Abstract
Objective
To demonstrate the feasibility of using a computerized system to detect the onset of a seizure and, in response, initiate Vagus nerve stimulation (VNS) in patients with medically refractory epilepsy.
Methods
We designed and built a non-invasive, computerized system that automatically initiates VNS following the real-time detection of a pre-identified seizure or epileptiform discharge. The system detects these events through patient-specific analysis of the scalp electroencephalogram (EEG) and electrocardiogram (ECG) signals.
Results
We evaluated the performance of the system on 5 patients (A–E). For patients A and B the computerized system initiated VNS in response to seizures; for patients C and D the system initiated VNS in response to epileptiform discharges; and for patient E neither seizures nor epileptiform discharges were observed during the evaluation period. During the 81 hour clinical test of the system on patient A, the computerized system detected 5/5 seizures and initiated VNS within 5 seconds of the appearance of ictal discharges in the EEG; VNS did not seem to alter the electrographic or behavioral characteristics of the seizures in this case. During the same testing session the computerized system initiated false stimulations at the rate of 1 false stimulus every 2.5 hours while the subject was at rest and not ambulating. During the 26 hour clinical test of the system on patient B, the computerized system detected 1/1 seizures and initiated VNS within 16 seconds of the appearance of ictal discharges; VNS did not alter the electrographic duration of the seizure but decreased anxiety and increased awareness during the post-seizure recovery phase. During the same testing session the computerized system did not declare any false detections.
Significance
Initiating Vagus nerve stimulation soon after the onset of a seizure may abort or ameliorate seizure symptoms in some patients; unfortunately, a significant number of patients cannot initiate VNS by themselves following the start of a seizure. A system that automatically couples automated detection of seizure onset to initiation of VNS may be helpful for seizure treatment.
Keywords: Seizure detection, Vagus nerve stimulation
1. Introduction
Epilepsy is a disorder of the central nervous system that predisposes individuals to experiencing recurrent seizures. Despite recent advances in the medical and surgical management of epilepsy more than 20% of individuals with epilepsy never achieve adequate control of their seizures. For this group of patients alternative treatment options such as neurostimulation are considered. One such therapy, Vagus nerve stimulation (VNS), has been in use since 1997.1–4
Vagus nerve stimulation therapy is delivered in two modes. In automatic mode VNS, the implanted pulse-generator automatically delivers vagus nerve stimuli at programmed intervals. In on-demand mode VNS, the patient or their caregiver initiates Vagus nerve stimulation in response to symptoms of a seizure; initiating on-demand stimulation requires holding a permanent magnet for 1–2 seconds over the implanted pulse generator. The VNS pulse generator is implanted in an infraclavicular, subcutaneous pocket and delivers stimuli to the midcervical portion of the left Vagus nerve.5
Several clinical trials have been conducted in order to assess the therapeutic efficacy of automatic mode Vagus nerve stimulation. The E03 and E05 trials demonstrated that automatic mode VNS reduces seizure frequency by more than 50% in more than 20% of patients within 3 months of device implantation.6,7 The XE5 trial further demonstrated that the efficacy of automatic Vagus nerve stimulation is both long-lasting and improves significantly with time.8 Based on these and other studies, Vagus nerve stimulation was deemed a safe and effective adjunctive therapy for intractable epilepsy.
Clinical studies designed to quantify the additional therapeutic impact of on-demand mode Vagus nerve stimulation have also been conducted. Hammond9 recorded an EEG tracing illustrating the abrupt termination of an electrographic and clinically-apparent seizure following the initiation of on-demand VNS. Morris10 retrospectively analyzed seizure diaries from the E04 trial11 and noted that 53% of patients capable of receiving on-demand stimulation reported experiencing seizure termination or diminution. Similarly Boon12 noted that two-thirds of patients receiving on-demand stimulation reported being able to interrupt seizures.
A significant proportion of VNS therapy patients depend on others to initiate on-demand stimulation following the onset of the first clinical signs of a seizure.12 Depending on a caregiver to initiate on-demand stimulation has two consequences: (1) It burdens caregivers and denies patients the therapeutic benefit of on-demand stimulation in the absence of caregivers, and (2) It leads to an inconsistent ability to terminate or attenuate seizures since caregivers, when present, may not always be able to initiate on-demand stimulation immediately upon the clinical (let alone the electrographic) onset of a seizure. Since there is anecdotal evidence suggesting that the likelihood of affecting seizure progression decreases as the time between seizure onset and the start of stimulation increases,9 this delay in detection may have significant consequences.
