Abstract
The mouse MHC class Ib gene H2-T11 is 95% identical at the DNA level to H2-T23, which encodes Qa-1, one of the most studied MHC class Ib molecules. H2-T11 mRNA was observed to be expressed widely in tissues of C57BL/6 mice, with highest levels in thymus. To circumvent the availability of a specific mAb, cells were transduced with cDNA encoding T11 with a substituted α3 domain. Hybrid T11D3 protein was expressed at high levels similar to control T23D3 molecules on the surface of both TAP+ and TAP− cells. Soluble T11D3 was generated by folding in vitro with Qdm, the dominant peptide presented by Qa-1. The circular dichroism spectrum of this protein was similar to that of other MHC class I molecules, and it was observed to bind labeled Qdm peptide with rapid kinetics. By contrast to the Qa-1 control, T11 tetramers did not react with cells expressing CD94/NKG2A, supporting the conclusion that T11 cannot replace Qa-1 as a ligand for NK cell inhibitory receptors. T11 also failed to substitute for Qa-1 in the presentation of insulin to a Qa-1-restricted T cell hybridoma. Despite divergent function, T11 was observed to share peptide-loading specificity with Qa-1. Direct analysis by tandem mass spectrometry of peptides eluted from T11D3 and T23D3 isolated from Hela cells demonstrated a diversity of peptides with a clear motif that was shared between the two molecules. Thus T11 is a paralog of T23 encoding an MHC class Ib molecule that shares peptide-binding specificity with Qa-1 but differs in function.
Keywords: MHC class Ib, T23/Qa-1b, T11, Qdm, peptide elution
Introduction
Major histocompatibility complex (MHC) class Ia molecules, including HLA-A, B, C in human and H2-K, D, L in mice, are expressed on surface of most nucleated cell, and present mainly endogenously derived antigenic peptides to CD8+ T cells, initiating signals required for the positive selection in the thymus and activation in the periphery. The assembly and cell surface expression of class I MHC heavy chain with peptide and β2m is dependent on TAP and other components of the endoplasmic reticulum (ER)-localized peptide-loading complex (1).
Another group of MHC class I molecules, known as non-classical or MHC class Ib, are encoded by genes mostly located at the telomeric end of the MHC gene region (2). Class Ib molecules have a similar structure, at both genomic and protein levels, to class Ia molecules, but Class Ib molecules generally have more limited tissue distribution, lower expression levels, and fewer alleles in comparison to MHC class Ia molecules (2-4). The mouse MHC class Ib genomic region is further divided to three subregions, H2-Q, T, and M (2, 5). There are ~40 MHC class Ib genes present in the C57BL/6 (B6) mouse genome, and only about half of them were reported to be transcribed (3). A number of MHC class Ib molecules have been studied and some have been shown to have specialized function, but the majority remain to be characterized. For example, H2-M3 preferentially binds N-formylated peptides that originate from bacterial or mitochondrial proteins, and it is not detectable on the cell surface until N-formylated peptide is bound (6, 7). H2-M3 has been reported to actively participate in anti-Listeria monocytogenes immune responses (8-12). By contrast, TL (encoded by T18d) assembles without bound peptide (13) and it serves as a ligand for CD8αα, regulating the function of a subset of CD8αα+ intestinal intraepithelial T cells (14, 15).
H2-T23 encodes one of the most well studied MHC class Ib proteins, Qa-1 (16). The T23 gene is ubiquitously transcribed (3), but the surface expression level of Qa-1 is lower than that of the MHC class Ia molecules. There are a number of identified alleles, but most laboratory mouse strains express Qa-1b or Qa-1a and other alleles are closely related to these prototypes (17-19). Unfortunately, the genes encoding Qa-1 are not mapped in strains other than C57BL/6 and BALB/c, therefore we do not know if they are allelic. Some of these Qa-1 molecules might be encoded by paralogous genes derived from a strain-specific gene duplication of the T23-like ancestral gene. Qa-1 appears to have a highly selective peptide-binding specificity, predominantly loading with Qdm (AMAPRTLLL), a peptide derived from the conserved leader sequence of H-2D and H-2L class Ia molecules (20, 21). Despite its origin in leader sequences, loading of Qdm is dependent on TAP, as well as tapasin and presumably other component of the class I peptide-loading complex (4, 22). The fragment of the leader sequence that contains Qdm is released into the cytoplasm after cleavage by signal peptidase and signal peptide peptidase, thus requiring TAP for transport into the ER lumen. Qa-1-Qdm complexes function as the sole ligand for CD94/NKG2 inhibitory and activating receptors on NK cells and recognition by CD94/NKG2 is highly specific for the sequence of bound Qdm peptide (23, 24). The expression of Qa-1-Qdm serves as a quality control system, such that cells lacking components of the peptide loading machinery required for generation of Qa-1-Qdm are killed by CD94/NKG2A+ NK cells (25).
Although Qdm is the dominant peptide presented by Qa-1 molecules, it is evident that Qa-1 has a capacity to present other peptides to CD8+ T cells. Qa-1-specific T cells have been reported to participate in immune responses to Listeria monocytogenes (9, 26) and Salmonella tryphimurium (27, 28), and Qa-1-restricted T cells with specificity for proinsulin (29) and insulin (30, 31) have been characterized. A number of studies have reported a role for Qa-1-restricted CD8+ T cells in regulating immune responses and self-tolerance (32-35), and in immune surveillance of TAP-deficient tumors (36, 37). Recently, Nagarajan et al. have demonstrated a role for Qa-1b-restricted T cells in monitoring the function of ERAAP, an aminopeptidase that mediates trimming of peptides presented by MHC class I molecules in the ER. Cytotoxic effector cells were shown to recognize a self-peptide (FL9) that is selectively presented by Qa-1 in ERAAP-deficient cells (38).
The MHC is shaped by successive rounds of segmental duplications. The mouse H2-T region, where Qa-1 is encoded, contains about 20 class I genes. This number varies greatly among haplotypes due to strain-specific deletions/duplications. The H2-T region of C57BL/6 and BALB/c contains two and A/J mice contain three highly similar segments (39-42). These duplicated segments were further modified by monogenic duplications, deletions, and single nucleotide changes, leading to strain-specific class I gene/pseudogene content. This process led to variable numbers of T23/T11, T22/T10, T25, and T18/T3-like paralogous genes, pseudogenes and gene fragments (42). For example, the TL antigen (43), which is expressed on intestinal epithelium and thymocytes, can be encoded by one (H2-T3), two (H2-T3 and -T18) or three genes (in A/J), depending on the strain. Qa-1b is encoded in BALB/c by the H2-T23d gene (originally Gene D37), whereas H2-T11d (originally named T10c) from BALB/c is a pseudogene due to a one base deletion leading to a frame-shift and early stop in exon 2 (44). Here we show that in C57BL/6 both H2-T23b and H2-T11b are functional genes.
Materials and methods
Mice
C57BL/6 mice (B6) were purchased from the Jackson Laboratory. Qa-1b−/− mice were gift from Dr. Harvey Cantor (Harvard Medical School) (35). All the mice were maintained in the University of Utah specific pathogen-free animal facility and used according to the protocol approved by the Institutional Animal Care and Use Committee of University of Utah.
Molecular cloning of the B6 genomic locus of H2-T11
Based on the genomic DNA sequence of the H2-T11 locus from 129 mice (Kumanovics, unpublished data), specific oligonucleotide primers were designed and synthesized from IDT. The whole region of the H2-T11 locus region from B6 mice was cloned, using TA cloning kits (Invitrogen), and subsequently sequenced. All DNA products were examined using 1 or 2% agarose gel.
Gene expression analysis
The primers used for amplifying H2-T11 and H2-T23 from the B6 mouse genome and total RNA were as follows: T11 forward, CGGTATTTCCACACCGTCGTA; T11 reverse, TAGAGATATGCGAGGCTAAGTTG; T23 forward, AGTATTGGGAGCGGGAGACTT; T23 reverse: AGCACCTCAGGGTGACTTCAT. PCR was performed using TaKaRa rTaq polymerase from Clontech. Murine RNA polymerase 2A (POLR2A) was used as the reference gene for quantitative-PCR (qPCR) analysis of T11. The primers for qPCR were: T11 forward, TAAACCTGAGGACCCTGCTC; T11 reverse, TAGGCCTCCTGACAATACCC; POLR2A forward, GACAAAACTGGCTCCTCTGC; POLR2A reverse, GCTTGCCCTCTACATTCTGC. The mouse tissues were collected and stored in RNAlater solution (Ambion) for less than a week at 4°C before RNA was extracted. The total RNA was extracted using RNeasy mini kit (Qiagen). The cDNA was synthesized using QuantiTect Reverse Transcription kit (Qiagen). The quantitative-PCR kit Absolute QPCR SYBR Green Mix (Thermal Scientific) was used in the qPCR analysis, which was performed on a Lightcycler 480 system (Roche).
Generation of hybrid H2-T11 and H2-T23 molecules
The α3 domain of H2-T11 or H2-T23 cDNA was replaced with the α3 domain of the H2-Db cDNA, and the hybrid molecules were named as H2-T11D3 or H2-T23D3, respectively. The H2-T11D3 and H2-T23D3 cDNAs were synthesized at Biomatik. The synthesized cDNAs were verified by sequencing before being cloned into expression vectors. The cDNAs were cloned into a retroviral vector MigR1 for expression in mammalian cells (45). The soluble forms (lack of the transmembrane and cytoplasmic domains) of H2-T11D3 or H2-T23D3 were generated by PCR and cloned into a bacteria expression vector pTCF (the NIH tetramer core facility), and the cloned products were verified by restriction enzyme digestion and sequencing. All restriction enzymes were from NEB laboratories. The plasmids were purified using Plasmid Mini Kit (Qiagen) for digestion and cloning, and by EndoFree Plasmid Maxi Kit (Qiagen) for transfection.
Abs, flow cytometry and cell sorting
Purified anti-Qa-1b (6A8, mouse IgG1, κ) and anti-Db α3 domain (28-14-8s, mouse IgG2a, κ), anti-human β2m (BB7.7) antibodies were purified from hybridoma supernatants using protein A affinity chromatography. FITC labeled anti-mouse CD3ε (145-2C11), PerCP-Cy5.5 labeled anti-mouse B220 (RA3-6B2), PE labeled anti-mouse NKp46 (29A1.4), PE-Cy7 labeled anti-mouse NK1.1 (PK136) antibodies were purchased from eBioscience or Biolegend. The Ab was diluted in a buffer composed of PBS, 0.5% BSA, and 2 mM EDTA. The suspended cells were incubated with the antibodies and/or the tetramer for 20 min at 4°C. The stained cells were washed twice with the above buffer and fixed with 1% paraformaldehyde. The fluorescence was detected on a FACS Canto II (BD). The data was analyzed by Flowjo (Tree Star). APC labeled TCRβ (H57-597), PE or APC labeled anti-mouse B220 (RA3-6B2), FITC or PerCP-Cy5.5 labeled anti-mouse CD4 (GK1.5), FITC or PE labeled anti-mouse CD8a (53-6.7), FITC labeled anti-mouse CD11c (HL3) and PE labeled anti-mouse NK1.1-PE (PK136) were purchased either from Biolegend, eBioscience or BD, and used for sorting of CD4−CD8− DN, CD4+CD8+ DP, CD4+ SP, CD8+ SP, B cells and DC subpopulations from thymus as well as T cells, CD4+ T cells, CD8+ T cells, B cells, DC, NK and NKT subpopulations from spleen. The cell sorting was performed using a BD FACSAria III.
