Abstract
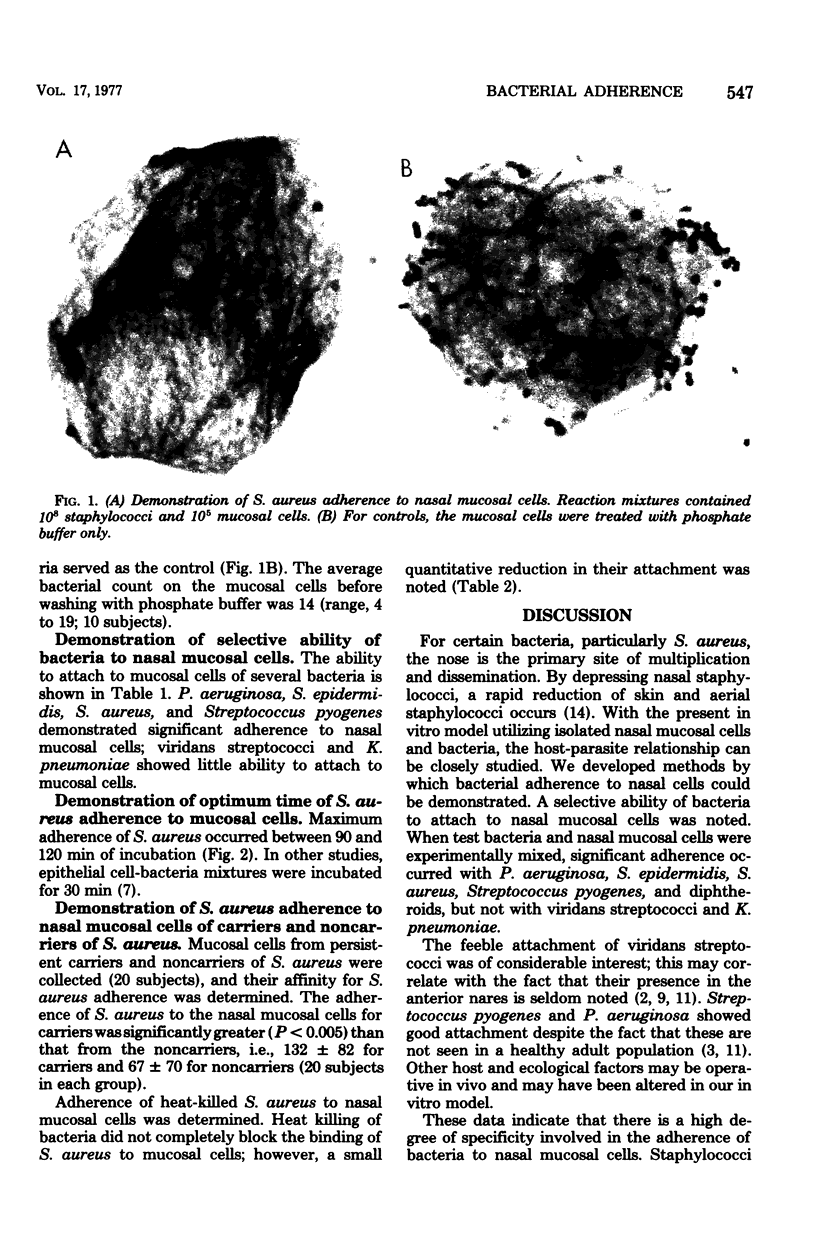
The ability of several bacterial species to adhere to human nasal mucosal cells and their distribution on nasal mucosal surfaces was studied. Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes, and Pseudomonas aeruginosa adhered to scraped nasal mucosal cells. In contrast, viridans streptococci and Klebsiella pneumoniae exhibited feeble or no adherence to nasal mucosal cells. S. aureus affinity for the nasal mucosal cells of carriers of S. aureus was greater than for those of the noncarriers (P less than 0.005). Heat treatment of S. aureus did not block, but slightly reduced, its binding to mucosal cells. The data suggest a high degree of specificity involved in the adherence of bacteria to nasal mucosal cells. The greater affinity of S. aureus for the nasal mucosal cells of carriers (than noncarriers) seems to be a property of mucosal cells rather than bacteria.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aly R., Maibach H. I., Shinefield H. R., Mandel A. D. Staphylococcus aureus carriage in twins. Am J Dis Child. 1974 Apr;127(4):486–488. doi: 10.1001/archpedi.1974.02110230032004. [DOI] [PubMed] [Google Scholar]
- Aly R., Maibach H. I., Shinefield H. R., Strauss W. G. Survival of pathogenic microorganisms on human skin. J Invest Dermatol. 1972 Apr;58(4):205–210. doi: 10.1111/1523-1747.ep12539912. [DOI] [PubMed] [Google Scholar]
- Aly R., Maibach H. I., Strauss W. G., Shinefield H. R. Effects of a systemic antibiotic on nasal bacterial ecology in man. Appl Microbiol. 1970 Aug;20(2):240–244. doi: 10.1128/am.20.2.240-244.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertschinger H. U., Moon H. W., Whipp S. C. Association of Escherichia coli with the small intestinal epithelium. I. Comparison of enteropathogenic and nonenteropathogenic porcine strains in pigs. Infect Immun. 1972 Apr;5(4):595–605. doi: 10.1128/iai.5.4.595-605.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Gibbons R. J. M protein-associated adherence of Streptococcus pyogenes to epithelial surfaces: prerequisite for virulence. Infect Immun. 1972 May;5(5):826–830. doi: 10.1128/iai.5.5.826-830.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect Immun. 1971 Apr;3(4):567–573. doi: 10.1128/iai.3.4.567-573.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973 Mar 1;137(3):571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]