A system that automatically initiates on-demand mode VNS following computerized seizure onset detection could relieve caregivers; increase a patient’s independence and sense of security; and more frequently terminate or diminish seizure symptoms in those who respond to on-demand stimulation. The system may also benefit patients who never realized they were responders since neither they nor their caregivers were able to initiate stimulation soon after the onset of a seizure.
In this report we describe the design and clinical evaluation of a non-invasive computerized system that automatically initiates on-demand VNS following the detection of a prespecified seizure or epileptiform discharges lasting at least one second in duration; epileptiform discharges were included since VNS has been shown to alter both their course and character.13,14 The computerized system detects these events through patient-specific analysis of the scalp electroencephalogram (EEG) and electrocardiogram (ECG) signals. Though the system relies primarily on EEG-based onset detection, the electrocardiogram signal is also examined since changes in the ECG signal have been noted to precede changes in the EEG signal in certain classes of seizures.15,16
This paper is organized as follows: In Sec. 2 we provide an overview of the computerized system; a description of the algorithms used to detect seizure onset; and an outline of the protocol used to evaluate the computerized system. In Sec. 3 we report the performance of the computerized system during clinical testing; we analyze in detail a testing session that involved automatic activation of on-demand VNS in response to ictal events, and then briefly discuss sessions involving activation of on-demand VNS in response to epileptiform discharges.
2. Methods
2.1. System overview
Figure 1 shows a block diagram of the computerized system; all the components of the system are external to the patient. The computerized system is composed of a commercial acquisition system (Digitrace 1800 Plus from SleepMed Inc.) that collects the EEG and ECG of a patient; a computer that receives and analyzes both the EEG and ECG in real-time using patient-specific algorithms; and an electromagnet that is worn by the patient and positioned so that it rests over the implanted VNS pulse generator.
Fig. 1.
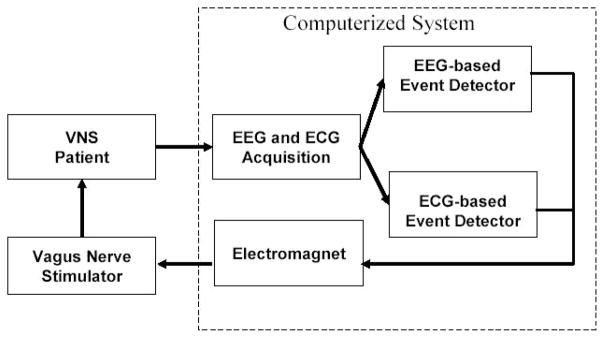
Block diagram of computerized, closed-loop VNS system.
When the computerized system detects the onset of a prespecified event (seizure or epileptiform discharge) through analysis of the EEG or ECG signal streams, it energizes the electromagnet worn by the patient. The magnetic field produced by the electromagnet triggers the implanted generator to initiate on-demand stimulation of the Vagus nerve. The electromagnet initiates on-demand stimulation through the same mechanism that is triggered when a permanent magnet is held over the implanted generator.
2.2. EEG-based detection of seizures and epileptiform discharges
In order to reliably detect a prespecified seizure or epileptiform discharge through EEG analysis, we adopted a patient-specific approach that utilizes machine-learning techniques. While non patient-specific algorithms can exhibit impressive detection performance on certain seizure types,17–19 commercially available patient non-specific algorithms20 tend to exhibit worse sensitivity, specificity, and longer detection delays when compared to patient-specific algorithms.21–23 This performance disparity is primarily due to the large variability in the character of seizure and non-seizure EEG that exists across patients.
In our patient-specific approach, a classifier determines whether an observed EEG segment resembles an individual’s non-epileptic EEG (captured during both awake and sleep periods), or one of their epileptic EEG events. The classifier accomplishes this task by comparing features of the current EEG segment with features of training epileptic and non-epileptic EEG segments taken from the individual; the classifier then assigns the observation to the class (epileptic or non-epileptic) whose features most resemble those of the observation.
