Abstract
Objective
Relative to recent successes in elucidating the genetic mechanisms associated with complex diseases including macular degeneration, Type II diabetes, heart disease and cancer, molecular genetic approaches to psychiatric illness have met with more limited success. While factors such as small allelic effects, allelic heterogeneity and variation in population sub-structure have received considerable attention in attempt to explain the paucity of significant results in psychiatric genetics, significantly less focus has been directed towards phenotypic factors.
Method
Data derived from molecular genetic studies of the psychosis phenotype in patients with a range of psychiatric illnesses are reviewed.
Results
Available data suggest that genes do not respect the boundaries of the current diagnostic system but may confer risk for symptom-based phenotypic variation that traverses those boundaries.
Conclusions
Molecular genetic studies offer convincing evidence for a relation between genetic variation and symptom-based phenotypic variation within psychiatric illness. These data may provide novel insights into the pathophysiology of schizophrenia and other related disorders. The exploration of relationships between genetic variation and symptom variation that traverses traditional diagnostic boundaries may ultimately lead to more refined classification systems that more closely reflect the genetic etiology of psychiatric illness.
Keywords: genetics, psychosis, dimensional models
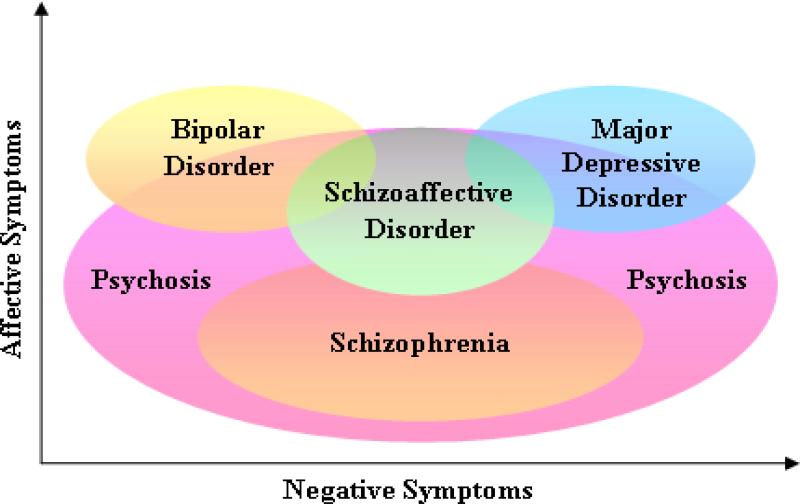
Despite its great utility for both research and practice in psychiatry, the conceptual basis of the Diagnostic and Statistical Manual of the American Psychiatric Association (DSM) remains controversial. The primary basis for this controversy has been the reliance on a categorical approach in which different diagnostic entities share common phenomenological features. This overlap in features associated with diagnostic categories is perhaps most prominent in regards to symptoms of psychosis. For example, psychotic phenomena including positive (hallucinations, delusions), negative (apathy, avolition, and asociality), and disorganized (disorganized speech or behavior) symptoms are the cornerstones of schizophrenia (SZ) but, as illustrated in Figure 1, are often observed across a broad spectrum of diagnoses outside of the nominal “psychotic disorders” category as defined by the current edition of the DSM. Because psychotic symptoms are not pathognomonic for a specific diagnostic category, classification of an individual experiencing these symptoms is based solely on aggregate clinical phenomena. Thus, an individual who presents with auditory hallucinations and delusional beliefs may be diagnosed with SZ, schizoaffective disorder (SAD), bipolar disorder (BPD) or major depressive disorder (MDD) depending upon the presence, course and severity of concomitant affective symptoms. Due to the limitations of the categorical approach, it is anticipated that the Fifth Edition of the DSM planned for publication in 2013, will include a dimensional approach for key aspects of psychopathology. The examination of such dimensional phenotypes would likely facilitate the identification of some of the molecular genetic mechanisms underlying these complex, syndromal diseases 1, 2, 3, 4, 5.
Figure 1.
Symptoms of psychosis are observed across multiple diagnostic categories. Psychosis is shown in the context of both affective and negative symptoms as usually manifested in the primary psychotic disorders (schizophrenia and schizoaffective disorder) and mood disorders (bipolar disorder and major depressive disorder). Also shown is the considerable degree of psychosis that is observed outside of these two major diagnostic categories. Such symptoms may be observed in other disorders and in non-clinical samples.
Heritability of psychosis
Initial support for a dimensional approach to elucidating the molecular genetic mechanisms associated with psychotic symptoms is derived from family studies demonstrating a partial overlap between the psychotic and affective disorders. Specifically, relatives of probands with SZ and BPD show some overlap in risk to the alternate disorder as well as an increased rate of both SAD and MDD relative to controls 6, 7. This overlap is even more pronounced when comparing psychotic affective illness and SZ. For example, the reanalysis of the Iowa 500 sample using DSM-III criteria found a significantly increased prevalence of psychotic affective illness in relatives of patients with SZ compared with controls 8. Similar findings were reported in the Roscommon family sample 9; affectively ill relatives of SZ probands were more likely to have psychotic affective illness than relatives of controls, and probands with psychotic affective illness were more likely to have relatives with SZ. Similarly, Erlenmeyer-Kimling and colleagues 10 found a significantly elevated prevalence of affective psychoses in the offspring of SZ probands compared with the offspring of control probands. Finally, Laursen et al. 7 demonstrated an increased risk of both BPD and SZ in relatives of patients with SAD. Taken together, these epidemiological data suggest considerable overlap in the heritability of psychiatric illness characterized by the presence of psychosis.
Family-based studies have also demonstrated considerable overlap in the heritability of psychotic symptom dimensions within diagnostic categories. For example symptom factors including positive, negative and affective symptoms 11, thought withdrawal, thought insertion, thought broadcasting and delusions of control 12 and disorganization 13, 14, 15 have been found to be significantly correlated in sibling pairs (including twins) concordant for psychotic illness. Moreover, a meta-analysis of family-based studies of BPD demonstrated statistically significant familial aggregation for the psychosis phenotype 5. Finally, subthreshold psychotic symptoms such as paranoid ideation, altered perceptual experiences, odd or magical thinking and social isolation are often observed in unaffected relatives of people with psychotic illness with family-based studies consistently indicating a moderate heritability of psychosis-related phenotypes even when diagnostic status is discordant 16. The convergence of these findings provides strong support for the notion that the psychosis dimension has a heritable component.
Despite the convincing evidence suggesting familial aggregation and heritability of psychosis phenotypes, relatively limited work has been conducted to identify the molecular underpinnings of this shared familial risk. In fact, the vast majority of research in psychiatric genetics has been predicated upon the assumption that psychiatric diagnoses are distinct disease entities with distinct etiologies. The overlap in diagnostic categories containing features of psychosis, however, suggests that psychiatric diagnoses are not distinct disease entities. It is therefore not surprising that considerable overlap in the molecular mechanisms associated with psychiatric disorders, particularly among the psychotic and affective disorders, has been repeatedly supported by linkage studies 5, candidate gene studies 17 and more recently, by genomewide association studies (GWAS) 18.