Cell culture
Phoenix-GP, Hela and T2 cells were cultured in DMEM complete media supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 292 μg/ml L-glutamine, 100 μM nonessential amino acids, 1 mM sodium pyruvate, and 55 μM 2-mercaptoethanol (all from Invitrogen). Splenocytes were cultured in RPMI-1640 complete media supplemented the same way as the DMEM complete media. To stimulate splenocytes, cells were cultured with plate-bound anti-CD3 (145-2C11) and anti-CD28 (37.57.1) Abs in RPMI-1640 complete media. All cells were maintained at 37°C humidified cell culture incubator containing 5% CO2.
Retroviral transduction
Phoenix-GP cells were added at 4×106 cells/dish to 6 cm collagen I coated cell culture dishes (BD). Eighteen hours later, 10 μg MigR1 empty vector or MigR1 containing the T11D3 or T23D3 cDNA was co-transfected with 5 μg Env plasmid into the Phoenix cells by calcium-phosphate precipitation as previously reported (46). Two days later, the supernatant containing the packaged retrovirus was harvested and filtered through 0.45 μm sterile filters (BD) before transduction. Half ml of 1×106/ml Hela or T2 cells were mixed with 0.5ml of the retroviral supernatant in the presence of 5 μg/ml polybrene (Sigma), and the cells were distributed evenly on collagen-I coated 6 cm dishes. Four hours later, 4 ml fresh DMEM complete media was added. After at least 3 days, the cells were harvested and the EGFP expression was examined by flow cytometry. The transduced cells were sorted at least twice according to the EGFP expression level for cells stably expressing high levels of target genes.
Expression of soluble recombinant proteins
The pTCF vector containing the T11D3 or T23D3 genes was transformed into BL21 (DE3) E.coli (Invitrogen). T11D3 or T23D3 positive clones were verified for producing recombinant proteins before they were expanded in 2 L Luria Broth media. IPTG (1 mM) was added to the culture when the OD600 reached ~0.6. The bacteria were harvested 4 hours later. The bacteria were pelleted by centrifugation, resuspended in resuspension buffer (50 mM pH 8.0 Tris-HCl, 25% (W/V) sucrose, 1 mM EDTA, 0.1% (W/V) NaAzide, 10 mM DTT) and stored at −80°C. The frozen bacteria were thawed and 1mg/ml lysozyme, 5 mM MgCl2, 33 μg/ml DNase I, 3.3% (V/V) Triton X-100, and 10 mM DTT were added. The bacteria were stirred and lysed at room temperature for 1 hour before sonication. The inclusion body was washed multiple times with wash buffer (50 mM pH8.0 Tris-HCl, 0.5% Triton X-100, 100 mM NaCl, 1 mM EDTA, 0.1% NaAzide, 1mM DTT) until the protein pellet was white and the supernatant was clear. The pellet was washed one more time with the same wash buffer without DTT. The recombinant inclusion body was solubilized in 6M guanidine chloride, analyzed using 12% SDS-polyacrylamide gel electrophoresis, and aliquoted and stored at −80°C until use.
In vitro MHC class I folding
Soluble recombinant T11D3 and T23D3 proteins were folded in vitro as described before (25). Briefly, 18 mg inclusion body of T11D3 or T23D3 heavy chain together with 6 mg human β2m light chain was diluted in 100 mM Tris folding buffer (pH 8.0) containing 400 mM L-arginine, 2 mM EDTA, 0.5 mM oxidized glutathione, 5 mM reduced glutathione, 0.2 mM PMSF in the absence or presence of Qdm peptides. The gram ratio of heavy chain:light chain:peptide was 3:1:1. The folding reaction was performed at 10°C for 2 days before it was harvested and concentrated by Amicon ultrafiltration cells (Millipore). The concentrated sample was filtered through a 0.45 μm filter and purified by a S300 gel filtration column. The purified folded products were concentrated by Amicon Ultra centrifugal filters (Millipore), buffer exchanged with PBS and stored at −80°C. The MHC folding products were analyzed using 4-20% gradient Tris-HCl polyacrylamide gel electrophoresis (Bio-Rad)
Antigen presentation assay
Transduced Hela cells (5×104) or B6 splenocytes (1×106) were used as antigen presenting cells, and co-cultured with 1×105 6C5 T cell hybridoma cells (31) overnight in the presence of different doses of bovine insulin (Sigma) in 96-well plates. The supernatant was harvested, and the production of IL-2 was measured by Europium-based immunoassay (47).
Eu-based peptide binding assay
The peptide binding capacity of folded recombinant T11D3 or T23D3 was examined using an Europium-based immunoassay (47). Briefly, 96-well ELISA plate was coated with 50 μl of 5 μg/ml anti-human β2m mAb (clone BB7.7) at 37°C for 2 h; the plate was blocked by 200 μl MTB (5% powdered skim milk, 1% BSA, 0.01% NaAzide in TTBS buffer, 50 mM Tris, 150 mM NaCl, 0.1% Tween-20, pH7.5) for 30 min at room temperature; one microgram of folded MHC monomers were diluted in 100 μl MTBN and incubated in the antibody coated plate for >2 hours at 4°C; the biotin-labeled peptides were diluted in PBS with 0.01% NP-40 and incubated the plate overnight at room temperature. The plate was washed extensively using TTBS before the addition of the next reagent. Finally, the plate was developed using Europium reagent and the fluorescence signal was recorded by a Victor3V plate reader (Perkin Elmer).
Fluorescence polarization assay
The folded MHC monomers were incubated with Alexa Fluro488-labeled Qdm-4C (ANACRTLLL) peptides in citrate/phosphate buffer (200mM citric acid, 200mM Na2HPO4, pH7). The parallel and perpendicular fluorescence signals (III and I⊥) were recorded at 60 second intervals for total 60000 seconds by an Infinite F200 microplate reader (Tecan) at 37°C or 25°C. Pure MHC monomer, AF488-labeled peptide, or buffer alone were also detected independently to record the background signals. After the background signals were subtracted, the anisotropy was calculated according to following formula: (48).
Circular dichroism (CD) assay
The CD spectrum was measured by an AVIV 410 CD instrument (AVIV Biomedical Inc.). The folded MHC monomer concentration was determined by OD280 and was diluted to 250 μg/ml in PBS. The far-UV CD spectrum was recorded in a cuvette with 1mm path-length at 25°C. The sample was scanned from 200 nm to 260 nm with a step of 1nm. The averaging time was 3 seconds and every step was scanned 3 times. The PBS background data was recorded in the same cuvette and was subtracted from the sample data.
The thermal stability of the folded MHC was measured by detecting the CD signal at 222nm. The temperature was increased by a step of 2°C from 25°C to 79°C. At each temperature point, the sample was equilibrated for 30 seconds before collecting the data. The averaging time was 30 seconds.
MHC class I tetramer preparation
T11D3 and T23D3 MHC tetramers were prepared as described before (49). The S300 purified MHC monomer was buffer exchanged to 10 mM Tris buffer (pH8.0) and concentrated to 2 mg/ml. One mg of the MHC monomer (8/10 volumes) was mixed with 1/10 volume of 10× BiomixA, 1/10 volume of 10× BiomixB and 5 μg BirA enzyme (GeneCopoeia). The reaction was kept at 25°C overnight. The product was further purified by a MonoQ anion exchange column. The biotinylated MHC was snap-frozen and stored at −80°C. When tetramer was generated, the biotinylated MHC monomer was thawed and tetramerized by gradually adding APC-labeled streptavidin to it. The final molar ratio of biotinylated MHC to streptavidin was 4:1. One tenth of the total required APC-labeled streptavidin was added to the sample each time and the sample was incubated in dark at room temperature for 10 min after each addition, so that each streptavidin was saturated. The tetramers were stored at 4°C, and used within 6 months.
Peptide elution and identification
The T11D3 and T23D3 bound peptides were eluted and identified using LC-tandem mass spectrometry as described (50). Briefly, Hela-MigR1, Hela-T11D3 and Hela-T23D3 cells were cultured in 20~25 T150 cell culture flasks, and the cells were harvested at about 80% to 90% confluent. The total cell numbers collected for each cell type were more than 1×109. The cells were pelleted, washed twice with cold DPBS, and was stored at −80°C until lysing within 2 months. The cells were lysed in NP-40 buffer (0.5% NP-40, 500 mM Tris-HCl, pH8.0, 150 mM NaCl and protease inhibitors). The lysate was centrifuged and cell debris was removed. The supernatant was passed through a Tris-blocked Sepharose column to pre-clear the lysate. The hybrid MHC was immunoprecipitated by 28-14-8s cross-linked on Protein A beads. The beads then were washed with four different buffers sequentially: (1) 0.005% NP-40, 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 5 mM EDTA; (2) 50 mM Tris-HCl (pH 8.0), 150 mM NaCl; (3) 50 mM Tris-HCl (pH 8.0), 450 mM NaCl; (4) 50 mM Tris-HCl (pH 8.0). The MHC-peptide complexes were eluted from the beads by 10% acetic acid, lyophilized, resuspended in 100μl DMSO, loaded and fractionated by HPLC (Beckman Coulter) into 27 fractions. Each fraction was concentrated to ~10μl before mass spectrometry. The sequences of the eluted peptides were gained by LC-tandem mass spectrometry.
Mass spectrometry analysis of peptide eluted from T11D3 or T23D3 was performed as our previously reported. Briefly, 1-2 μl of each HPLC fraction was analyzed by tandem mass spectrometry (Agilent 6510 quadrupole time-of-flight (Q-TOF) instrument with Chip Cube electrospray ionization; Agilent Technologies). The samples were injected using nanospray protein chip no.1 (40-nl trap, 75 × 43 mm, C-18SB-ZX chip, 5-mm particles) at a flow rate of 400 nl/min. Data acquisition was done using MassHunter (version B.01.03) in a 2-GHz extended dynamic range at a rate of three scans per second followed by data-dependent tandem mass spectrometric fragment scans of the three most intense ions. Precursor ion exclusion was set for 12 s after two consecutive tandem mass spectrometric scans. Before each experiment, the Q-TOF analyzer was tuned to a resolution of >12,000, and mass accuracy was calibrated to <2 ppm. Acquired tandem mass spectrometric spectra were searched with no enzyme specificity using Spectrum Mill (Agilent Technologies) against the UniProt human FASTA protein database (August 2007 download). Raw peptide data files generated were converted into Excel format (Microsoft) and sorted according to their corresponding mass-to-charge ratio (m/z) values, charge state, retention time, and intensity (50, 51). A user-defined intensity threshold (7.0) above the background noise was fixed to limit false-positive identification. All identified peptides sequences above this score were manually verified (51). In addition, peptides found in the fractions from control Hela-MigR1 lysates were considered contaminants and subtracted from the final list of peptides.