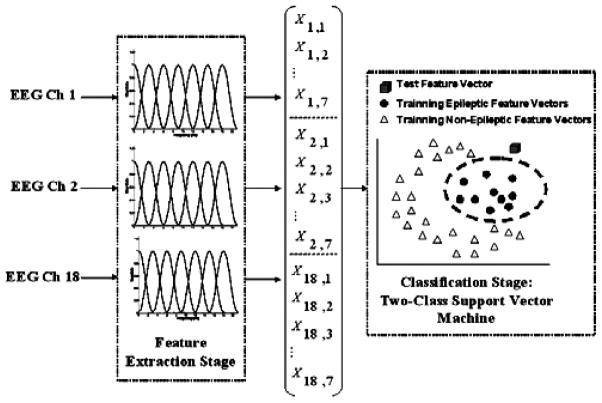
The block diagram in Fig. 2 illustrates more concretely the processing stages that comprise our patient-specific detector. The detector passes 2 second epochs from each of N EEG channels (typically N = 18) through a feature extractor. In turn, the feature extractor uses a filter bank to assemble for each channel a feature vector whose 7 elements correspond to the energies in 7 overlapping frequency bands. The frequency bands are each 3 Hz wide and collectively span 0–20 Hz; most physiologic and pathophysiologic scalp EEG activity can be observed within the 0–20 Hz frequency interval.24 The 7 features extracted from each of the N channels are then concatenated to form a 7N dimensional feature vector that captures spatial and spectral information within a 2-second epoch of EEG.
Fig. 2.
Block diagram of patient-specific algorithm for detecting seizures and epileptiform discharges.
Finally, the feature vector is assigned to the epileptic or non-epileptic class using a support vector machine (SVM) classifier25 trained to differentiate between an individual’s non-epileptic EEG and one type of epileptic EEG event.
If an individual has several types of epileptic EEG events (e.g. three types of seizures each with a distinct electrographic signature) then three instances of the detector shown in Fig. 2 are constructed. Each detector is tasked with differentiating between the individual’s non-epileptic EEG and one of their epileptic EEG events (e.g. one of the three seizure types). Now when one of the target epileptic events occurs the appropriate detector will initiate on-demand VNS.
Two retrospective evaluations of our patient-specific detector have been conducted. In the first evaluation26 we tested our detector on 60 hours of EEG from thirty-six pediatric epilepsy patients. The detector recognized 131 of 139 seizure events within 8.0 +/− 3.2 seconds, and declared 15 false detections. In the second evaluation23 we tested the detector on 536 hours of continuously recorded EEG from a second set of 16 pediatric epilepsy patients. The detector noted 132/143 seizures with a 6.8 +/− 2.4 second detection latency and 0.2 +/− 0.7 false alarms per hour.
2.3. ECG-based detection of seizures
An adaptive algorithm was used to detect accelerations in heart rate associated with the onset of a seizure. The algorithm compares the N most recent, instantaneous heart rate measurements {HR(t), HR(t − 1), …, HR(t − N + 1)} to the average heart rate HRavg computed over the preceding 3 minute period. The onset of seizure-associated tachycardia is declared if the N most recent heart rate measurements are successively increasing and the terminal heart rate measurement is greater than HRavg + L1. More specifically, the algorithm declares seizure-associated tachycardia whenever the following two conditions are met: HR(t−N + 1) < HR(t−N + 2) < · · · < HR(t) and HR(t) > HRavg + L1. In the study N = 5 and L1 = 15.
2.4. Study protocol
The clinical evaluation of the computerized system followed a protocol approved by the committee on clinical investigations (IRB) at the Beth Israel Deaconess Medical Center (BIDMC), Boston, Massachusetts, USA. Study participants were adult, long-term users of VNS who reported no adverse effects from on-demand VNS. Participants were admitted to the BIDMC General Clinical Research Center (GCRC) for a period lasting up to five days. Admission to the GCRC took place after study staff obtained informed consent, and confirmed that energizing the computerized system’s electromagnet initiates on-demand VNS reliably. No changes to a study participant’s AED regimen or VNS stimulation parameters, which varied across patients, were made during the study period.
During the admission period the participant’s Video, EEG, and ECG signals were recorded using the Digitrace 1800 Plus Recorder and Digital Video System (Sleep Med Inc.). After the first 24 hours of the study, an electroencephalographer reviewed the collected EEG and highlighted seizures or epilepti-form discharges; a subset of these events was then chosen to be the “target events.” Next, the computerized system was trained to detect the target events using both the EEG gathered in the first 24 hours of the study, and prerecorded EEG from the study participant available on record at BIDMC. On subsequent days of the study, the computerized system was set to automatically activate the participant’s VNS Therapy System whenever real-time detection of the target events occurred.
At the conclusion of the study we evaluated the sensitivity, specificity, and delay with which the computerized system detected the target events and initiated on-demand VNS. When the target EEG event was a seizure, we also evaluated whether on-demand VNS altered the electrographic signature or clinical behavior associated with the seizure.