Evidence derived from genetic linkage studies
The linkage evidence in support of shared genetic risk factors for psychosis comes primarily from linkage studies of BPD and SZ. Initial evidence for overlap in the genetics of these disorders came from a large meta-analysis of SZ and BPD that indicated strong combined linkage evidence at several loci including 1p13.3-q23.3, 2q22.1-q23.3, 3p25.3-p22.1, 5q23.2-q34, 6pterp22.3, 6p22.3-p21.1, 8p22-p21.1, 11q22.3-q24.1, 14pter-q13.1, 20p12.3-p11, and 22pter-q12.3 19. Based on independent findings from multiple studies in both disorders other chromosomal regions including 1q42, 6q22, and 15q14 have also been shown to overlap in SZ and BPD 20. Follow up studies testing the hypothesis that BPD pedigrees enriched for psychotic symptoms would show increased evidence of linkage to chromosomal regions of prior overlap between BPD and SZ, demonstrated that there were at least four overlapping chromosomal regions that could harbor shared susceptibility genes. These included regions on 13q31, 22q12, 10p12-14 and 18p11.2 6. The psychotic BPD subtype was further studied in a genome-wide linkage analysis of 40 extended BP pedigrees with psychotic features; significant evidence was reported for linkage at 9q31 and 8p21, with suggestive evidence at 13q32, all of which had been previously implicated in SZ 21. Further, it should be noted that the region on chromosome 13q has also been linked to MDD 22. Unfortunately, of the few linkage studies that have been conducted on extended major depressive disorder pedigrees, none have focused on the psychosis phenotype within the broader diagnostic category. Finally, Fanous et al 23 used a genome scan correlation method to test for a genetic correlation between SZ and schizotypy The results of these analyses suggested that a subset of schizophrenia susceptibility genes also affects schizotypy in non-psychotic relatives.
Evidence derived from candidate gene studies
Consistent with the aforementioned linkage evidence, data from candidate gene studies also suggest considerable overlap in genetic susceptibility across diagnostic categories 17 and implicate several well known candidate genes including: DTNBP1, DISC1, NRG1, DAOA, COMT, RGS4 and others in shared risk for the psychotic illnesses across traditional DSM boundaries. Moreover, several of these candidate genes have now been linked to specific clinical profiles within, and in some cases across, diagnostic categories.
D-amino acid oxidase activator (DAOA/ G72;G30)
Chumakov and colleagues 24 first reported associations between SZ and markers in DAOA/G72 and G30, overlapping genes transcribed in opposite directions on chromosome 13q34, in a relatively large European cohort. Associations to SZ in this region are supported by at least two meta-analyses 25, 26, although there is no consensus concerning the specific risk alleles or haplotypes across studies. Schumacher and colleagues 27 reported an overlap in the association between SNPs within DAOA and risk for both SZ and BPD. Specifically, these authors assessed a sample of 599 patients (299 schizophrenic, 300 bipolar) and 300 controls and reported an association between SZ and several single nucleotide polymorphisms (SNPs) in a four-marker haplotype, with the most significant SNP, as well as the haplotype, also showing nominal association to BPD. Follow-up studies of DAOA have consistently replicated the association between DAOA and BPD and it is now considered one of the best supported candidate gene loci for BPD 17. Finally, Rietschel and colleagues 28 assessed the relation between DAOA and major depression in 500 patients and 1,030 population-based controls and reported a significant association at the same haplotype identified by Schumacher and colleagues. The convergence of these data suggest that variation at DAOA may be more closely linked to specific aspects of psychiatric illness that cross the diagnostic boundaries of the DSM-IV than to a specific diagnostic category.
Data also suggests that DAOA may be associated with specific phenotypes of psychiatric illness rather than a specific diagnostic group. Specifically, Shulze and colleagues 29 found that persecutory delusions were the only significant explanatory variable for a previously identified risk genotype shared in SZ and BPD. Moreover, in a study of SZ and BPD, Williams and colleagues 30 found that variation at DAOA was more significantly associated with major mood episodes across these disorders rather than categorical diagnoses. Finally, Corvin et al 31 assessed the relationship between a previously identified risk variant in DAOA and symptom factors derived from Positive and Negative Symptom Scale (PANSS) in a sample of patients with SZ and SAD. These authors found that carriers of the DAOA risk variant scored significantly higher on a depression and anxiety factor than non-carriers (p = 0.01). Again, these data suggest that DAOA may influence phenotypic variation that traverses the traditional DSM-IV categories.
Neuregulin 1 (NRG1)
NRG1 was first implicated in SZ after a systematic study of 8p21-22 revealed an association between a haplotype at the 5' end of NRG1 and SZ 32. Additional support for this association was later provided by findings in 2 separate case-control samples 33, 34. Moreover, a review of the NRG1 literature 35 concluded that despite some differences in the associated haplotypes, the evidence for an association between NRG1 and SZ is quite strong. Although NRG1 has not yet been extensively studied in bipolar disorder, at few studies 36, 37 found significant evidence for association between a SZ risk haplotype within NRG1 and BPD. Moreover, in the Green et al 36 study the strength of the association was substantially increased in BPD cases with predominantly mood-incongruent psychotic features, and SZ patients who had experienced mania. Goes et al 38 also reported findings suggesting that NRG1 may be specifically associated with the psychotic subset of BPD probands. Thus, these data suggest that NRG1 may play a role in susceptibility to both SZ and BPD and further suggest that it may exert a specific effect on phenotypic variation that crosses diagnostic boundaries.
Dysbindin (DTNBP1)
DTNBP1 was first identified by Straub and colleagues 39 as a susceptibility gene for SCZ in a follow-up analysis of their initial linkage findings on chromosome 6p. Using a family based association analysis including 270 families derived from the Irish Study of High Density Schizophrenia Families several SNPs within DTNBP1, alone or in combination, increased the risk for SZ. To date, several replication studies in different populations of European descent have been reported, and a comprehensive meta-analysis suggests that DTNBP1 is one of the most robust association signals in the literature 40. Although the data linking DTNBP1 variation to BPD are limited, Raybould and colleagues 41 found modestly significant evidence for association in a subset of bipolar cases with predominantly psychotic episodes. Moreover, Domshke et al 42 recently found modest evidence for an association between variants in DTNBP1 and psychotic MDD. Collectively, these findings suggests that variation in DTNBP1 may confer risk to the psychotic syndrome rather than a specific DSM-IV diagnosis.
Available data also suggests that DTNBP1 may be more closely related to a dimension of negative symptoms than a specific diagnostic category. Negative symptoms are a particularly attractive phenotype for genetic investigation, as they tend to be more stable across the duration of illness, may present prior to the onset of illness, and are often unaffected by antipsychotic drug treatment 43. Moreover, heritability studies of clinical symptomatology have indicated that affected relative pairs with schizophrenia may share similar severity of negative symptoms more often than expected by chance 11, indicating a substantial genetic component. Data from several groups suggest that negative symptoms may be influenced by DTNBP1 genotype. For example, Fanous and colleagues 44 assessed 755 subjects with psychotic illness in the Irish Study of High Density Schizophrenia Families with the Operational Criteria Checklist for Psychiatric Illness (OPCRIT) 45 with factor analyses revealing that an 8-locus schizophrenia risk haplotype was over-transmitted to subjects with greater negative symptom factor scores. These findings have been replicated by multiple groups using measures of negative symptoms including lifetime severity 46, 47 longitudinal course 48 and cross-sectional severity 49, 50, 51. Taken together, these independent studies suggest that DTNBP1 variants may influence negative symptom dimensions rather than a specific diagnostic category.