Results
Transcription of T11 gene in B6 and T23/Qa-1b knockout mice
H2-T11 and H2-T23 genes appear to have been duplicated from a common ancestor gene in evolution (42). Since the genomic sequence of the T11 from B6 mice is not available from the NCBI database, we cloned and sequenced the T11 genomic region from B6 mice, using the H2-T11 locus genomic DNA sequence from 129 mice as the template (Kumanovics, unpublished data). The H2-T11 and H2-T23 cDNA sequences showed a high degree of homology with a 95% identity (data not shown). At the amino acid level, the T11 putative protein is 91% identical to H2-T23 (Qa-1b) (Fig. 1).
Fig. 1.

Alignment of amino acid sequences of Qa-1b, Qa-1a and T11 ectodomains. The amino acid sequences of Qa-1b, Qa-1a and T11 ectodomains were aligned, using CLUSTALW, SDSC Biology WorkBench 3.2 (http://workbench.sdsc.edu). Alpha 1, 2 and 3 domains are boxed. Consensus key: * fully conserved;: strongly conserved; weakly conserved.
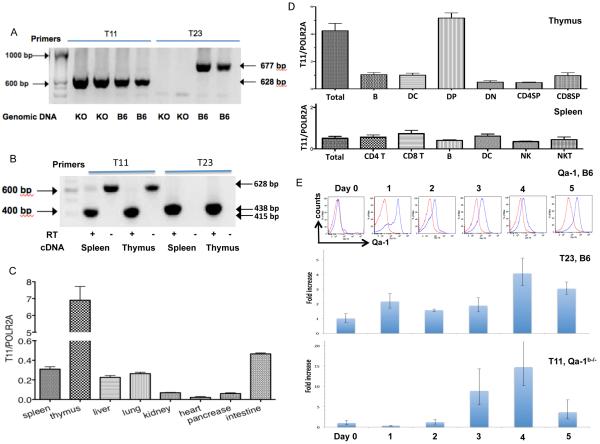
PCR analysis was performed using oligonucleotide primers specific for T11 or T23 gene, respectively, as reported previously (42). Genomic DNA analysis showed both T11 (628bp band) and T23 (677bp band) genes were detected in wild type B6 mice, while only T11 but not T23 was detected in T23/Qa-1b knockout (Qa-1b−/−) mice, since the amplified region (exons 1 to 3 of T23) is deleted in Qa-1b−/− mice (35) (Fig. 2A). Reverse-transcriptional PCR (RT-PCR) analysis showed that the T11 gene was transcribed, as represented as a 415 bp band in spleen and thymus. The T23 transcript (a 438 bp band) was also detected in spleen and thymus as expected (Fig. 2B). All of the amplified PCR products were confirmed by sequencing, and the different sizes of the PCR products from genomic DNA and cDNA reflected correctly spliced products. Therefore, T11 is not a pseudogene in B6 mice, and has the potential to encode a functional protein.
Fig. 2.
Expression of T11 gene. (A) PCR amplification of T11 and T23 genes from the wild type C57BL/6 (B6) and Qa-1b−/− (KO) mouse genomic DNA. The T11 amplicon size was 628bp and the T23 amplicon size was 677bp. (B) Reverse-transcriptional PCR amplification of T11 and T23 transcripts from B6 mouse spleen and thymus. The amplicon sizes are 415 bp and 438 bp for T11 and T23 respectively. RT: reverse transcriptase. (C) Quantitative-PCR to determine the T11 transcription level in different tissues. The total RNA was extracted from Qa-1b−/− mice. The reference gene was RNA polymerase 2A (POLR2A). The ratio of T11 to POLR2A was calculated according to the Pfaffl method. The qPCR was done in triplicates (n=3) and the experiment was repeated twice with similar results. One of them was shown. (D) Quantitative-PCR to determine the T11 transcription level in different cell subpopulations of thymus and spleen. The total RNA was extracted from thymus and spleen of Qa-1b−/− mice. The qPCR was done as above, at least in triplicates. (E) Qa-1/T23 and T11 expression after stimulation. Splenocytes of B6 or Qa-1b−/− mice were stimulated with plate-bound anti-CD3/28 Ab. Surface expression of Qa-1 on B6 splenocytes was determined by FACS. The total RNA was extracted from unstimulated or stimulated B6 or Qa-1b−/− splenocytes. The qPCR for T23 used RNA from B6 or for T11 used RNA from Qa-1b−/− splenocytes, respectively. The qPCR was done as same as above, in triplicates (n= 3) and the experiment was repeated twice with similar results. One of surface staining of Qa-1was shown.
Next qPCR analysis was performed to compare the mRNA expression levels of T11 in multiple tissues. Because of a high similarity of T23 and T11, we were not able to design T11 specific primers to selectively amplify the short amplicon needed in qPCR. A pairs of primers flanking T11 exon 2 and 3 were used in qPCR to produce a 127 bp amplicon, but they also cross-amplified a product from the T23 gene. To circumvent this problem, we used Qa-1b−/− mice to evaluate the T11 expression levels. Murine RNA polymerase 2A (POLR2A) was used as the reference gene (52). The qPCR result showed that T11 was expressed at relatively high level in spleen, thymus and intestine, and at lower level in kidney, heart and pancreas. Among 8 different tissues examined, the highest T11 expression level was detected in the thymus (Fig. 2C).
Furthermore, CD4−CD8− double negative (DN), CD4+CD8+ double positive (DP), CD4+ single positive (SP), CD8+ SP, B cells and DC subpopulations as well as T cells, CD4+ T cells, CD8+ T cells, B cells, DC, NK and NKT subpopulations were sorted from thymus or spleen of Qa-1b−/− mice, respectively. Expression levels of H2-T11 of those subpopulations were compared by qPCR and shown in Fig. 2D (thymus, upper panel, and spleen, lower panel). Expression levels are similar across all tested cell subpopulations with the exception of DP thymocytes, which express relatively high levels of T11 mRNA. Upon activation by TCR engagement with CD3 and CD28 Ab, surface Qa-1 expression on splenocytes of B6 mice was increased, as reported previously. Similarly, using qPCR, T23 transcripts were also increased after activation. T11 transcripts from Qa-1b−/− splenocytes were increased by activation as well, but with delayed kinetics, peaking on day 3 and day 4 (Fig. 2E).
Expression of H2-T11 encoded protein on the surface of TAP+/+ and TAP−/− cells
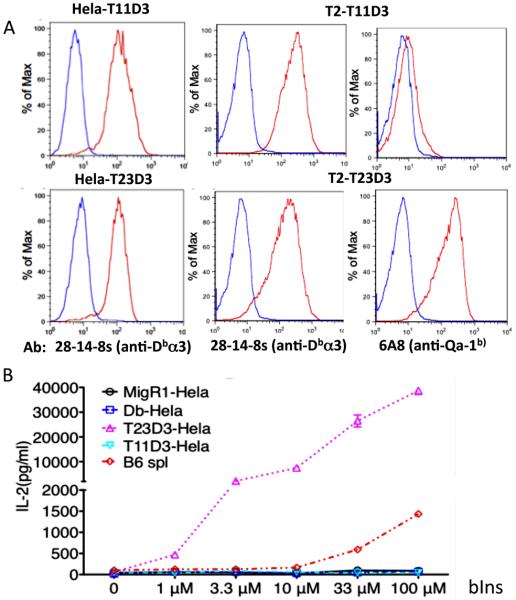
Our PCR and sequencing results indicated that T11 might encode a functional protein. We generated cDNA expression constructs encoding a chimeric T11 protein substituted with the α3 domain of H2-Db to circumvent the absence of a mAb that recognizes T11 (37). The H2-Db α3 domain is recognized by mAb 28-14-8s. Chimeric Qa-1 molecules were generated as a control and these hybrid molecules were designated H2-T11D3 and H2-T23D3, respectively. The hybrid T11D3 and T23D3 were transduced into Hela (TAP+) and T2 (TAP deficient) cells using the MigR1 retroviral transduction system, which has an EGFP reporter gene following an IRES (45).
High levels of T23D3 were detected on the surface of Hela cells with mAb 28-14-8s demonstrating that the chimeric Qa-1 heavy chain efficiently assembles with endogenous human β2m to form a stable complex (Fig. 3A, HeLa-T23D3 panel). As previously reported for wild type Qa-1 (23, 25, 53, 54), cell surface T23D3 can also be expressed at high levels in TAP-deficient cells (Fig. 3A, T2-T23D3 panels). Presentation of the dominant Qa-1 associated peptide Qdm is strictly TAP dependent (20, 53). However, Qa-1 has been reported to assemble with alternative non-Qdm peptides in TAP-deficient cells (37, 55). Cell surface T23D3 was also detected with mAb 6A8, which recognizes an epitope in the α2 domain of Qa-1b (Fig. 3A, T2-T23D3 panels).
Fig. 3.
Expression of hybrid T11D3 and T23D3 molecules and test of the function of hybrid molecule expressing cells as Ag presenting cells. (A) FACS analysis of hybrid T11D3 and T23D3 on the surface of Hela and T2 cells. Transduced Hela cells were stained with 28-14-8s (α-Db α3) and T2 cells were stained with 28-14-8s and 6A8 (α-Qa-1b α2), respectively, shown as marked (red lines). The staining of isotype control Ab is blue. (B) Antigen presentation assay to test capability of the hybrid MHC-Ib molecule expressing cells to present insulin to 6C5 T hybridoma cells specific to bovine insulin (bINS). The assay was set up in triplicates (n=3) and the experiment was repeated twice with similar results. One of them was shown.
The chimeric T11D3 protein was expressed at levels similar to T23D3 on the surface of both Hela and T2 cells, as determined by mAb 28-14-8s staining (Fig. 3A, top panels). Significantly reduced staining was observed with the anti-Qa-1b mAb 6A8, suggesting that the epitope recognized by this mAb is not fully preserved in T11. These results suggest that T11 can efficiently assemble and be expressed as a stable cell surface protein, with the caveat that the α3 domain has been substituted in our experiments. Like T23D3, high levels of T11D3 were expressed in TAP-deficient T2 cells. It is possible that T11, like TL (T18d), assembles without bound peptide antigen (13, 56). Alternatively, like Qa-1, T11 may assemble with peptides through a TAP-independent mechanism.
T11 cannot substitute for Qa-1 in T cell antigen presentation
Previously findings from our laboratory showed that a subset of CD8+ T cells with specificity for insulin are selected by Qa-1b in mice (30, 31). The Qa-1b-restricted CD8+ T cell hybridoma 6C5 recognizes an epitope in the B chain of insulin. Hela cells expressing the chimeric Qa-1 T23D3 molecules were able to efficiently present insulin to 6C5 T cells, with even greater function than B6 splenocytes (Fig. 3B). Thus, the substituted to α3 domain does not affect the antigen presentation function of Qa-1 from these T cells. By contrast, no T cell response was observed in experiments with Hela cells expressing similar levels of T11D3. We concluded that T11 cannot substitute for Qa-1 in antigen presentation to insulin-specific 6C5 T cells.
