi: 10.1196/annals.1429.023. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro CL, Manola J, Leboff M. Ovarian failure after adjuvant chemotherapy is associated with rapid bone loss in women with early-stage breast cancer. J Clin Oncol. 2001;19:3306–11. doi: 10.1200/JCO.2001.19.14.3306. [DOI] [PubMed] [Google Scholar]
- 8.Eastell R. Management of osteoporosis due to ovarian failure. Med Pediatr Oncol. 2003;41:222–7. doi: 10.1002/mpo.10341. [DOI] [PubMed] [Google Scholar]
- 9.Fogelman I, Blake GM, Blamey R, et al. Bone mineral density in premenopausal women treated for node-positive early breast cancer with 2 years of goserelin or 6 months of cyclophosphamide, methotrexate and 5-fluorouracil (CMF) Osteoporos Int. 2003;14:1001–6. doi: 10.1007/s00198-003-1508-y. [DOI] [PubMed] [Google Scholar]
- 10.Hirbe A, Morgan EA, Uluckan O, et al. Skeletal Complications of Breast Cancer Therapies. Clin Cancer Res. 2006;12:6309s–6314. doi: 10.1158/1078-0432.CCR-06-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reid DM, Doughty J, Eastell R, et al. Guidance for the management of breast cancer treatment-induced bone loss: a consensus position statement from a UK Expert Group. Cancer Treat Rev. 2008;34 (Suppl 1):S3–18. doi: 10.1016/j.ctrv.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Eastell R, Adams JE, Coleman RE, et al. Effect of Anastrozole on Bone Mineral Density: 5-Year Results From the Anastrozole, Tamoxifen, Alone or in Combination Trial 18233230. J Clin Oncol. 2008;26:1051–1057. doi: 10.1200/JCO.2007.11.0726. [DOI] [PubMed] [Google Scholar]
- 13.Francucci CM, Romagni P, Camilletti A, et al. Effect of natural early menopause on bone mineral density. Maturitas. 2008;59:323–8. doi: 10.1016/j.maturitas.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher JC. Effect of early menopause on bone mineral density and fractures. Menopause. 2007;14:567–71. doi: 10.1097/gme.0b013e31804c793d. [DOI] [PubMed] [Google Scholar]
- 15.Hershman DL, McMahon DJ, Crew KD, et al. Zoledronic Acid Prevents Bone Loss in Premenopausal Women Undergoing Adjuvant chemotherapy for Early-Stage Breast Cancer. J Clin Oncol. 2008;26:4739–45. doi: 10.1200/JCO.2008.16.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hines SL, Mincey BA, Sloan JA, et al. Phase III Randomized, Placebo-Controlled, Double-Blind Trial of Risedronate for the Prevention of Bone Loss in Premenopausal Women Undergoing Chemotherapy for Primary Breast Cancer. J Clin Oncol. 2009;27:1947–53. doi: 10.1200/JCO.2008.19.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuleihan Gel H, Salamoun M, Mourad YA, et al. Pamidronate in the prevention of chemotherapy-induced bone loss in premenopausal women with breast cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2005;90:3209–14. doi: 10.1210/jc.2004-1444. [DOI] [PubMed] [Google Scholar]
- 18.Powles T, McCloskey E, Paterson A, et al. Oral clodronate and reduction in loss of bone mineral density in women with operable primary breast cancer. J Natl Cancer Inst. 1998;90:704–8. doi: 10.1093/jnci/90.9.704. [DOI] [PubMed] [Google Scholar]
- 19.Saarto T, Blomqvist C, Valimaki M, et al. Chemical castration induced by adjuvant cyclophosphamide, methotrexate, and fluorouracil chemotherapy causes rapid bone loss that is reduced by clodronate: a randomized study in premenopausal breast cancer patients. J Clin Oncol. 1997;15:1341–1347. doi: 10.1200/JCO.1997.15.4.1341. [DOI] [PubMed] [Google Scholar]
- 20.Pocock SJ. Allocation of patients to treatment in clinical trials. Biometrics. 1979;35:183–87. [PubMed] [Google Scholar]