Disrupted in Schizophrenia 1 (DISC1):
The gene encoding DISC1 is located at the breakpoint of a balanced translocation between chromosomes 1 and 11, t(1;11)(q42;q14) that was originally identified in an extended psychiatric pedigree and was shown to co-segregate with multiple major psychiatric disorders including SZ, SAD, BPD and MDD 53. Genome scans 53, 54, 55 and candidate gene analyses 56, 57 have consistently linked DISC1 with risk for several psychiatric disorders including a report of multiple haplotypes within DISC1 associated with both affective and psychotic disorders 58. The considerable overlap in findings relating DISC1 to a broad spectrum of affective and psychotic disorders suggests that variation in DISC1 may confer risks that traverse diagnostic boundaries.
Initial support for the relation between variation in DISC1 and specific clinical profiles was provided by Hennah and colleagues 56. These researchers assessed 458 Finnish schizophrenia families and identified a single common, under-transmitted haplotype (HEP3) that significantly increased risk for the development of SZ. Additional analyses using more refined phenotypes that included factor scores derived the OPCRIT, however, indicated that the risk haplotype was more significantly associated with a specific clinical profile characterized by high loading on factor scores that included delusions and hallucinations, manic symptoms, depressive symptoms and negative symptoms. The strongest of these findings indicated association of DISC1 HEP3 to the delusions and hallucination factor and within this factor, the strongest association to HEP 3 was persecutory delusions (p < 0.00001). Follow up work in at least one additional patient sample has replicated these results suggesting that a known DISC1 SZ risk haplotype is more closely linked to lifetime measure of delusions 59. Perhaps more convincing however, is the finding that DISC1 may associate to dimensions of psychosis in a general population sample. Specifically, Tomppo et al 60 found that in a large, unselected birth cohort, variation at DISC1 was strongly associated with social anhedonia, a cardinal symptom of SZ. Collectively, these data suggest that DISC1 risk genotypes may be more closely related to dimensions of psychosis rather than a specific diagnostic category.
Catechol-O-methyltransferase (COMT)
COMT is an enzyme involved in the degradation of the neurotransmitter dopamine and has received considerable attention in molecular genetic studies of psychiatric populations. The gene encoding COMT is located in the region on chromosome 22q11 commonly deleted in velo-cardio-facial/DiGeorge syndrome (VCFS/DGS) whose phenotypic spectrum includes severe psychiatric disease that has been described as both SZ and BPD 61. To date, a plethora of candidate gene studies have found evidence for a link between variation in COMT and psychiatric illness and in contrast to other candidate gene studies, the SNPs and haplotypes implicated in the different diseases are strikingly similar. For example, Shifman and colleagues identified a single haplotype within COMT that increased risk for both SZ 62 and BPD 63. These data suggest that it is likely that the association between COMT genotype and psychiatric illness may be better characterized by assessing the effect of COMT genetic variation on the overlapping clinical profiles of psychiatric illness.
To date, much of the work relating variation in COMT to symptom dimensions within psychiatric samples has focused on its relation to affective symptoms 64, 65, 66. However, data derived from non-clinical samples suggest that variation at COMT may be associated with dimensions of psychosis. Stefanis and colleagues 67 first reported an association of the Val158Met allele of COMT to measures of schizotypy in a sample of 543 males derived from a non-clinical population. These authors reported a significant effect of the high-activity Val allele load on negative and disorganized dimensions of schizotypy as measured by the Schizotypal Personality Questionnaire (SPQ) 68. These data have been replicated by the same group in much larger sample comprised of 2,130 young males 69 as well as independent samples 70, 71. Moreover, Van Winkel and colleagues 72 recently tested for an association between variants of COMT and psychosis as measured by the Brief Psychiatric Rating Scale (BPRS) in unaffected relatives of SZ patients. These authors reported that sharing of the met allele of COMT between affected and unaffected relative pairs was a significant predictor of an association between the affected and unaffected's scores on the BPRS. These data suggest that genetic variation may be, at least in populations with a genetic predisposition to psychosis, related to the pathophysiology of psychosis.
Other candidate genes
The evidence reviewed above suggests that delineation of the role of specific genes on symptom dimensions, rather than diagnostic entities, may lead to more refined approaches and may suggest novel treatment targets for specific domains of illness. It should also be noted that in addition to the overlapping effects of the aforementioned risk genotypes, a number of studies have also demonstrated associations between symptom domains and variants within genes not associated with diagnostic categories. These include relations between negative symptoms and BDNF, DAT1 73 and HkCa3 74; positive symptoms and DRD4 75, DRD2 76, CCK-A 77 and 5-HTT 78; and disorganization with DRD2 76. Thus, specific genetic variants may act to modify 79 the clinical presentation of illness without increasing the risk for the illness.
Evidence derived from linkage using quantitative traits for symptom dimensions
An alternative approach to candidate gene studies is the use of genetic linkage approaches that have the capacity to detect novel loci located within relatively large chromosomal regions. To date, a very limited number of studies have used this approach. Results of these studies, however, have identified several regions on chromosomes 6 80, 81; 8 82, 81, 9, 12 81 and 20 81, 23 that are associated with dimensions of psychosis in SZ suggesting that several regions of the genome may harbor genes that influence the clinical features rather than diagnosis. Although some of the regions suggestive of linkage to these quantitative traits, including several loci on chromosomes 6 and 8, had previously been linked to SZ, the regions identified on chromosomes 9, 12 and 20 had not been previously linked to the disease. Thus, these data suggest that these regions on the genome may harbor genes that influence the clinical features of schizophrenia without increasing the risk for the illness and follow-up analyses have supported these findings. Specifically, our group 83 identified an association between positive psychotic symptoms is patients with SZ and a SNP in the gene encoding for the origin recognition complex (ORC3L) located within the linkage regions previously associated with positive symptoms 80. Moreover, we also identified and association between disorganization in patients with SZ and several SNPs in the gene encoding for brain specific angiogenesis inhibitor (BAI3) located within the linkage region previously associated with disorganization 81. Although preliminary, these data point towards specific candidate genes located within previously implicated linkage peaks for psychotic symptoms and provide additional support for the role of genetic variation in dimensional measures of psychosis.