Folding of soluble T11D3 with or without Qdm peptide in vitro
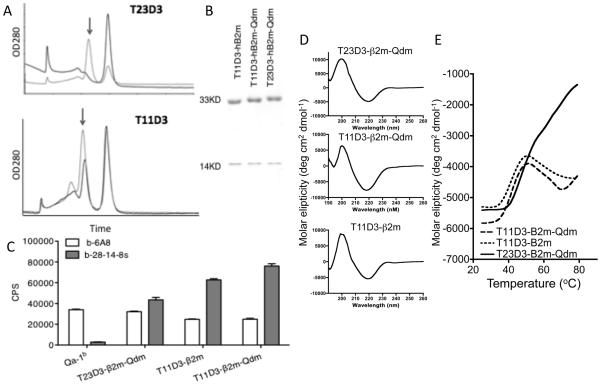
The class Ia leader sequence derived peptide Qdm has an optimal sequence for binding to Qa-1 (24, 57) and it is the dominant peptide bound to Qa-1 molecules in TAP-expressing cells (21). As previously demonstrated for wild type Qa-1b (23, 24), T23D3 heavy chain assembles efficiently under standard folding conditions in vitro in the presence of Qdm peptide and β2m based on size exclusion chromatography (Fig. 4A). No folded protein was detected in the absence of Qdm indicating that the in vitro folding reaction is strictly dependent on the presence of an appropriate peptide ligand. Given the high degree of sequence similarity with Qa-1, we set up folding reactions with T11D3 under identical conditions. A large peak corresponding to folded MHC class I molecules was observed with T11D3 in the presence of Qdm. In contrast to T23D3, a peak corresponding to folded T11D3 protein was also observed in the absence of Qdm peptide, although the yield was lower than that observed in the presence of Qdm. The appropriate peaks from size exclusion chromatography were concentrated and examined by SDS-PAGE. Both heavy and light chain of MHC class I were detected, indicating the assembly of T11D3 with β2m, even in the absence of Qdm (Fig. 4B). The products were also analyzed using a Europium-based fluorescence immunoassay (Fig. 4C). In this assay, the folding product was captured with an anti-β2m mAb (BB7.7), washed and detected by 28-14-8s (anti Dbα3) or 6A8 (anti Qa-1b) mAbs. The results showed that folded T11D3 and T23D3 products can be recognized by both 28-14-8s and 6A8 antibodies, further confirming assembly with β2m.
Fig. 4.
In vitro folding of the hybrid T11D3 and T23D3 molecules. (A) S300 spectrum of the folding products. Arrows indicate the correct folding product peak. Top panel: black line, T23D3-β2m folding, gray line, T23D3-β2m-Qdm folding; bottom panel: T11D3-β2m folding, black line, T23D3-β2m folding, gray line, T11D3-β2m-Qdm folding. (B) SDS-PAGE analysis of the purified folding products. The MHC heavy chain is ~33kD and the β2m light chain is −14kD. (C) Eu-based immunoassay to examine the folding products. Folding MHC monomer was captured by the anti-β2m mAb and detected by biotinylated 28-14-8s and 6A8 (b-28-14-8s and b-6A8). The assay was set up in triplicates (n=3) and the experiment was repeated twice with similar results. One of them was shown.
(D) In vitro folded MHC monomers were analyzed using far-UV circular dichroism. Each spectrum curve was the average of three independent scans, and a representative one was shown.
(E) Thermal denaturation curves were generated from CD signals recorded at 222nm and the temperature was increased at 2°C interval from 25°C to 79°C. Each curve presented the average of three independent experiments.
To further characterize the structure of the folded T11D3 and T23D3 proteins, circular dichroism (CD) was used to analyze secondary structure. The CD results showed that all three folded proteins, T11D3-β2m, T11D3-β2m-Qdm, and T23D3-β2m-Qdm displayed wavelength spectrums (Fig. 4D) similar to those previously published for MHC class I molecules (13, 58-60). The CD spectrums each showed a single maximum signal at ~220nm, which was in conformity with a β-sheet dominated structure in the MHC heavy chain. Thermal stability studies showed that folded T11D3 products had abnormal stability profiles (Fig. 4E). For most class I MHC proteins, the molecule loses its regular secondary structure, forming random coils, as the temperature increases, such that the molar ellipticity gradually approaches zero (58, 59, 61). With increasing temperature, T11D3 displayed evidence of denaturation, as shown by an increased molar ellipticity, but the denaturation was not complete at 80°C. Instead, the molar ellipticity stabilized at ~50oC, possibly reflecting entry into a relatively stable misfolded conformation.
The capacity of chimeric T11 and T23 proteins to bind Qdm peptide
The peptide binding capacity of folded T23D3 and T11D3 was evaluated using biotin-labeled Qdm in Eu-streptavidin based immunoassays. Folded proteins were incubated for 18h at RT in microtiter wells coated with anti-β2m capture mAb in the presence or absence of biotin-Qdm or the control Kb-binding peptide biotin-SIINFEKL. After washing, bound peptide was detected with Eu-streptavidin. As shown in Fig. 5A, wild type Qa-1b and T23D3 bind Qdm but not SIINFEKL through a peptide exchange reaction. T11D3 that had been folded in the presence of Qdm was also able to bind biotin-Qdm. No binding activity, however, was observed with T11D3 that was generated by folding in the absence of peptide. This suggests that the “empty” T11D3 may assume a conformation (acquired before or during the binding assay) that is not receptive to peptide binding.
Fig. 5.
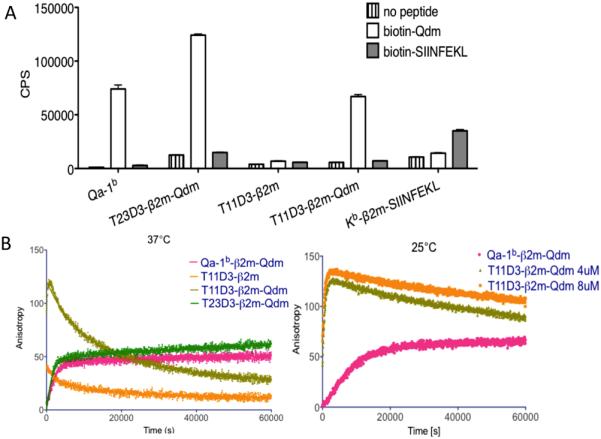
Qdm-binding capability of T11D3. (A) Eu-based immunoassay to test the ability of folded T11 and T23 monomers of binding Qdm peptide. Folded MHC Ib monomers were captured on plates by the coated the anti-b2m mAb and incubated with the biotin-labeled peptides at room temperature overnight. The assay was set up in triplicates (n=3) and the experiment was repeated twice with similar results. One of them was shown. (B) and (C) Fluorescence polarization (FP) assay. Folded MHC Ib monomers were incubated with Alexfluro488 labeled Qdm peptides and the FP signal was recorded every 60 seconds at 37°C or 25°C, respectively. The experiments were repeated twice (37°C) and three times (25°C) with similar results. One of each was shown.
Peptide binding was further analyzed in fluorescence polarization (FP) assays. Alexa Fluor 488 labeled Qdm peptide was incubated with the folded MHC proteins and peptide binding as measured by fluorescence anisotropy was recorded in real-time. Peptide binding to Qa-1-Qdm and T23D3-Qdm was rapid at 37°C, approaching saturation in ~1h (Fig. 5 left). The kinetics is consistent with an exchange reaction in which unlabeled Qdm is replaced by the labeled peptide. Previous results have indicated that Qdm dissociates from Qa-1 with a half-life of 40-100 minutes at 37°C (25), a relatively rapid rate of dissociation despite having an optimal sequence for binding to Qa-1 (24). The observed peptide association rates probably reflect rate of dissociation of Qdm, a step necessary for binding of labeled peptide.
The kinetics of binding of labeled Qdm to T11D3-Qdm was highly unusual, displaying a rapid initial kinetics followed by a rapid decay in binding signal. This pattern suggests that T11D3-Qdm complexes are unstable at 37°C, rapidly undergoing denaturation or conversion to a peptide unreceptive conformation. Consistent with the results of Eustreptavidin peptide binding experiments, T11D3 that was folded in the absence of peptide showed little or no peptide binding activity. This protein may initially bind some labeled Qdm, but it very rapidly assumes a peptide unreceptive state. Complexes formed with labeled peptide and T11D3-Qdm were considerably more stable at 25°C relative to 37°C, yet the unusual decay in signal was still observed at this temperature (Fig. 5 right). Overall, the results indicated that T11D3 can bind Qdm peptide, but T11D3-Qdm complexes are highly unstable and subject to denaturation or conversion to a peptide unreceptive conformation.
Evidence that T11 is not a ligand for CD94/NKG2 receptors
Qa-1 plays a key role in regulating NK cell activation as the exclusive ligand for CD94/NKG2 signaling receptors, which are expressed on a major fraction of NK cells, NKT cells, as well as a subpopulation of CD8+ T cells. CD94/NKG2 receptors display a high degree of specificity for the sequence of Qa-1-bound peptide, specifically recognizing Qdm (24). Several Qa-1 alleles have been identified and they appear to share the capacity to bind Qdm and serve as ligands for CD94/NKG2 receptors (19). Given the similarity of T11 to Qa-1 and the capacity of T11D3 to bind Qdm peptide, we were interested in determining whether T11 could serve as an alternative ligand for CD94/NKG2.
CD94/NKG2 ligand binding can be demonstrated by flow cytometry using tetramers generated from in vitro folded Qa-1-Qdm complexes to stain NK cells (23, 24). Similar to wild type Qa-1, tetramers generated with chimeric T23D3-Qdm molecules were observed to stain a major fraction of CD3−NKp46+ NK cells from the spleen and liver of B6 mice (Fig. 6). Thus, substitution of the T23 α3 domain does not disrupt receptor recognition. A substantial fraction of CD3+ lymphocytes from liver was also tetramer positive, reflecting the large number of CD94/NKG2+ NKT cells present in liver. By contrast, no staining was observed with tetramers generated from T11D3-Qdm molecules. The T11D3 molecules used to generate the tetramers where confirmed to contain β2m and Qdm, and they were appropriately biotinylated and tetramerized with streptavidin (data not shown). Nevertheless, it is possible that a fraction of T11D3 tetramers had loss of function from dissociation of Qdm. Despite this caveat, our results support the conclusion that T11 cannot substitute for Qa-1 as an alternative ligand for CD94/NKG2 receptors. In addition, no T11D3 tetramer positive lymphocytes were identified in lymphocytes from thymus, inguinal lymph node, mesenteric lymph node, Peyer’s patches, or bone marrow (data not shown).
Fig. 6.
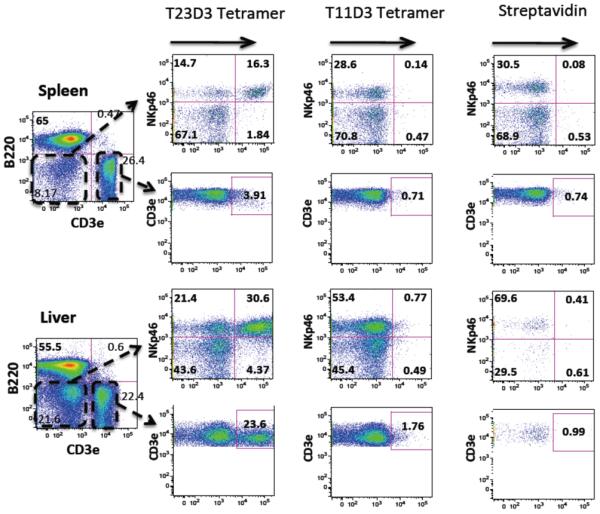
MHC Ib-Qdm tetramer staining of lymphocytes. B6 spleen and liver lymphocytes were stained with surface markers for B cells (B220), T cells (CD3ε), NK cells (NKp46) and the hybrid T23D3-Qdm or T11D3-Qdm tetramers. APC labeled streptavidin was used as a negative control. The lymphocytes were gated out for FACS analysis. The staining was repeated twice with similar results and one of each was shown.