3. Results
In this section we report the performance of the system during a clinical study that included five patients {A–E}. We first discuss the performance of the system during testing sessions with seizures as the target event (patients A–B). We then discuss the performance of the system during sessions with epileptiform discharges as the target event (patients C–D). We omit discussion of patient E since neither seizures nor frequent epileptiform discharges were observed during the admission.
3.1. Patient A: Medical history
Patient A is a 39-year-old woman with a long history of refractory complex partial seizures. At the time of admission to our study, she was experiencing 30–40 seizures per month. Patient A’s seizures last for 1–2 minutes and consist of repeatedly asking questions and blank stares; she may also demonstrate automatic behaviors and her seizures are not accompanied by tonic-clonic movements. Following a seizure, patient A is confused for a few minutes and requires reorientation by friends or family. Patient A is a VNS therapy patient; however, since she does not experience a warning prior to the onset of a seizure she does not use the on-demand mode of VNS.
3.1.1. Patient A: Automatic, EEG-based activation of on-demand VNS
Figure 3 illustrates an ictal EEG trace recorded from patient A prior to participation in the study. The seizure begins following the 1511 second mark as a 3–4 Hz rhythmic oscillation most prominent on the right-sided temporal channels {T4-T6; T6-O2}, and the right-sided central channels {F4-C4; C4-P4}. The seizure is also accompanied by eye-flutter that can be observed on the frontal channels {FP1-F3; FP2-F4} following the 1506 second mark.
Fig. 3.
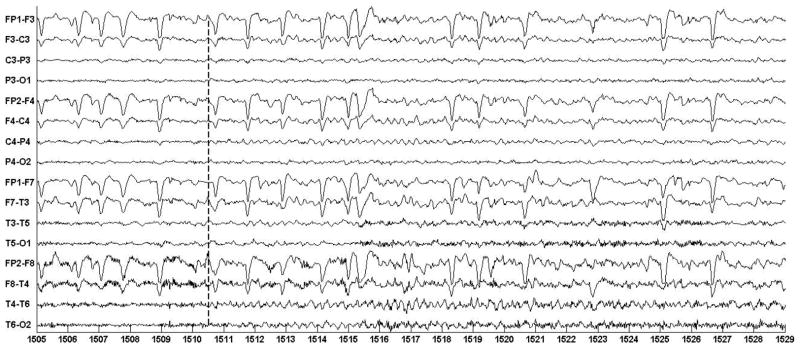
EEG trace illustrating electrographic signature associated with patient A’s seizures.
A total of 5 prerecorded seizures as well as two days of prerecorded awake and sleep EEG were available to train one patient-specific detector to differentiate between patient A’s seizures and non-epileptic EEG. The training seizure and non-seizure data were recorded six months prior to patient A’s admission to the study. For the first 4 seizures recorded during the study the computerized system used EEG exclusively to initiate on-demand VNS.
Figure 4 shows the first of four seizures that the system detected and automatically initiated on-demand VNS. The seizure in Fig. 4 begins following the 322 second mark, and similar to the training seizure recorded six months earlier, is characterized by 3–4 Hz rhythmic activity on channels {T4-T6; F4-C4; C4-P4}. The system noted seizure activity at the 324.5 second mark (2.5 second detection delay relative to seizure onset time) and then energized the electromagnet for a period of 1.5 seconds; this initiated on-demand VNS. On-demand VNS begins following the 328 second mark and appears as a spike train on the VNS channel. The 6 second delay between seizure onset and the appearance of the VNS spike train is a composite of the following delays: 6 sec delay = 2.5 sec detection delay + 1.5 sec electromagnet on-time delay + ~ 2 sec VNS start-up time delay. Note that the system promptly detected the seizure in Fig. 4 even though it was trained on seizures recorded from the same individual six months prior to the study. The second and third automatic seizure detections and vagus nerve stimulator activations were similar to the one illustrated in Fig. 4.
Fig. 4.
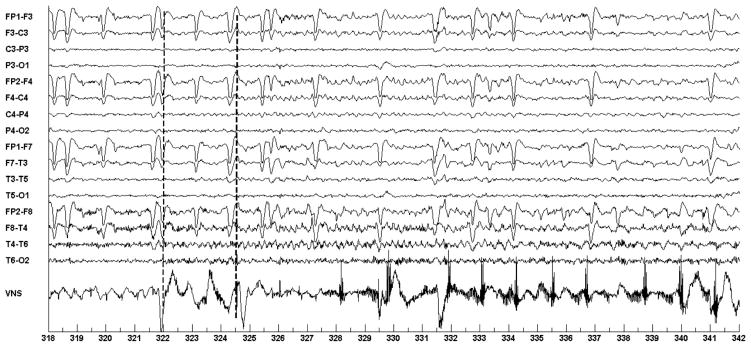
Automatic initiation of VNS following automatic detection of ictal discharges in the EEG of patient A. Seizure starts at the 322 second mark; Seizure is detected at 324.5; VNS is initiated at 328.