Summary and future directions
Accumulating evidence supports the role of genetic factors in variation across the psychosis phenotype and may suggest an overlap in the pathophysiological mechanisms of psychotic illness. Such findings challenge the current concepts of disease classification and demonstrate the potential utility of a symptom-based, dimensional approach. Data derived from large-scale, collaborative genome-wide association studies (GWAS) in psychiatry, albeit quite limited, provide strong support for such an approach. Indeed, many of the genetic variants that have been identified in GWAS support what had previously been observed in both linkage and candidate gene studies; they suggest considerable overlap between diagnostic categories. For example, Williams et al 84 recently reported that of the eight robust associations that have been identified in GWAS studies for SZ or BPD, six of them show strong trans-disorder effects. Indeed, the largest psychiatric GWAS to date 85 recently reported that when SZ and BPD are analyzed jointly, the number of genome-wide significant loci increases with and enhancement of signals at loci previously shown to confer risk to both disorders. These data are consistent with data derived from the International Schizophrenia Consortium (ISC) that showed that common polygenic variation explained approximately 1/3 of the variation in liability to SZ and the same polygenic variation was substantially shared with BPD 86. Follow-up analyses using the same polygenic model in an independent sample demonstrated that the observed overlap may relate more closely to the sharing of a ‘schizophrenia-like’ phenotype 87. Given the clinical heterogeneity and molecular complexity of psychiatric illness it is unlikely that any single genetic paradigm will account for a large degree of the variance to be explained. However, the exploration of relationships between genetic variation and symptom variation that traverses traditional diagnostic boundaries may ultimately lead to more refined classification systems that more closely reflect the genetic etiology of psychiatric illness.
Clinical Implications.
The psychosis phenotype traverses traditional diagnostic boundaries
Studies seeking to assess the relationship between genetic variation and the psychosis phenotype may provide insight into the pathophysiological mechanisms of psychiatric illness.
Limitations
Despite its potential utility, there is a very limited amount of work that has been conducted to parse the specific effects of genetic variants on phenotypic factors that are shared amongst diagnostic groups.
Large-scale studies using genome-wide association, next-generation sequencing and other emerging analytic tools targeted at specific phenotypes are needed.
Acknowledgments
Funding/Support: This work was supported by grant MH086756 to PD.
References
- 1.Carpenter WT, Jr, Buchanan RW, Kirkpatrick B, Tamminga C, Wood F. Strong inference, theory testing, and the neuroanatomy of schizophrenia. Arch Gen Psychiatry. 1993;50(10):825–31. doi: 10.1001/archpsyc.1993.01820220081009. [DOI] [PubMed] [Google Scholar]
- 2.Potash JB, Willour VL, Chiu YF, Simpson SG, MacKinnon DF, Pearlson GD, DePaulo JR, Jr, McInnis MG. The familial aggregation of psychotic symptoms in bipolar disorder pedigrees. Am J Psychiatry. 2001;158(8):1258–1264. doi: 10.1176/appi.ajp.158.8.1258. [DOI] [PubMed] [Google Scholar]
- 3.Potash JB, Zandi PP, Willour VL, Lan TH, Huo Y, Avramopoulos D, Shugart YY, MacKinnon DF, Simpson SG, McMahon FJ, DePaulo JR, Jr, McInnis MG. Suggestive linkage to chromosomal regions 13q31 and 22q12 in families with psychotic bipolar disorder. Am J Psychiatry. 2003;160(4):680–686. doi: 10.1176/appi.ajp.160.4.680. [DOI] [PubMed] [Google Scholar]
- 4.Hamet P, Merlo E, Seda O, Broeckel U, Tremblay J, Kaldunski M, Gaudet D, Bouchard G, Deslauriers B, Gagnon F, Antoniol G, Pausova Z, Labuda M, Jomphe M, Gossard F, Tremblay G, Kirova R, Tonellato P, Orlov SN, Pintos J, Platko J, Hudson TJ, Rioux JD, Kotchen TA, Cowley AW., Jr Quantitative founder-effect analysis of French Canadian families identifies specific loci contributing to metabolic phenotypes of hypertension. Am J Hum Genet. 2005;76:815–832. doi: 10.1086/430133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Potash JB, Toolan J, Steele J, Miller EB, Pearl J, Zandi PP, Schulze TG, Kassem L, Simpson SG, Lopez V. NIMH Genetics Initiative Bipolar Disorder Consortium, Mackinnon DF, McMahon FJ: The bipolar disorder phenome database: a resource for genetic studies. Am J Psychiatry. 2007;164(8):1229–1237. doi: 10.1176/appi.ajp.2007.06122045. [DOI] [PubMed] [Google Scholar]
- 6.Berrettini W. Susceptibility loci for bipolar disorder: Overlap with inherited vulnerability to schizophrenia. Biol Psychiatry. 2000;47(3):245–51. doi: 10.1016/s0006-3223(99)00226-7. [DOI] [PubMed] [Google Scholar]
- 7.Laursen TM, Labouriau R, Licht RW, Bertelsen A, Munk-Olsen T, Mortensen PB. Family history of psychiatric illness as a risk factor for schizoaffective disorder: a Danish register-based cohort study. Arch Gen Psychiatry. 2005 Aug;62(8):841–8. doi: 10.1001/archpsyc.62.8.841. [DOI] [PubMed] [Google Scholar]
- 8.Kendler KS, Gruenberg AM, Tsuang MT. Psychiatric illness in first-degree relatives of schizophrenic and surgical control patients. A family study using DSM-III criteria. Arch Gen Psychiatry. 1985;42(8):770–9. doi: 10.1001/archpsyc.1985.01790310032004. [DOI] [PubMed] [Google Scholar]
- 9.Kendler KS, McGuire M, Gruenberg AM, O'Hare A, Spellman M, Walsh D. The Roscommon Family Study. IV. Affective illness, anxiety disorders, and alcoholism in relatives. Arch Gen Psychiatry. 1993;50(12):952–60. doi: 10.1001/archpsyc.1993.01820240036005. [DOI] [PubMed] [Google Scholar]