Analysis of peptides eluted from T11D3 and T23D3 expressed in Hela cells
T11D3 (like T23D3) can be expressed at high levels on the surface of TAP-deficient T2 cells and some degree of folding of T11D3 heavy chain and β2m was observed in vitro in the absence of peptide. The T11D3-Qdm folding product was observed to bind labeled Qdm peptide, yet the resulting complexes appeared to be unstable (Fig. 5). These results leave the unanswered question of whether T11 normally assembles with peptides in cells and, if so, it would be relevant to determine the nature of the peptides and the extent to which the peptide loading specificity differs from T23. In addition, there is limited information on the nature of peptides other than Qdm that can be presented by T23 (Qa-1). To directly address these questions, T11D3 and T23D3 bound peptides were eluted from Hela-T11D3 and Hela-T23D3 cells, and identified by LC-MS/MS tandem mass spectrometry. Background peptides identified in eluates from control Hela-MigR1 cells were subtracted from the eluted peptide pools.
Acquired MS/MS spectra form peptide pools eluted from T23D3 were initially searched using Spectrum Mill Proteomics Workbench (Agilent Technologies, Santa Clara, CA) yielding 190 peptide sequences. These were further culled through a stringent validation process, involving expert manual inspection of the LC-MS/MS fragmentation spectra as previously described (51), resulting in 82 peptides with 41 unique sequences (Table I). The two HLA class Ia leader peptide derived sequences VMAPRTLIL and VMAPRTLVL from HLA-C and HLA-A together represented ~11% of the validated peptide “hits”. These well-established ligands for HLA-E and CD94/NKG2A in humans have previously been shown to bind Qa-1 with affinities similar to Qdm. This result is consistent with the dominant presentation of Qdm (or related peptides) in TAP-expressing cells. However, it is noteworthy that a large variety of other peptides were also identified. Peptides ranged in length from 8-12 amino acids, with a strong predominance of 9-mers (Fig. 7A). The T23D3-bound peptides shared a clear motif, with strong preference for leucine at P9, as well as a preference for alanine at P2 (Fig.7C). Proline is prominent at P4, and this amino acid is conserved in the mouse and human class Ia leader peptides recognized by CD94/NKG2A receptors. Hydrophobic amino acids are present in the C-terminal position in all the peptides, regardless of length, with similar representation of amino acids as shown for 9-mers. The peptides identified are derived from proteins with broad intracellular distribution, including slightly >50% from the cytoplasm and/or nucleus, and the remainder with predominant localization in plasma member, ER, Golgi, or mitochondria. The two class Ia leader sequence derived peptides were the only peptides identified from ER leader sequences. These results indicate that even in TAP-expressing cells, Qa-1b can load with a diverse repertoire of peptide sequences sharing a common motif.
Table 1.
Peptide eluted from T23 and T11
| Sequence | Start amino acid |
Length | T23 Hits |
T11 Hits |
Accession | Name |
|---|---|---|---|---|---|---|
| VMAPRTLIL | 3 | 9 | 6 | 0 | P04222 | HLA class I histocompatibility antigen, Cw-3 alpha chain |
| VMAPRTLVL | 3 | 9 | 3 | 1 | P01892 | HLA class I histocompatibility antigen, A-2 alpha chain precursor |
| KLFGSTSSF | 195 | 9 | 4 | 0 | P08174 | Complement decay-accelerating factor |
| SAIPHPLIM | 226 | 9 | 4 | 0 | P61201 | COP9 signalosome complex subunit 2 |
| VFGPILASLL | 166 | 10 | 3 | 0 | O43147 | Small G protein signaling modulator 2 |
| ETYPDAVKI | 182 | 9 | 3 | 0 | Q8WV22 | Non-structural maintenance of chromosomes element 1 homolog |
| FVPAEKIVI | 301 | 9 | 3 | 0 | Q9UHF4 | Interleukin-20 receptor subunit alpha |
| AKYPEIKSL | 28 | 9 | 2 | 0 | O15121 | Sphingolipid delta(4)-desaturase DES1 |
| AAFHEEFVV | 217 | 9 | 2 | 0 | P51114 | Fragile X mental retardation syndrome-related protein 1 |
| AQLPEKVEY | 986 | 9 | 2 | 0 | P51532 | Transcription activator BRG1 |
| LPMFIIVV | 308 | 8 | 2 | 0 | P53794 | Sodium/myo-inositol cotransporter |
| GQLPGLHEY | 561 | 9 | 2 | 0 | Q8TAT6 | Nuclear protein localization protein 4 homolog |
| FGFHKPKMY | 2 | 9 | 2 | 0 | Q9NP50 | Protein FAM60A |
| YAYDGKDYIA | 140 | 10 | 1 | 0 | P01892 | HLA class I histocompatibility antigen, A-2 alpha chain |
| YAYDGKDYIAL | 140 | 11 | 1 | 0 | P01892 | HLA class I histocompatibility antigen, A-2 alpha chain |
| RKLEAAEDIAY | 239 | 11 | 1 | 0 | P35232 | Prohibitin |
| FAYPAIRYL | 117 | 9 | 1 | 0 | P51398 | 28S ribosomal protein S29, mitochondrial |
| HSAEILAEI | 75 | 9 | 1 | 0 | Q6FI81 | Anamorsin |
| HDLIRVVY | 155 | 8 | 1 | 0 | Q8IXS8 | Protein FAM126B |
| AAFAYTVKY | 243 | 9 | 1 | 0 | Q8N2K1 | Ubiquitin-conjugating enzyme E2 J2 |
| HTANIQTLI | 313 | 9 | 1 | 0 | Q8NG31 | Protein CASC5 |
| LAAQILAVL | 202 | 9 | 1 | 0 | Q96IK0 | Transmembrane protein 101 |
| SKLPIGDVATQY | 291 | 12 | 1 | 0 | Q99832 | T-complex protein 1 subunit eta |
| SLINEFKL | 147 | 8 | 1 | 0 | Q9NYW6 | Taste receptor type 2 member 3 |
| RAFDQGADAIY | 34 | 11 | 1 | 0 | Q9UDW1 | Cytochrome b-c1 complex subunit 9 |
| GKAPLNVQF | 876 | 9 | 3 | 1 | O75369 | Filamin-B |
| AAFLKAIGY | 744 | 9 | 3 | 1 | O75533 | Splicing factor 3B subunit 1 |
| SAIDRIFTL | 403 | 9 | 4 | 2 | Q86TU7 | Histone-lysine N-methyltransferase setd3 |
| TASPLVKSV | 866 | 9 | 3 | 1 | Q6P4F7 | Rho GTPase-activating protein 11A |
| NIFRNVEV | 80 | 8 | 3 | 2 | Q7L523 | Ras-related GTP-binding protein A |
| AAFDKIQQL | 121 | 9 | 3 | 2 | Q9P2I0 | Cleavage and polyadenylation specificity factor subunit 2 |
| SAKTPGFSV | 143 | 9 | 2 | 1 | Q9NY61 | Protein AATF |
| ISTPVIRTF | 989 | 9 | 2 | 1 | Q9NZB2 | Constitutive coactivator of PPAR-gamma-like protein 1 |
| MTPEIIQKL | 168 | 9 | 2 | 2 | Q9UMS4 | Pre-mRNA-processing factor 19 |
| SAVPFKILY | 1030 | 9 | 1 | 1 | P10586 | Receptor-type tyrosine-protein phosphatase F |
| ALIEFIRSEY | 355 | 10 | 1 | 1 | P26639 | Threonine--tRNA ligase, cytoplasmic |
| ETFNTPAMYV | 825 | 10 | 1 | 1 | Q6S8J3 | POTE ankyrin domain family member E |
| AAMPRPVSY | 576 | 9 | 1 | 1 | Q6TFL4 | Kelch-like protein 24 |
| EDDNISVTI | 239 | 9 | 1 | 1 | Q9NWH9 | SAFB-like transcription modulator |
| RQADFVQVL | 414 | 9 | 1 | 1 | Q9Y2V7 | Conserved oligomeric Golgi complex subunit 6 |
| EIFADPRTV | 228 | 9 | 1 | 2 | Q92871 | Phosphomannomutase 1 |
| AQRMTTQLL | 2 | 9 | 0 | 1 | P15328 | Folate receptor alpha |
| AASSIQRVL | 124 | 9 | 0 | 1 | Q5BKT4 | Dol-P-Glc:Glc(2)Man(9)GlcNAc(2)-PP-Dol alpha-1,2- glucosyltransferase |
| QAVAKCAQLL | 336 | 10 | 0 | 1 | Q6ZU64 | Coiled-coil domain-containing protein 108 |
| DVIYPMAVV | 177 | 9 | 0 | 2 | P78406 | mRNA export factor |
| GAFGKPSSL | 442 | 9 | 0 | 2 | Q8IVT2 | Uncharacterized protein C19orf21 |
| VAAPQVQQV | 231 | 9 | 0 | 3 | Q12772 | Sterol regulatory element-binding protein 2 |
Fig. 7.
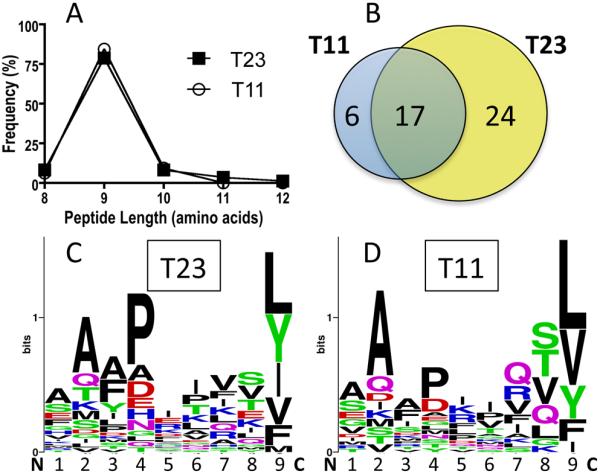
Peptide elution from Hela-T11D3 and Hela-T23D3 cells. (A) Length distribution of the peptides eluted from Hela-T11D3 and Hela-T23D3 cells. (B) The unique peptides from the T23 eluted and T11 eluted peptide pool were analyzed. Numbers of 8mer, 9mer and 10mer peptides were shown in the Venn diagram. (C) Sequence logo of the total eluted unique peptides from T23 and (D) T11. Each column represents one amino acid position in the peptide. Amino acids with different properties were labeled with different colors.