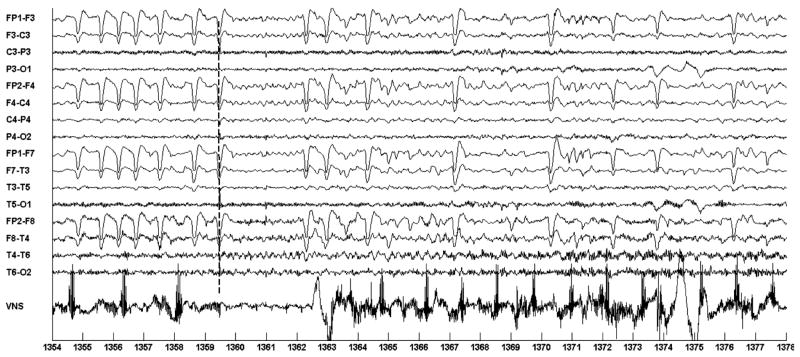
The fourth automatic on-demand activation is illustrated in Fig. 5. In this case the system noted seizure activity, through the detection of eye-flutter, immediately preceding the onset of ictal discharges in the right temporal region at the 1359.5 second mark, and subsequently initiated on-demand VNS. The association of eye-flutter with seizure onset was automatically learned through the analysis of the 5 training seizures. In this case, associating eye-flutter with seizure onset resulted in rapid seizure detection, but, as will be discussed in Sec. 3.1.4, this association also resulted in false detections whenever eye-flutter occurred outside the context of a seizure. The first sign of the onset of on-demand VNS occurs most clearly following the 1364.5 (5 second delay) second mark. Coincidently, prior to the onset of the seizure, the last few pulses of the automatic mode VNS cycle appear between the 1354–1358 second marks.
Fig. 5.
Automatic initiation of VNS following automatic detection of seizure-associated eye-flutter in the EEG of patient A. Seizure starts at the 1359 second mark; seizure is detected at 1359; VNS is initiated at 1364.
Table 1 summarizes the system’s performance on the four test seizures discussed in this section. On average, on-demand VNS was initiated within 6 seconds of the onset of seizure activity in the EEG. Since no human subject studies on how soon following seizure onset should on-demand VNS begin, we cannot state whether the system’s VNS activation latency is good or bad. However, anecdotal evidence suggests that the likelihood of affecting seizure progression using on-demand VNS decreases as the time between seizure onset and the start of stimulation increases.9
Table 1.
Latency of seizure detection and VNS activation.
| Seizure number | Seizure detection latency | VNS activation latency |
|---|---|---|
| 1 | 2.5 sec | 6 sec |
| 2 | 1.5 sec | 5 sec |
| 3 | 2 sec | 8 sec |
| 4 | 0 sec | 5 sec |
| Mean | 1.5 sec | 6 sec |
3.1.2. Patient A: Automatic, ECG-based activation of on-demand VNS
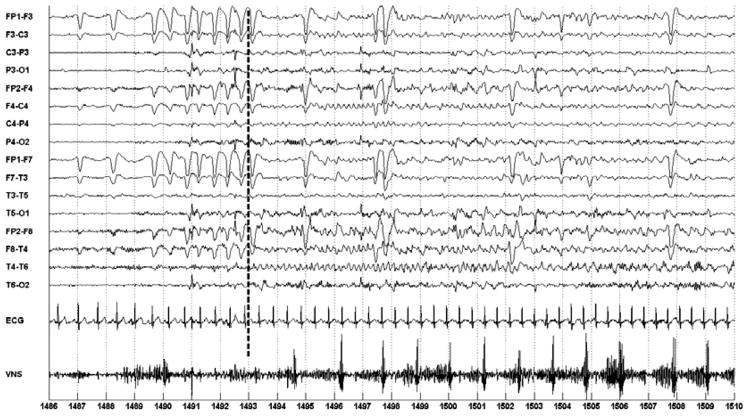
For the fifth seizure in the study, the ECG was used to initiate on-demand VNS. Figure 6 illustrates on-demand activation of the VNS following computerized detection of seizure-associated heart rate acceleration in the ECG. Seizure onset occurs following the 1493 second mark, and the system notes seizure activity at the 1491 second mark (−2 second detection delay relative to time of seizure onset in EEG).