- 10.Erlenmeyer-Kimling L, Adamo UH, Rock D, Roberts SA, Bassett AS, Squires-Wheeler E, Cornblatt BA, Endicott J, Pape S, Gottesman II. The New York High-Risk Project. Prevalence and comorbidity of axis I disorders in offspring of schizophrenic parents at 25-year follow-up. Arch Gen Psychiatry. 1997;54(12):1096–102. doi: 10.1001/archpsyc.1997.01830240052008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kendler KS, Karkowski-Shuman L, O'Neill FA, Straub RE, MacLean CJ, Walsh D. Resemblance of psychotic symptoms and syndromes in affected sibling pairs from the Irish Study of High-Density Schizophrenia Families: evidence for possible etiologic heterogeneity. Am J Psychiatry. 1997;154(2):191–198. doi: 10.1176/ajp.154.2.191. [DOI] [PubMed] [Google Scholar]
- 12.Loftus J, Delisi LE, Crow TJ. Factor structure and familiality of first-rank symptoms in sibling pairs with schizophrenia and schizoaffective disorder. Br J Psychiatry. 2000;177:15–19. doi: 10.1192/bjp.177.1.15. [DOI] [PubMed] [Google Scholar]
- 13.Cardno AG, Jones LA, Murphy KC, Sanders RD, Asherson P, Owen MJ, McGuffin P. Dimensions of psychosis in affected sibling pairs. Schizophr Bull. 1999;25(4):841–850. doi: 10.1093/oxfordjournals.schbul.a033423. [DOI] [PubMed] [Google Scholar]
- 14.Cardno AG, Sham PC, Murray RM, McGuffin P. Twin study of symptom dimensions in psychoses. Br J Psychiatry. 2001;179:39–45. doi: 10.1192/bjp.179.1.39. [DOI] [PubMed] [Google Scholar]
- 15.Rijsdijk FV, Gottesman II, McGuffin P, Cardno AG. Heritability estimates for psychotic symptom dimensions in twins with psychotic disorders. Am J Med Genet B Neuropsychiatr Genet. 2010;156B(1):89–98. doi: 10.1002/ajmg.b.31145. [DOI] [PubMed] [Google Scholar]
- 16.Krabbendam L, Isusi P, Galdos P, Echevarria E, Bibao JR, Martin-Pagola A, Papiol S, Castano L, Van Os J. Associations between COMTVal158Met polymorphism and cognition: direct or indirect effects? Eur Psychiatry. 2006;21(5):338–42. doi: 10.1016/j.eurpsy.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Owen MJ, Craddock N, Jablenski A. The genetic deconstruction of psychosis. Schizophr Bull. 2007;33(4):905–11. doi: 10.1093/schbul/sbm053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams HJ, Owen MJ, O'Donovan MC. Schizophrenia genetics: new insights from new approaches. Br Med Bull. 2009;91:61–74. doi: 10.1093/bmb/ldp017. [DOI] [PubMed] [Google Scholar]
- 19.Lewis CM, Levinson DF, Wise LH, DeLisi LE, Straub RE, Hovatta I, Williams NM, Schwab SG, Pulver AE, Faraone SV, Brzustowicz LM, Kaufmann CA, Garver DL, Gurling HM, Lindholm E, Coon H, Moises HW, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. Am J Hum Genet. 2003;73(1):34–48. doi: 10.1086/376549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potash JB. Carving Chaos: genetics and the classification of mood and psychotic syndromes. Harv Rev Psychiatry. 2006;14(2):47–63. doi: 10.1080/10673220600655780. [DOI] [PubMed] [Google Scholar]
- 21.Park N, Juo SH, Cheng R, Liu J, Loth JE, Lilliston B, Nee J, Grunn A, Kanyas K, Lerer B, Endicott J, Gilliam TC, Baron M. Linkage analysis of psychosis in bipolar pedigrees suggests novel putative loci for bipolar disorder and shared susceptibility with schizophrenia. Mol Psychiatry. 2004;9(12):1091–9. doi: 10.1038/sj.mp.4001541. [DOI] [PubMed] [Google Scholar]
- 22.McGuffin P, Knight J, Breen G, Brewster S, Boyd PR, Craddock N, Gill M, Korszun A, Maier W, Middleton L, Mors O, Owen MJ, Perry J, Preisig M, Reich T, Rice J, Rietschel M, Jones L, Sham P, Farmer AE. Whole genome linkage scan of recurrent depressive disorder from the depression network study. Hum Mol Genet. 2005;14(22):3337–45. doi: 10.1093/hmg/ddi363. [DOI] [PubMed] [Google Scholar]
- 23.Fanous AH, Neale MC, Gardner CO, Webb BT, Straub RE, O'Neill FA, Walsh D, Riley BP, Kendler KS. Significant correlation in linkage signals from genome-wide scans of schizophrenia and schizotypy. Mol Psychiatry. 2007;12(10):958–65. doi: 10.1038/sj.mp.4001996. [DOI] [PubMed] [Google Scholar]
- 24.Chumakov I, Blumenfield M, Guerassimenko O, Cavarec L, Palicio M, Abderrahim H, Bougueleret L, Barry C, Tanaka H, La Rosa P, Puech A, Tahri N, Cohen-Akenine A, Delabrosse S, Lissarrague S, Picard FP, Maurice K, Essioux L, Millasseau P, Grel P, Debailleul V, Simon AM, Caterina D, Dufaure I, Malekzadeh K, Belova M, Luan JJ, Bouillot M, Sambucy JL, et al. Genetic and physiologic data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. Pro Natl Acad Sci USA. 2002;99(21):13675–80. doi: 10.1073/pnas.182412499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Detera-Wadleigh SD, McMahon FJ. G72/G30 in schizophrenia and bipolar disorder: Review and meta-analysis. Biol Psychiatry. 2006;60(2):106–14. doi: 10.1016/j.biopsych.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 26.Müller DJ, Zai CC, Shinkai T, Strauss J, Kennedy JL. Association between the DAOA/G72 gene and bipolar disorder and meta-analyses in bipolar disorder and schizophrenia. Bipolar Disord. 2011;13(2):198–207. doi: 10.1111/j.1399-5618.2011.00905.x. [DOI] [PubMed] [Google Scholar]
- 27.Schumacher J, Jamra RA, Freudenberg J, Becker T, Ohlraun S, Otte AC, Tullius M, Kovalenko S, Bogaert AV, Maier W, Rietschel M, Propping P, Nöthen MM, Cichon S. Examination of g72 and D-amino-acid oxidase as genetic risk factors for schizophrenia and bipolar affective disorder. Mol Psychiatry. 2004;9(2):203–7. doi: 10.1038/sj.mp.4001421. [DOI] [PubMed] [Google Scholar]
- 28.Rietschel M, Beckmann L, Strohmaier J, Georgi A, Karpushova A, Schirmbeck F, Boesshenz KV, Schmäl C, Bürger C, Jamra RA, Schumacher J, Höfels S, Kumsta R, Entringer S, Krug A, Markov V, Maier W, Propping P, Wüst S, Kircher T, Nöthen MM, Cichon S, Schulze TG. G72 and its association with major depression and neuroticism in large population-based groups from Germany. Am J Psychiatry. 2008;165(6):753–62. doi: 10.1176/appi.ajp.2008.07060883. [DOI] [PubMed] [Google Scholar]
- 29.Schulze TG, Ohlraun S, Czerski PM, Schumacher J, Kassem L, Deschner M, Gross M, Tullius M, Heidmann V, Kovalenko S, Jamra RA, Becker T, Leszczynska-Rodziewicz A, Hauser J, Illig T, Klopp N, Wellek S, Cichon S, Henn FA, McMahon FJ, Maier W, Propping P, Nöthen MM, Rietschel M. Genotype phenotype studies in bipolar disorder showing association between the DAOA/G30 locus and persecutory delusions: a first step toward a molecular genetic classification of psychiatric phenotypes. Am J Psychiatry. 2005;162(11):2101–8. doi: 10.1176/appi.ajp.162.11.2101. [DOI] [PubMed] [Google Scholar]