Eighty-nine peptide sequences were identified on initial analysis of samples from T11D3, with 32 confirmed by manual validation, including 23 unique sequences (Table I). The length distribution was similar to that observed with T23D3, with dominant representation of 9-mers (Fig. 7A). There was a striking degree of overlap in peptides isolated from T11 and T23 (Fig. 7B), including 17 sequences shared between the two samples (Table I). The motif identified with peptides eluted from T11 is very similar to that obtained with T23 (Fig. 7C and 7D), and there was no discernable difference in the subcellular localization of the source proteins. In contrast to T23, class Ia leader peptides were not as prominent among the validated peptides, with only one “hit” representing 3% of the total peptides. These results provide direct evidence that T11 normally assembles with peptides and that it shares a peptide-binding motif very similar to that of Qa-1.
Discussion
A large number of MHC class Ib genes remain to be characterized with respect to expression and potential function. Analysis is challenging because of the high level of sequence homology among class I genes and the limited availability of specific mAbs. In the current study, we investigated the potential for expression and function of the H2-T11 gene from C57BL/6 mice, a gene with a high degree of homology to the Qa-1b-encoding H2-T23 gene. After cloning and sequencing the T11 gene, RT-PCR was used to demonstrate that correctly spliced T11 mRNA is expressed widely in tissues and leukocyte subpopulations, and that expression is particularly high in thymus. Expression was also prominent in spleen, intestine, lung and liver. The highest expression of T11 was observed in the DP subpopulation of the thymocytes. The possibility that T11 may have a special function in thymic selection remains to be explored. Previous studies have demonstrated that, in contrast to conventional class Ia-restricted T cells, class Ib-restricted T or NKT cells can be positively selected by thymic hematopoietic cells (including DP thymocytes) expressing Qa-1, H2-M3, or CD1 (31, 62, 63).
Further support for the conclusion that H2-T11 encodes a functional MHC protein was obtained using cDNA encoding a chimeric T11 protein with a substituted α3 domain that allowed detection by an existing mAb. The chimeric T11D3 protein was expressed at high levels on the surface of both TAP-expressing Hela and TAP-deficient T2 cells, at levels very similar to those observed with the control T23D3 cDNA. Confirmation that the natural T11 protein is assembled and expressed on the surface of primary tissues will require the generation of an appropriate mAb. Our study does not exclude the possibility that the T11 α3 domain contains substitutions that interfere with protein assembly. T11 differs from the known Qa-1 alleles at four amino acid positions in the N-terminal segment of the α3 domain (residues 191, 195, 197, and 198). However, the amino acid present at each of these positions in T11 are also present in other mouse class I molecules. For example, 191R is present in H-2Kk and 195P/197G/198D are present in H-2Kd and H-2Dd. In addition, these residues are largely surface exposed. Thus it is likely that the T11 α3 domain is functional and that the T11 protein is express at the surface of cells in tissues.
Given the high degree of sequence similarity between T11 and Qa-1, we were interested in determining whether Qa-1 function is conserved in T11. In addition to its function in regulating NK cell activation, Qa-1 has been demonstrated to function in T cell antigen presentation. Control experiments demonstrated that Hela cells expressing chimeric T23D3 molecules were highly functional in presenting antigen to insulin-specific, Qa-1b-restricted 6C5 T cells. Thus, substitution of the α3 domain did not disrupt that antigen presentation function of T23. By contrast, no responses were observed in antigen presentation experiments with cells expressing T11D3, indicating the T11 cannot substitute for Qa-1 in antigen presentation to 6C5 T cells. In addition to two substitutions in the β-sheet floor of the peptide-binding groove, T11 has six substitutions in surface exposed positions of the α1α2 helices as compared to Qa-1b. Any of these substitutions could impact TCR recognition. While it is possible that some T cells may cross-recognize Qa-1 and T11, there are enough amino acid substitutions in the α1α2 domain to make it unlikely that T cell cross-recognition is common.
Qa-1 plays a major role as the exclusive ligand for CD94/NKG2 inhibitory and activating receptors on NK cells. Receptor recognition is highly specific for the sequence of the Qa-1-bound peptide. Thus, it was important to determine whether the capacity to bind to the canonical class Ia leader sequence derived peptide Qdm is conserved in T11. Recombinant T11D3 heavy chain was observed to fold in vitro in the presence of Qdm peptide and β2m with an efficiency similar to that observed with the control T23D3 heavy chain. The capacity of T11 to bind Qdm was further confirmed in peptide exchange reactions with folded T11D3-Qdm protein and labeled Qdm peptide. The possibility that T11 can serve as an alternative ligand for CD94/NKG2 receptors was investigated using MHC tetramers and flow cytometry. Control T23D3-Qdm tetramers where observed to stain large populations of NK cells and NKT cells from spleen and liver, demonstrating that α3 domain substitution does not prevent binding of Qa-1 to CD94/NKG2 receptors. By contrast, no staining was observed with comparable T11D3-Qdm tetramers, supporting the conclusion that T11 cannot serve as an alternative ligand for CD94/NKG2.
The co-crystal structure of HLA-E bound to human CD94/NKG2A demonstrates that the inhibitory receptor binds to the same general surface of the MHC molecule as do TCRs (64). Examination of this structure suggests T11 position 65 as a candidate receptor contact residue that might preclude recognition by CD94/NKG2A (57). This position contains a non-conservative arginine substitution in T11 replacing tryptophan in all Qa-1 alleles. It is noteworthy that, like T11 and unlike Qa-1, arginine is also present at this position in HLA-E. It has previously been demonstrated that while peptide binding specificity is conserved between Qa-1 and HLA-E, CD94/NKG2 receptors do not cross-recognize species mismatched MHC class Ib molecules, even if they are bound to a species-matched leader peptide (65). Thus there has been co-evolution of CD94/NKG2, its MHC class Ib ligand, and the MHC class Ia leader sequences that provide the source of Qdm and related peptides.
Our results demonstrate that recombinant T11D3 has the capacity to bind Qdm peptide. Size exclusion chromatography, immunoassays, and far-UV CD analysis indicated that recombinant T11D3, generated in vitro by the folding of heavy chain with β2m and Qdm, assembles similarly to conventional MHC class I molecules. In addition, this protein can bind labeled Qdm through a peptide exchange reaction. However, an atypical thermal denaturation profile was observed in CD measurements with recombinant T11D3-Qdm consistent with entry into a relatively stable misfolded conformation with preservation of some β-sheet secondary structure at increasing temperatures. In addition, the kinetics of binding of labeled Qdm to T11D3-Qdm was unusual, displaying very rapid association kinetics followed by decay in the binding signal consistent with rapid conversion to a peptide-unreceptive conformation at 37oC. Given these findings, one must consider the possibility that T11 is in general unstable at physiological temperature. This possibility, however, is strongly countered by the observation that high levels of T11D3 are expressed at steady state on the surface of transduced cells, and these molecules were observed to be associated with bound peptides. Thus we favor the hypothesis that T11 can function as a stable peptide presentation molecule.
LC-MS analysis of peptides eluted from T11D3 isolated from transduced Hela cells demonstrated that T11 loads with a diversity of predominantly 9-mer peptides that share a common motif, with dominant preference for aliphatic and hydrophobic amino acids at P9 and preference for alanine in P2. This motif is very similar if not identical to that obtained with T23D3. Indeed, a large majority of the peptide sequences identified from T11D3 were also identified in the T23D3 peptide sample. T11 differs from Qa-1b in only two positions inside the peptide-binding groove, T9H and A11V. These residues are located in the floor of the peptide-binding groove, interacting primarily with the α1 helix but not with bound peptide. A11V is a conservative substitution and 9H is present in the a, c and d alleles of Qa-1 that, like the b allele, bind Qdm and serve as functional ligands for CD94/NKG2 receptors. Based on the crystal structure of Qa-1b-Qdm, none of the amino acids that differ between T11b and all Qa-1 alleles directly contact peptide (57). Thus, it appears that the peptide binding specificity of Qa-1 is conserved in T11.
Early work demonstrated that Qa-1 predominantly loads with Qdm, in striking contrast to that large diversity of peptides that assemble with class Ia molecules (21). Crystal structures of Qa-1 and its human ortholog HLA-E with bound leader peptides have demonstrated five primarily hydrophobic anchor site distributed throughout the length of the groove that accommodate side chains from peptide positions P2, P3, P6, P7, and P9, with P2, P7, and P9 being relatively deep (57, 66). This distribution of pockets may in part account for the exceptionally restricted peptide binding specificity of these MHC molecules, favoring sequences closely related to Qdm. It is also important to note that, despite having ideal sequences for binding Qa-1 and HLA-E, the Qdm-related peptides dissociate relatively rapidly from the MHC molecules (25). Thus, only a relatively small fraction of Qa-1-binding peptides with near optimal affinities may form complexes that survive long enough to be present in appreciable quantities at steady state.
An important finding in the present study is the identification of a relatively large number of different Qa-1 (T23D3)-bound peptides. Qdm-related HLA-C and HLA-A derived leader sequences were readily detected in the eluted peptide samples, representing ~11% of the identified peptides, yet many other peptide sequences were also present. A number of studies have characterized T cells with specificity for alternative peptide antigens presented by Qa-1, demonstrating that Qa-1 can bind and present peptides other than Qdm. An Hsp60-derived peptide was identified by mass spectrometry from Qa-1 isolated from cells lacking a source of Qdm (55). In the present study the peptides isolated from T23D3 were predominantly 9-mers and a clear motif was identified, with dominant preference for hydrophobic amino acids at P9 and preference for alanine at P2 and P3. Some degree of selectivity was also observed at the other anchor positions (P6 and P7). The anchor positions in Qa-1 have been defined by binding experiments with substituted peptides (24) and from the Qa-1b-Qdm crystal structure (57). The results are generally consistent with findings from a previous study analyzing the relative preference for specific amino acids at each anchor position based on in vitro folding reactions with pools of substituted Qdm peptides randomized at individual positions (24). In the latter study, the greatest observed specificity was for leucine at P9. Previously described Qa-1-restricted T cell epitopes, as well as the Qdm-related leader peptides from mice and humans, contain leucine at P9 and hydrophobic residues at P2. These include the ERAAP-sensitive self-peptide FL9 (FYAEATPML) (38), a salmonella peptide (GMQFDRGYL) (27), peptides from proinsulin (ALWMRFLPL) (29), influenza (FYAEATPML) (24) and an epitope recognized by T cells with specificity for TAP-deficient tumor cells (FAPLPRLPTL) (37).
Recently, Oliveira et al., reported a large number of peptides identified by LC-MS/MS isolated from TAP-deficient EC7.1 cells expressing a chimeric Qa-1b protein containing the H-2Db alpha-3 domain, analogous to the constructs used in our experiments (37). The distribution of peptide lengths was greater as compared to our results. The frequent presence of Leu at the C-terminus as well as Ala at P2 was consistent with our current results with TAP-expressing cells. By contrast, a very strong signature for Asn at P5 was present in their peptides but absent from our results (which showed no specificity at the P5 position). As noted above, P5 is not an anchor position in Qa-1b. EC7.1 is a Kb- and Db-negative variant of the TAP-deficient H-2b RMA-S cell line. Given that the anti-Db mAb 28-14-8S was used to isolate chimeric Qa-1 molecules in the Oliveira et al. study, it is possible that Db associated peptides were also present in the samples analyzed in that study from low level surface or intracellular pools of residual Db molecules. The Db peptide-binding motif (Asn at P5 and Met, Ile, or Leu at the C-terminus) overlaps with Qa-1b at the C-terminus, but the dominant Asn anchor at P5 is not observed with Qa-1.