- 21.Zelen M. The randomization and stratification of patients to clinical trials. J Chron Dis. 1974;27:365–75. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 22.Freidlin B, Korn EL, George SL. Data monitoring committees and interim monitoring guidelines. Control Clin Trials. 1999;20:395–407. doi: 10.1016/s0197-2456(99)00017-3. [DOI] [PubMed] [Google Scholar]
- 23.Hui SL, Slemenda CW, Johnston CC., Jr Age and bone mass as predictors of fracture in a prospective study. J Clin Invest. 1988;81:1804–9. doi: 10.1172/JCI113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanis JA, McCloskey EV, Powles T, et al. A high incidence of vertebral fracture in women with breast cancer. British Journal of Cancer. 1999;79:1179–81. doi: 10.1038/sj.bjc.6690188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Z, Maricic M, Aragaki AK, et al. Fracture risk increases after breast cancer survivors. Arch Int Med. 2005;165:552–8. doi: 10.1001/archinte.165.5.552. [DOI] [PubMed] [Google Scholar]
- 26.Vondracek SF, Hansen LB, McDermott MT. Osteoporosis risk in premenopausal women. Pharmacotherapy. 2009;29:305–17. doi: 10.1592/phco.29.3.305. [DOI] [PubMed] [Google Scholar]
- 27.Bischoff L, Derk CT. Premenopausal osteoporosis. Minerva Med. 2008;99:55–63. [PubMed] [Google Scholar]
- 28.Lewiecki EM, Kendler DL, Kiebzak GM, et al. Special report on the official positions of the International Society for Clinical Densitometry. Osteoporos Int. 2004;15:779–84. doi: 10.1007/s00198-004-1677-3. [DOI] [PubMed] [Google Scholar]
- 29.Pritchard KI. Risk of chemotherapy-induced menopause: more detailed data will lead to improved quality of life. Eur J Cancer. 2007;43:1644–5. doi: 10.1016/j.ejca.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 30.Swain SM, Land SR, Ritter MW, et al. Amenorrhea in premenopausal women on the doxorubicin-and-cyclophosphamide-followed-by-docetaxel arm of NSABP B-30 trial. Breast Cancer Res Treat. 2009;115:315–20. doi: 10.1007/s10549-008-9937-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith IE, Dowsett M, Yap Y-S, et al. Adjuvant aromatase inhibitors for early breast cancer after chemotherapy-induced amenorrhoea: Caution and suggested guidelines. J Clin Oncol. 2006;24:2444–2447. doi: 10.1200/JCO.2005.05.3694. [DOI] [PubMed] [Google Scholar]
- 32.Powles T, Hickish T, Kanis J, et al. Effect of tamoxifen on bone mineral density measured by dual-energy x-ray absorptiometry in healthy premenopausal and postmenopausal women. J Clin Oncol. 1996;14:78–84. doi: 10.1200/JCO.1996.14.1.78. [DOI] [PubMed] [Google Scholar]
- 33.Vehmanen L, Elomaa I, Blomqvist C, et al. Tamoxifen treatment after adjuvant chemotherapy has opposite effects on bone mineral density in premenopausal patients depending on menstrual status. J Clin Oncol. 2006;24:675–80. doi: 10.1200/JCO.2005.02.3515. [DOI] [PubMed] [Google Scholar]
- 34.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–22. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 35.Brown JE, Ellis SP, Lester JE, et al. Prolonged efficacy of a single dose of the bisphosphonate zoledronic acid. Clin Cancer Res. 2007;13:5406–10. doi: 10.1158/1078-0432.CCR-07-0247. [DOI] [PubMed] [Google Scholar]
- 36.Shapiro CL, Phillips G, Van Poznak CH, et al. Baseline bone mineral density of the total spine may predict for chemotherapy-induced ovarian failure. Breast Cancer Res Treat. 2005;90:41–6. doi: 10.1007/s10549-004-2625-9. [DOI] [PubMed] [Google Scholar]
- 37.Gnant M, Mlineritsch B, Luschin-Ebengreuth G, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudy. Lancet Oncology. 2008;9:890–9. doi: 10.1016/S1470-2045(08)70204-3. [DOI] [PubMed] [Google Scholar]
- 38.Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–91. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]