- 30.Williams NM, Green EK, Macgregor S, Dwyer S, Norton N, Williams H, Raybould R, Grozeva D, Hamshere M, Zammit S, Jones L, Cardno A, Kirov G, Jones I, O'Donovan MC, Own MJ, Craddock N. Variation at the DAOA/G30 locus influences susceptibility to major mood episodes but not psychosis in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2006;63(4):366–73. doi: 10.1001/archpsyc.63.4.366. [DOI] [PubMed] [Google Scholar]
- 31.Corvin A, Donohoe G, McGhee K, Murphy K, Kenny N, Schwaiger S, Nangle JM, Morris D, Gill M. D-amino acid oxidase (DAO) genotype and mood symptomatology in schizophrenia. 2007;426(2):97–100. doi: 10.1016/j.neulet.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, Hjaltason O, Birgisdottir B, Jonsson H, Gudnadottir VG, Gudmundsdottir E, Bjornsson A, Ingvarsson B, Ingason A, Sigfusson S, Hardardottir H, Harvey RP, Lai D, Zhou M, Brunner D, Mutel V, Gonzalo A, Lemke G, Sainz J, Johannesson G, Andresson T, Gudbjartsson D, Manolescu A, Frigge ML, Gurney ME, Kong A, Gulcher JR, Petursson H, Stefansson K. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71(4):877–92. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stefansson H, Sarginson J, Kong A, Yates P, Steinthorsdottir V, Gudfinnsson E, Gunnarsdottir S, Walker N, Petursson H, Crombie C, Ingason A, Gulcher JR, Stefansson K, St Clair D. Association of neuregulin 1 with schizophrenia confirmed in a Scottish population. Am J Hum Genet. 2003;72(1):83–7. doi: 10.1086/345442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams NM, Preece A, Spurlock G, Norton N, Williams HJ, Zammit S, O'Donovan MC, Owen MJ. Support for genetic variation in neuregulin 1 and susceptibility to schizophrenia. Mol Psychiatry. 2003;8(5):485–7. doi: 10.1038/sj.mp.4001348. [DOI] [PubMed] [Google Scholar]
- 35.Tosato S, Dazzan P, Collier D. Association between the neuregulin 1 gene and schizophrenia: a systematic review. Schizophr Bull. 2005;31(3):613–7. doi: 10.1093/schbul/sbi043. [DOI] [PubMed] [Google Scholar]
- 36.Green EK, Raybould R, Macgregor S, Gordon-Smith K, Heron J, Hyde S, Grozeva D, Hamshere M, Williams N, Owen MJ, O'Donovan MC, Jones L, Jones I, Kirov G, Craddock N. Operation of the schizophrenia susceptibility gene, neuregulin 1, across traditional diagnostic boundaries to increase risk for bipolar disorder. Arch Gen Psychiatry. 2005;62(6):642–8. doi: 10.1001/archpsyc.62.6.642. [DOI] [PubMed] [Google Scholar]
- 37.Walker RM, Christoforou A, Thomson PA, McGhee KA, Maclean A, Mühleisen TW, Strohmaier J, Nieratschker V, Nöthen MM, Rietschel M, Cichon S, Morris SW, Jilani O, Stclair D, Blackwood DH, Muir WJ, Porteous DJ, Evans KL. Association analysis of Neuregulin 1 candidate regions in schizophrenia and bipolar disorder. Neurosci Lett. 2010;478(1):9–13. doi: 10.1016/j.neulet.2010.04.056. [DOI] [PubMed] [Google Scholar]
- 38.Goes FS, Willour VL, Zandi PP, Belmonte PL, MacKinnon DF, Mondimore FM, Schweizer B, Gershon ES, McMahon FJ, Potash JB, Bipolar Disorder Phenome Group NIMH Genetics Initiative Bipolar Disorder Consortium. Family-based association study of Neuregulin 1 with psychotic bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(5):693–702. doi: 10.1002/ajmg.b.30895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Straub RE, Jiang Y, MacLean CJ, Ma Y, Webb BT, Myakishev MV, Harris-Kerr C, Wormley B, Sadek H, Kadambi B, Cesare AJ, Gibberman A, Wang X, O'Neill FA, Walsh D, Kendler KS. Genetic variation in the 6p22.3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. Am J Hum Genet. 2002;71(2):337–48. doi: 10.1086/341750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen NC, Bagade S, McQueen MB, Ioannidis JP, Kavvoura FK, Khoury MJ, Tanzi RE, Bertram L. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. 2008;40(7):827–34. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- 41.Raybould R, Green EK, MacGregor S, Gordon-Smith K, Heron J, Hyde S, Caesar S, Nikolov I, Williams N, Jones L, O'Donovan MC, Owen MJ, Jones I, Kirov G, Craddock N. Bipolar disorder and polymorphisms in the dysbindin gene (DTNBP1). Biol Psychiatry. 2005;57(7):696–701. doi: 10.1016/j.biopsych.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 42.Domschke K, Lawford B, Young R, Voisey J, Morris CP, Roehrs T, Hohoff C, Birosova E, Arolt V, Baune BT. Dysbindin (DTNBP1)—a role in psychotic depression? J Psychiatry Research. 2011;45(5):588–95. doi: 10.1016/j.jpsychires.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 43.Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophr Bull. 2007;33(1):69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fanous AH, van den Oord EJ, Riley BP, Aggen SH, Neale MC, O'Neill FA, Walsh D, Kendler KS. Relationship between a high-risk haplotype in the DTNBP1 (Dysbindin) gene and clinical features of schizophrenia. Am J Psychiatry. 2005;162:1824–32. doi: 10.1176/appi.ajp.162.10.1824. [DOI] [PubMed] [Google Scholar]
- 45.McGuffin P, Farmer A, Harvey I. A polydiagnostic application of operational criteria in studies of psychotic illness. Development and reliability of the OPCRIT system. Arch Gen Psychiatry. 1991;48(8):764–70. doi: 10.1001/archpsyc.1991.01810320088015. [DOI] [PubMed] [Google Scholar]
- 46.DeRosse P, Funke B, Burdick KE, Lencz T, Ekholm JM, Kane JM, Kucherlapati R, Malhotra AK. Dysbindin genotype and negative symptoms in schizophrenia. Am J Psychiatry. 2006;163(3):532–4. doi: 10.1176/appi.ajp.163.3.532. [DOI] [PubMed] [Google Scholar]
- 47.Wessman J, Paunio T, Tuulio-Henriksson A, Koivisto M, Partonen T, Suvisaari J, Turunen JA, Wedenoja J, Hennah W, Pietiläinen OP, Lönnqvist J, Mannila H, Peltonen L. Mixture model clustering of phenotype features reveals evidence for association of DTNBP1 to a specific subtype of schizophrenia. Biol Psychiatry. 2009;66(11):990–6. doi: 10.1016/j.biopsych.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 48.Tosato S, Ruggeri M, Bonetto C, Bertani M, Marrella G, Lasalvia A, Cristofalo D, Aprili G, Tansella M, Dazzan P, Diforti M, Murray RM, Collier DA. Association study of dysbindin gene with clinical and outcome measures in a representative cohort of Italian schizophrenic patients. Am J Med Genet B Neuropsychiatr Genet. 2007;144(5):647–59. doi: 10.1002/ajmg.b.30484. [DOI] [PubMed] [Google Scholar]