It is interesting to note that there appears to be selectivity for Pro at the solvent-exposed non-anchor P4 position in T23D3-bound peptides. It is possible that Pro at this position may constrain the conformation of the peptide, favoring optimal positioning of anchor residues. Alternatively, cellular mechanisms impacting peptide processing or loading might introduce bias favoring Pro at P4.
Overall, our results establish a common peptide-binding motif shared by Qa-1b and T11b. It appears very likely that T11 is a functional MHC class Ib molecule with a capacity for peptide binding and cell surface expression. T11 differs from all Qa-1 alleles through substitutions at a number of positions on the α-helical receptor-contact surface of the peptide-binding domain that may prevent T11 from serving as an alternative ligand for CD94/NKG2 or Qa-1-restricted TCRs. We cannot rule out the possibility that T11 might have specialized function, serving as a ligand for a yet to be identified receptor. Given that T11 shares a very similar peptide-binding specificity with Qa-1, it is also interesting to consider the possibility that T11-restricted CD8+ T cells might have regulatory functions that parallel those identified for Qa-1-restricted T cells. These possibilities will require further investigation.
Acknowledgements
We thank Xiaomin Wang for strong technical support and Matthew Weinstock for the help with the circular dichroism experiments.
Abbreviations
- β2m
β2-microglobulin
- CD
circular dichroism
- ER
endoplasmic reticulum
- DC
dendritic cells
- Qdm
Qa-1 determinant modifier
Footnotes
This work was supported by National Institutes of Health Research Grants AI30554 and AI33614.
References
- 1.Jensen PE. Recent advances in antigen processing and presentation. Nat. Immunol. 2007;8:1041–1048. doi: 10.1038/ni1516. [DOI] [PubMed] [Google Scholar]
- 2.Kumanovics A, Takada T, Lindahl KF. Genomic organization of the mammalian MHC. Annu. Rev. Immunol. 2003;21:629–657. doi: 10.1146/annurev.immunol.21.090501.080116. [DOI] [PubMed] [Google Scholar]
- 3.Howcroft T, Singer D. Expression of nonclassical MHC class Ib genes: comparison of regulatory elements. Immunol. Res. 2003;27:1–30. doi: 10.1385/IR:27:1:1. [DOI] [PubMed] [Google Scholar]
- 4.Rodgers JR, Cook RG. MHC class Ib molecules bridge innate and acquired immunity. Nat. Rev. Immunol. 2005;5:459–471. doi: 10.1038/nri1635. [DOI] [PubMed] [Google Scholar]
- 5.Shawar SM, Vyas JM, Rodgers JR, Rich RR. Antigen presentation by major histocompatibility complex class I-B molecules. Annu. Rev. Immunol. 1994;12:839–880. doi: 10.1146/annurev.iy.12.040194.004203. [DOI] [PubMed] [Google Scholar]
- 6.Pamer EG, Wang CR, Flaherty L, Lindahl KF, Bevan MJ. H-2M3 presents a Listeria monocytogenes peptide to cytotoxic T lymphocytes. Cell. 1992;70:215–223. doi: 10.1016/0092-8674(92)90097-v. [DOI] [PubMed] [Google Scholar]
- 7.Chiu NM, Chun T, Fay M, Mandal M, Wang CR. The majority of H2-M3 is retained intracellularly in a peptide-receptive state and traffics to the cell surface in the presence of N-formylated peptides. J. Exp. Med. 1999;190:423–434. doi: 10.1084/jem.190.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu H, Chun T, Choi HJ, Wang B, Wang CR. Impaired response to Listeria in H2-M3-deficient mice reveals a nonredundant role of MHC class Ib-specific T cells in host defense. J. Exp. Med. 2006;203:449–459. doi: 10.1084/jem.20051866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seaman MS, Wang CR, Forman J. MHC class Ib-restricted CTL provide protection against primary and secondary Listeria monocytogenes infection. J. Immunol. 2000;165:5192–5201. doi: 10.4049/jimmunol.165.9.5192. [DOI] [PubMed] [Google Scholar]
- 10.Cho H, Choi HJ, Xu H, Felio K, Wang CR. Nonconventional CD8+ T cell responses to Listeria infection in mice lacking MHC class Ia and H2-M3. J. Immunol. 2011;186:489–498. doi: 10.4049/jimmunol.1002639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gulden PH, Fischer P, 3rd, Sherman NE, Wang W, Engelhard VH, Shabanowitz J, Hunt DF, Pamer EG. A Listeria monocytogenes pentapeptide is presented to cytolytic T lymphocytes by the H2-M3 MHC class Ib molecule. Immunity. 1996;5:73–79. doi: 10.1016/s1074-7613(00)80311-8. [DOI] [PubMed] [Google Scholar]
- 12.Lenz LL, Dere B, Bevan MJ. Identification of an H2-M3-restricted Listeria epitope: implications for antigen presentation by M3. Immunity. 1996;5:63–72. doi: 10.1016/s1074-7613(00)80310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber DA, Attinger A, Kemball CC, Wigal JL, Pohl J, Xiong Y, Reinherz EL, Cheroutre H, Kronenberg M, Jensen PE. Peptide-independent folding and CD8 alpha alpha binding by the nonclassical class I molecule, thymic leukemia antigen. J. Immunol. 2002;169:5708–5714. doi: 10.4049/jimmunol.169.10.5708. [DOI] [PubMed] [Google Scholar]
- 14.Leishman AJ, Naidenko OV, Attinger A, Koning F, Lena CJ, Xiong Y, Chang HC, Reinherz E, Kronenberg M, Cheroutre H. T cell responses modulated through interaction between CD8alphaalpha and the nonclassical MHC class I molecule, TL. Science. 2001;294:1936–1939. doi: 10.1126/science.1063564. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, Park Y, Wang-Zhu Y, Larange A, Arens R, Bernardo I, Olivares-Villagomez D, Herndler-Brandstetter D, Abraham N, Grubeck-Loebenstein B, Schoenberger SP, Van Kaer L, Kronenberg M, Teitell MA, Cheroutre H. Mucosal memory CD8(+) T cells are selected in the periphery by an MHC class I molecule. Nat. Immunol. 2011;12:1086–1095. doi: 10.1038/ni.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen PE, Sullivan BA, Reed-Loisel LM, Weber DA. Qa-1, a nonclassical class I histocompatibility molecule with roles in innate and adaptive immunity. Immunol. Res. 2004;29:81–92. doi: 10.1385/IR:29:1-3:081. [DOI] [PubMed] [Google Scholar]
- 17.Connolly DJ, Cotterill LA, Hederer RA, Thorpe CJ, Travers PJ, McVey JH, Dyson J, Robinson PJ. A cDNA clone encoding the mouse Qa-1a histocompatibility antigen and proposed structure of the putative peptide binding site. J. Immunol. 1993;151:6089–6098. [PubMed] [Google Scholar]
- 18.Hermel E, Hart AJ, Miller R, Aldrich CJ. CTL and sequence analyses of MHC class IB antigens Qa1(c) (H2-T23(r)) and Qa1(d) (H2-T23(f)) Immunogenetics. 1999;49:712–717. doi: 10.1007/s002510050671. [DOI] [PubMed] [Google Scholar]
- 19.Hermel E, Hart AJ, Gunduz I, Acton H, Kim C, Wurth M, Uddin S, Smith C, Fischer Lindahl K, Aldrich CJ. Polymorphism and conservation of the genes encoding Qa1 molecules. Immunogenetics. 2004;56:639–649. doi: 10.1007/s00251-004-0722-x. [DOI] [PubMed] [Google Scholar]
- 20.Aldrich CJ, DeCloux A, Woods AS, Cotter RJ, Soloski MJ, Forman J. Identification of a Tap-dependent leader peptide recognized by alloreactive T cells specific for a class Ib antigen. Cell. 1994;79:649–658. doi: 10.1016/0092-8674(94)90550-9. [DOI] [PubMed] [Google Scholar]
- 21.DeCloux A, Woods AS, Cotter RJ, Soloski MJ, Forman J. Dominance of a single peptide bound to the class I(B) molecule, Qa-1b. J. Immunol. 1997;158:2183–2191. [PubMed] [Google Scholar]
- 22.Li L, Sullivan BA, Aldrich CJ, Soloski MJ, Forman J, Grandea AG, 3rd, Jensen PE, Van Kaer L. Differential requirement for tapasin in the presentation of leader- and insulin-derived peptide antigens to Qa-1brestricted CTLs. J. Immunol. 2004;173:3707–3715. doi: 10.4049/jimmunol.173.6.3707. [DOI] [PubMed] [Google Scholar]
- 23.Vance RE, Kraft JR, Altman JD, Jensen PE, Raulet DH. Mouse CD94/NKG2A is a natural killer cell receptor for the nonclassical major histocompatibility complex (MHC) class I molecule Qa-1(b) J. Exp. Med. 1998;188:1841–1848. doi: 10.1084/jem.188.10.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraft JR, Vance RE, Pohl J, Martin AM, Raulet DH, Jensen PE. Analysis of Qa-1(b) peptide binding specificity and the capacity of CD94/NKG2A to discriminate between Qa-1-peptide complexes. J. Exp. Med. 2000;192:613–624. doi: 10.1084/jem.192.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kambayashi T, Kraft-Leavy JR, Dauner JG, Sullivan BA, Laur O, Jensen PE. The nonclassical MHC class I molecule Qa-1 forms unstable peptide complexes. J. Immunol. 2004;172:1661–1669. doi: 10.4049/jimmunol.172.3.1661. [DOI] [PubMed] [Google Scholar]
- 26.Bouwer HG, Seaman MS, Forman J, Hinrichs DJ. MHC class Ibrestricted cells contribute to antilisterial immunity: evidence for Qa-1b as a key restricting element for Listeria-specific CTLs. J. Immunol. 1997;159:2795–2801. [PubMed] [Google Scholar]
- 27.Lo WF, Ong H, Metcalf ES, Soloski MJ. T cell responses to Gram-negative intracellular bacterial pathogens: a role for CD8+ T cells in immunity to Salmonella infection and the involvement of MHC class Ib molecules. J. Immunol. 1999;162:5398–5406. [PubMed] [Google Scholar]
- 28.Lo WF, Woods AS, DeCloux A, Cotter RJ, Metcalf ES, Soloski MJ. Molecular mimicry mediated by MHC class Ib molecules after infection with gram-negative pathogens. Nat. Med. 2000;6:215–218. doi: 10.1038/72329. [DOI] [PubMed] [Google Scholar]
- 29.Chun T, Aldrich CJ, Baldeon ME, Kawczynski LV, Soloski MJ, Gaskins HR. Constitutive and regulated expression of the class IB molecule Qa-1 in pancreatic beta cells. Immunology. 1998;94:64–71. doi: 10.1046/j.1365-2567.1998.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tompkins SM, Kraft JR, Dao CT, Soloski MJ, Jensen PE. Transporters associated with antigen processing (TAP)-independent presentation of soluble insulin to alpha/beta T cells by the class Ib gene product, Qa-1(b) J. Exp. Med. 1998;188:961–971. doi: 10.1084/jem.188.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan BA, Kraj P, Weber DA, Ignatowicz L, Jensen PE. Positive selection of a Qa-1-restricted T cell receptor with specificity for insulin. Immunity. 2002;17:95–105. doi: 10.1016/s1074-7613(02)00343-6. [DOI] [PubMed] [Google Scholar]