- 49.Corvin A, Donohoe G, Nangle JM, Schwaiger S, Morris D, Gill M. A dysbindin risk haplotype associated with less severe manic-type symptoms in psychosis. Neurosci Lett. 2008;431(2):146–9. doi: 10.1016/j.neulet.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 50.Wirgenes KV, Djurovic S, Agartz I, Jonsson EG, Werge T, Melle I, Andreassen OA. Dysbindin and d-amino-acid-oxidase gene polymorphisms associated with positive and negative symptoms in schizophrenia. Neuropsychobiology. 2009;60(1):31–6. doi: 10.1159/000235799. [DOI] [PubMed] [Google Scholar]
- 51.Réthelyi JM, Bakker SC, Polgár P, Czobor P, Strengman E, Pásztor PI, Kahn RS, Bitter I. Association study of NRG1, DTNBP1, RGS4, G72/G30, and PIP5K2A with schizophrenia and symptom severity in a Hungarian sample. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(3):792–801. doi: 10.1002/ajmg.b.31049. [DOI] [PubMed] [Google Scholar]
- 52.St Clair D, Blackwood D, Muir W, Carothers A, Walker M, Spowart G, Gosden C, Evans HJ. Association within a family of a balanced autosomal translocation with major mental-illness. Lancet. 1990;336:13–16. doi: 10.1016/0140-6736(90)91520-k. [DOI] [PubMed] [Google Scholar]
- 53.Ekelund J, Hovatta I, Parker A, Paunio T, Varilo T, Martin R, Suhonen J, Ellonen P, Chan G, Sinsheimer JS, Sobel E, Juvonen H, Arajärvi R, Partonen T, Suvisaari J, Lönnqvist J, Meyer J, Peltonen L. Chromosome 1 loci in Finnish schizophrenia families. Hum Mol Genet. 2001;10(15):1611–7. doi: 10.1093/hmg/10.15.1611. [DOI] [PubMed] [Google Scholar]
- 54.Ekelund J, Hennah W, Hiekkalinna T, Parker A, Meyer J, Lönnqvist J, Peltonen L. Replication of 1q42 linkage in Finnish schizophrenia pedigrees. Mol Psychiatry. 2004;9(11):1037–41. doi: 10.1038/sj.mp.4001536. [DOI] [PubMed] [Google Scholar]
- 55.Hamshere ML, Bennett P, Williams N, Segurado R, Cardno A, Norton N, Lambert D, Williams H, Kirov G, Corvin A, Holmans P, Jones L, Jones I, Gill M, O'Donovan MC, Owen MJ, Craddock N. Genomewide linkage scan in schizoaffective disorder: significant evidence for linkage at 1q42 close to DISC1, and suggestive evidence at 22q11 and 19q13. Arch Gen Psychiatry. 2005;62(10):1081–8. doi: 10.1001/archpsyc.62.10.1081. [DOI] [PubMed] [Google Scholar]
- 56.Hennah W, Varilo T, Kestila M, Paunio T, Arajarvi R, Haukka J, Parker A, Martin R, Levitzky S, Partonen T, Meyer J, Lonnqvist J, Peltonen L, Ekelund J. Haplotype transmission analysis provides evidence of association for DISC1 to schizophrenia and suggests sex-dependent effects. Hum Mol Genet. 2003;12(23):3151–9. doi: 10.1093/hmg/ddg341. [DOI] [PubMed] [Google Scholar]
- 57.Thomson PA, Harris SE, Starr JM, Whalley LJ, Porteous DJ, Deary IJ. Association between genotype at an exonic SNP in DISC1 and normal cognitive aging. Neurosci Lett. 2005;389(1):41–5. doi: 10.1016/j.neulet.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 58.Hodgkinson CA, Goldman D, Jaeger J, Persaud S, Kane JM, Lipsky RH, Malhotra AK. Disrupted in schizophrenia 1 (DISC1): Association with schizophrenia, schizoaffective disorder, and bipolar disorder. Am J Hum Genet. 2004;75:862–872. doi: 10.1086/425586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeRosse P, Hodgkinson CA, Lencz T, Burdick KE, Kane JM, Goldman D, Malhotra AK. Disrupted in schizophrenia 1 genotype and positive symptoms in schizophrenia. Biol Psychiatry. 2007;61:1208–10. doi: 10.1016/j.biopsych.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 60.Tomppo L, Hennah W, Miettunen J, Jarvelin MR, Veijola J, Ripatti S, Lahermo P, Lichtermann D, Peltonen L, Ekelund J. Association of variants in DISC1 with psychosis-related traits in a large population cohort. Arch Gen Psychiatry. 2009;66(2):134–41. doi: 10.1001/archgenpsychiatry.2008.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Funke B, Malhotra AK, Finn CT, Plocik AM, Lake SL, Lencz T, DeRosse P, Kane J, Kucherlapati R. COMT genetic variation confers risk for psychotic and affective disorders: a case control study. Behav Brain Funct. 2005;1:19. doi: 10.1186/1744-9081-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shifman S, Bronstein M, Sternfeld M, Pisante-Shalom A, Lev-Lehman E, Weizman A, Reznik I, Spivak B, Grisaru N, Karp L, Schiffer R, Kotler M, Strous RD, Swartz-Vanetik M, Knobler HY, Shinar E, Beckmann JS, Yakir B, Risch N, Zak NB, Darvasi A. A highly significant association between a COMT haplotype and schizophrenia. Am J Hum Genet. 2002;7(6):1296–1302. doi: 10.1086/344514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shifman S, Bronstein M, Sternfeld M, Pisante A, Weizman A, Reznik I, Spivak B, Grisaru N, Karp L, Schiffer R, Kotler M, Strous RD, Swartz-Vanetik M, Knobler HY, Shinar E, Yakir B, Zak NB, Darvasi A. COMT: a common susceptibility gene in bipolar disorder and schizophrenia. Am J Med Genet B. 2004;128(1):61–64. doi: 10.1002/ajmg.b.30032. [DOI] [PubMed] [Google Scholar]
- 64.DeRosse P, Hodgkinson CA, Lencz T, Burdick KE, Kane JM, Goldman D, Malhotra AK. Disrupted in schizophrenia 1 genotype and positive symptoms in schizophrenia. Biol Psychiatry. 2007;61:1208–10. doi: 10.1016/j.biopsych.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 65.Goghari VM, Sponheim SR. Differential association of the COMT Val158Met polymorphism with clinical phenotypes in schizophrenia and bipolar disorder. Schizophr Res. 2008;103(1-3):186–91. doi: 10.1016/j.schres.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benedetti F, Dallaspezia S, Locatelli C, Radaelli D, Poletti S, Lorenzi C, Pirovano A, Colombo C, Smeraldi E. Recurrence of bipolar mania is associated with catechol-O-methyltransferase Val(108/158)Met polymorphism. J Affect Disord. 2011;132(1-2):293–6. doi: 10.1016/j.jad.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 67.Stefanis NC, Van Os J, Avramopoulos D, Smyrnis N, Evdokimidis I, Hantoumi I, Stefanis CN. Variation in catechol-o-methyltransferase val158 met genotype associated with schizotypy but not cognition: a population study in 543 young men. Biol Psychiatry. 2004;56(7):510–5. doi: 10.1016/j.biopsych.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 68.Raine A. The Schizotypal Personality Questionnaire (SPQ) : A measure of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17:555–564. doi: 10.1093/schbul/17.4.555. [DOI] [PubMed] [Google Scholar]