- 32.Jiang H, Chess L. Regulation of immune responses by T cells. N. Engl. J. Med. 2006;354:1166–1176. doi: 10.1056/NEJMra055446. [DOI] [PubMed] [Google Scholar]
- 33.Wu Y, Zheng Z, Jiang Y, Chess L, Jiang H. The specificity of T cell regulation that enables self-nonself discrimination in the periphery. Proc. Natl. Acad. Sci. U. S. A. 2009;106:534–539. doi: 10.1073/pnas.0811843106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HJ, Verbinnen B, Tang X, Lu L, Cantor H. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature. 2010;467:328–332. doi: 10.1038/nature09370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu D, Ikizawa K, Lu L, Sanchirico ME, Shinohara ML, Cantor H. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat. Immunol. 2004;5:516–523. doi: 10.1038/ni1063. [DOI] [PubMed] [Google Scholar]
- 36.van Hall T, Wolpert EZ, van Veelen P, Laban S, van der Veer M, Roseboom M, Bres S, Grufman P, de Ru A, Meiring H, de Jong A, Franken K, Teixeira A, Valentijn R, Drijfhout JW, Koning F, Camps M, Ossendorp F, Karre K, Ljunggren HG, Melief CJ, Offringa R. Selective cytotoxic T-lymphocyte targeting of tumor immune escape variants. Nat. Med. 2006;12:417–424. doi: 10.1038/nm1381. [DOI] [PubMed] [Google Scholar]
- 37.Oliveira CC, van Veelen PA, Querido B, de Ru A, Sluijter M, Laban S, Drijfhout JW, van der Burg SH, Offringa R, van Hall T. The nonpolymorphic MHC Qa-1b mediates CD8+ T cell surveillance of antigenprocessing defects. J. Exp. Med. 2010;207:207–221. doi: 10.1084/jem.20091429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagarajan NA, Gonzalez F, Shastri N. Nonclassical MHC class Ibrestricted cytotoxic T cells monitor antigen processing in the endoplasmic reticulum. Nat. Immunol. 2012;13:579–586. doi: 10.1038/ni.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brorson KA, Hunt SW, 3rd, Hunkapiller T, Sun YH, Cheroutre H, Nickerson DA, Hood L. Comparison of exon 5 sequences from 35 class I genes of the BALB/c mouse. J. Exp. Med. 1989;170:1837–1858. doi: 10.1084/jem.170.6.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teitell M, Cheroutre H, Panwala C, Holcombe H, Eghtesady P, Kronenberg M. Structure and function of H-2 T (Tla) region class I MHC molecules. Crit. Rev. Immunol. 1994;14:1–27. [PubMed] [Google Scholar]
- 41.Hammerling U, Ronne H, Widmark E, Servenius B, Denaro M, Rask L, Peterson PA. Gene duplications in the TL region of the mouse major histocompatibility complex. EMBO J. 1985;4:1431–1434. doi: 10.1002/j.1460-2075.1985.tb03798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohtsuka M, Inoko H, Kulski JK, Yoshimura S. Major histocompatibility complex (Mhc) class Ib gene duplications, organization and expression patterns in mouse strain C57BL/6. BMC Genomics. 2008;9:178. doi: 10.1186/1471-2164-9-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis BK, Cook RG, Rich RR, Rodgers JR. Hyperconservation of the putative antigen recognition site of the MHC class I-b molecule TL in the subfamily Murinae: evidence that thymus leukemia antigen is an ancient mammalian gene. J. Immunol. 2002;169:6890–6899. doi: 10.4049/jimmunol.169.12.6890. [DOI] [PubMed] [Google Scholar]
- 44.Nakayama K, Tokito S, Jaulin C, Delarbre C, Kourilsky P, Nakauchi H, Gachelin G. Comparative structure of two duplicated T1a class I genes (T10c and 37) of the murine H-2d MHC. Implications on the evolution of the T1a region. J. Immunol. 1990;144:2400–2408. [PubMed] [Google Scholar]
- 45.Pear WS, Miller JP, Xu L, Pui JC, Soffer B, Quackenbush RC, Pendergast AM, Bronson R, Aster JC, Scott ML, Baltimore D. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood. 1998;92:3780–3792. [PubMed] [Google Scholar]
- 46.Zhou Z, Callaway KA, Weber DA, Jensen PE. Cutting edge: HLA-DM functions through a mechanism that does not require specific conserved hydrogen bonds in class II MHC-peptide complexes. J. Immunol. 2009;183:4187–4191. doi: 10.4049/jimmunol.0901663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jensen PE, Moore JC, Lukacher AE. A europium fluoroimmunoassay for measuring peptide binding to MHC class I molecules. J. Immunol. Methods. 1998;215:71–80. doi: 10.1016/s0022-1759(98)00062-3. [DOI] [PubMed] [Google Scholar]
- 48.Rossi AM, Taylor CW. Analysis of protein-ligand interactions by fluorescence polarization. Nat. Protoc. 2011;6:365–387. doi: 10.1038/nprot.2011.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 50.Escobar H, Crockett DK, Reyes-Vargas E, Baena A, Rockwood AL, Jensen PE, Delgado JC. Large scale mass spectrometric profiling of peptides eluted from HLA molecules reveals N-terminal-extended peptide motifs. J. Immunol. 2008;181:4874–4882. doi: 10.4049/jimmunol.181.7.4874. [DOI] [PubMed] [Google Scholar]
- 51.Escobar H, Reyes-Vargas E, Jensen PE, Delgado JC, Crockett DK. Utility of characteristic QTOF MS/MS fragmentation for MHC class I peptides. J. Proteome Res. 2011;10:2494–2507. doi: 10.1021/pr101272k. [DOI] [PubMed] [Google Scholar]
- 52.Liu G, Gramling S, Munoz D, Cheng D, Azad AK, Mirshams M, Chen Z, Xu W, Roberts H, Shepherd FA, Tsao MS, Reisman D. Two novel BRM insertion promoter sequence variants are associated with loss of BRM expression and lung cancer risk. Oncogene. 2011;30:3295–3304. doi: 10.1038/onc.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aldrich CJ, Waltrip R, Hermel E, Attaya M, Lindahl KF, Monaco JJ, Forman J. T cell recognition of QA-1b antigens on cells lacking a functional Tap-2 transporter. J. Immunol. 1992;149:3773–3777. [PubMed] [Google Scholar]
- 54.Sivakumar PV, Gunturi A, Salcedo M, Schatzle JD, Lai WC, Kurepa Z, Pitcher L, Seaman MS, Lemonnier FA, Bennett M, Forman J, Kumar V. Cutting edge: expression of functional CD94/NKG2A inhibitory receptors on fetal NK1.1+Ly-49-cells: a possible mechanism of tolerance during NK cell development. J. Immunol. 1999;162:6976–6980. [PubMed] [Google Scholar]
- 55.Davies A, Kalb S, Liang B, Aldrich CJ, Lemonnier FA, Jiang H, Cotter R, Soloski MJ. A peptide from heat shock protein 60 is the dominant peptide bound to Qa-1 in the absence of the MHC class Ia leader sequence peptide Qdm. J. Immunol. 2003;170:5027–5033. doi: 10.4049/jimmunol.170.10.5027. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y, Xiong Y, Naidenko OV, Liu JH, Zhang R, Joachimiak A, Kronenberg M, Cheroutre H, Reinherz EL, Wang JH. The crystal structure of a TL/CD8alphaalpha complex at 2.1 A resolution: implications for modulation of T cell activation and memory. Immunity. 2003;18:205–215. doi: 10.1016/s1074-7613(03)00027-x. [DOI] [PubMed] [Google Scholar]
- 57.Zeng L, Sullivan LC, Vivian JP, Walpole NG, Harpur CM, Rossjohn J, Clements CS, Brooks AG. A structural basis for antigen presentation by the MHC class Ib molecule, Qa-1b. J. Immunol. 2012;188:302–310. doi: 10.4049/jimmunol.1102379. [DOI] [PubMed] [Google Scholar]
- 58.Fahnestock ML, Tamir I, Narhi L, Bjorkman PJ. Thermal stability comparison of purified empty and peptide-filled forms of a class I MHC molecule. Science. 1992;258:1658–1662. doi: 10.1126/science.1360705. [DOI] [PubMed] [Google Scholar]
- 59.Bouvier M, Wiley DC. Importance of peptide amino and carboxyl termini to the stability of MHC class I molecules. Science. 1994;265:398–402. doi: 10.1126/science.8023162. [DOI] [PubMed] [Google Scholar]
- 60.Crowley MP, Reich Z, Mavaddat N, Altman JD, Chien Y. The recognition of the nonclassical major histocompatibility complex (MHC) class I molecule, T10, by the gammadelta T cell, G8. J. Exp. Med. 1997;185:1223–1230. doi: 10.1084/jem.185.7.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dedier S, Reinelt S, Reitinger T, Folkers G, Rognan D. Thermodynamic stability of HLA-B*2705. Peptide complexes. Effect of peptide and major histocompatibility complex protein mutations. J. Biol. Chem. 2000;275:27055–27061. doi: 10.1074/jbc.M002777200. [DOI] [PubMed] [Google Scholar]
- 62.Urdahl KB, Sun JC, Bevan MJ. Positive selection of MHC class Ib-restricted CD8(+) T cells on hematopoietic cells. Nat. Immunol. 2002;3:772–779. doi: 10.1038/ni814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J. Exp. Med. 1995;182:2091–2096. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Petrie EJ, Clements CS, Lin J, Sullivan LC, Johnson D, Huyton T, Heroux A, Hoare HL, Beddoe T, Reid HH, Wilce MC, Brooks AG, Rossjohn J. CD94-NKG2A recognition of human leukocyte antigen (HLA)-E bound to an HLA class I leader sequence. J. Exp. Med. 2008;205:725–735. doi: 10.1084/jem.20072525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller JD, Weber DA, Ibegbu C, Pohl J, Altman JD, Jensen PE. Analysis of HLA-E peptide-binding specificity and contact residues in bound peptide required for recognition by CD94/NKG2. J. Immunol. 2003;171:1369–1375. doi: 10.4049/jimmunol.171.3.1369. [DOI] [PubMed] [Google Scholar]
- 66.O’Callaghan CA, Tormo J, Willcox BE, Braud VM, Jakobsen BK, Stuart DI, McMichael AJ, Bell JI, Jones EY. Structural features impose tight peptide binding specificity in the nonclassical MHC molecule HLA-E. Mol. Cell. 1998;1:531–541. doi: 10.1016/s1097-2765(00)80053-2. [DOI] [PubMed] [Google Scholar]