- 69.Smyrnis N, Avramopoulos D, Evdokimidis I, Stefanis CN, Tsekou H, Stefanis NC. Effect of schizotypy on cognitive performance and its tuning by COMT val158 met genotype variations in a large population of young men. Biol Psychiatry. 2007;61(7):845–53. doi: 10.1016/j.biopsych.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 70.Avramopoulos D, Stefanis NC, Hantoumi I, Smyrnis N, Evdokimidis I, Stefanis CN. Higher scores of self reported schizotypy in healthy young males carrying the COMT high activity allele. Mol Psychiatry. 2002;7(7):706–11. doi: 10.1038/sj.mp.4001070. [DOI] [PubMed] [Google Scholar]
- 71.Schurhoff F, Szöke A, Chevalier F, Roy I, Meary A, Bellivier F, Giros B, Lebover M. Schizotypal dimensions: an intermediate phenotype associated with the COMT high activity allele. Am J Med Genet B Neuropsychiatr Genet. 2007;144.B(1):64–8. doi: 10.1002/ajmg.b.30395. [DOI] [PubMed] [Google Scholar]
- 72.van Winkel R, Isusi P, Galdos P, Echevarria E, Bilbao JR, Martin-Pagola A, Castaño L, Papiol S, Mengelers R, Krabbendam L, van Os J, Myin-Germeys I. Evidence that the COMTVal158Met polymorphism moderates subclinical psychotic and affective symptoms in unaffected first-degree relatives of patients with schizophrenia. Eur Psychiatry. 2008;23(3):219–22. doi: 10.1016/j.eurpsy.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 73.Fanous AH, Neale MC, Straub RE, Webb BT, O'Neill AF, Walsh D, Kendler KS. Clinical features of psychotic disorders and polymorphisms in HT2A, DRD2, DRD4, SLC6A3, (DAT1), and BDNF: a family based association study. Am J Med Genet B Neuropsychiatr Genet. 2004;125B(1):69–78. doi: 10.1002/ajmg.b.20103. [DOI] [PubMed] [Google Scholar]
- 74.Cardno AG, Bowen T, Guy CA, Jones LA, McCarthy G, Williams NM, Murphy KC, Spurlock G, Gray M, Sanders RD, Craddock N, McGuffin P, Owen MJ, O'Donovan MC. CAG repeat length in the hKCa3 gene and symptom dimensions in schizophrenia. Biol Psychiatry. 1999;45(12):1592–6. doi: 10.1016/s0006-3223(99)00033-5. [DOI] [PubMed] [Google Scholar]
- 75.Serretti A, Lilli R, Lorenzi C, Lattuada E, Smeraldi E. DRD4 exon 3 variants associated with delusional symptomatology in major psychoses: a study on 2,011 affected subjects. Am J Med Genet. 2001;105(3):283–90. doi: 10.1002/ajmg.1321. [DOI] [PubMed] [Google Scholar]
- 76.Serretti A, Lattuada E, Lorenzi C, Lilli R, Smeraldi E. Dopamine receptor D2 Ser/Cys 311 variant is associated with delusion and disorganization symptomatology in major psychoses. Mol Psychiatry. 2000;5(3):270–4. doi: 10.1038/sj.mp.4000726. [DOI] [PubMed] [Google Scholar]
- 77.Zhang XY, Zhou DF, Zhang PY, Wei J. The CCK-A receptor gene possibly associated with positive symptoms of schizophrenia. Mol Psychiatry. 2000;5(3):239–240. doi: 10.1038/sj.mp.4000677. [DOI] [PubMed] [Google Scholar]
- 78.Malhotra AK, Goldman D, Mazzanti C, Clifton A, Breier A, Pickar D. A functional serotonin transporter (5-HTT) polymorphism is associated with psychosis in neuroleptic-free schizophrenics (1998). Mol Psychiatry. 1998;3(4):328–32. doi: 10.1038/sj.mp.4000412. [DOI] [PubMed] [Google Scholar]
- 79.Fanous AH, Kendler KS. Genetic heterogeneity, modifier genes, and quantitative phenotypes in psychiatric illness: searching for a framework. Mol Psychiatry. 2005;10(1):6–13. doi: 10.1038/sj.mp.4001571. [DOI] [PubMed] [Google Scholar]
- 80.Brzustowicz LM, Honer WG, Chow EW, Hogan J, Hodgkinson K, Bassett AS. Use of a quantitative trait to map a locus associated with severity of positive symptoms in familial schizophrenia to chromosome 6p. Am J Hum Genet. 1997;61(6):1388–1396. doi: 10.1086/301623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilcox MA, Faraone SV, Su J, Van Eerdewegh P, Tsuang MT. Genome scan of three quantitative traits in schizophrenia pedigrees. Biol Psychiatry. 2002;52(9):847–854. doi: 10.1016/s0006-3223(02)01465-8. [DOI] [PubMed] [Google Scholar]
- 82.Kendler KS, Myers JM, O'Neill FA, Martin R, Murphy B, MacLean CJ, Walsh D, Straub RE. Clinical features of schizophrenia and linkage to chromosomes 5q, 6.p, 8p, and 10p in the Irish Study of High-Density Schizophrenia Families. Am J Psychiatry. 2000;157(3):402–408. doi: 10.1176/appi.ajp.157.3.402. [DOI] [PubMed] [Google Scholar]
- 83.DeRosse P, Lencz T, Burdick KE, Kane JM, Malhotra AK. The Genetics of Symptom-Based Phenotypes: Towards a Molecular Classification of Schizophrenia. Schizophr Bull. 2008;34(6):1047–53. doi: 10.1093/schbul/sbn076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Williams HJ, Craddock N, Russo G, Hamshere ML, Moskvina V, Dwyer S, Smith RL, Green E, Grozeva D, Holmans P, Owen MJ, O'Donovan MC. Most genome-wide significant susceptibility loci for schizophrenia and bipolar disorder reported to date cross-traditional diagnostic boundaries. Hum Mol Genet. 2011;20(2):387–91. doi: 10.1093/hmg/ddq471. [DOI] [PubMed] [Google Scholar]
- 85.Ripke S, Sanders AR, Kendler KS, Levinson DF, Sklar P. Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43(10):969–76. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, Sullivan PF, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–52. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hamshere ML, O'Donovan MC, Jones IR, Jones L, Kirov G, Green EK, Moskvina V, Grozeva D, Bass N, McQuillin A, Gurling H, St Clair D, Young AH, Ferrier IN, Farmer A, McGuffin P, Sklar P, Purcell S, Holmans PA, Owen MJ, Craddock N. Polygenic dissection of the bipolar phenotype. Br J Psychiatry. 2011;198:284–8. doi: 10.1192/bjp.bp.110.087866. [DOI] [PMC free article] [PubMed] [Google Scholar]