Fig. 6.
Automatic initiation of VNS following automatic detection of seizure-associated heart rate acceleration in the ECG of patient A. Seizure starts at the 1493 second mark; seizure is detected at 1491; VNS is initiated 1494.
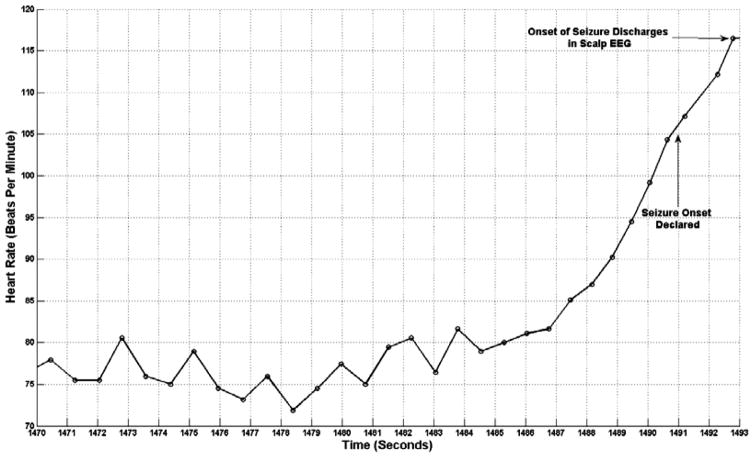
To appreciate the heart rate acceleration compare the smaller R-R interval formed by the QRS complexes between the 1490–1491 second marks with the larger R-R interval formed by the QRS complexes between the 1486–1487 second marks. Heart acceleration is more clearly illustrated in the plot of heart rate shown in Fig. 7. On-demand VNS begins following the 1494 second mark (1 second delay) and appears as a spiking train on the VNS channel.
Fig. 7.
A time profile of patient A’s heart rate prior to appearance of ictal discharges in the EEG. Seizure discharges appear in the EEG at the 1493 second mark, but the seizure is detected at the 1491 second mark.
In the case of patient A, the ECG-based event detector initiated on-demand VNS earlier than the EEG-based event detector. However, as will be discussed in Sec. 3.1.4, the EEG-based event detector had better specificity relative to the ECG-based event detector.
3.1.3. Patient A: Comparing stimulated and unstimulated seizures
Intentionally, the system did not initiate on-demand VNS following the onset of patient A’s sixth seizure. This allowed us to compare the electro-graphic and behavioral character of the stimulated seizures (seizures 1–5) and the non-stimulated seizure (seizure 6). We did not appreciate a difference in the electrographic or behavioral character of the stimulated seizures and the non-stimulated seizure.
Three possible explanations for why on-demand VNS did not alter the course of patient A’s sixth seizure are: (1) The on-demand stimulus was not initiated early enough in the course of the seizure (2) The on-demand VNS stimulus parameters (pulse current, frequency, and duration) were not set optimally (3) Patient A is not a responder to on-demand mode VNS.
3.1.4. Patient A: Sensitivity and specificity analysis of computerized system
The system was operated for a total of 81.13 hours (~ 3.4 days). For the majority of the study period, only the EEG-based detector was allowed to initiate on-demand VNS; however, for the entirety of the study period both the EEG and ECG detectors logged detections.
During the monitoring period the system delivered 37 on-demand stimuli. Five of these on-demand stimuli were in response to seizures; four triggered by the EEG-based detector and one triggered by the ECG-based detector. The remaining 32 stimuli were due to false detections and all triggered by the EEG-based detector. Consequently, the system demonstrated 100% sensitivity and a specificity of 0.39 False Stimulations/Hour (or 1 false stimulus every 2.5 hours). During the same monitoring period, 3245 automatic-mode VNS stimuli (each of nearly equal strength to a magnet-mode stimulus, but lasting only half the duration) were delivered by the implanted generator.
A majority of the 32 false stimulations were caused by rapid eye-movements or slow, high-amplitude activity that involves the frontal channels. A false detection due to rapid eye movement is illustrated in Fig. 8. Note the similarity between the eye-movement pattern in Fig. 8 and the eye-movement pattern associated with the seizures in Figs. 4–6.
Fig. 8.
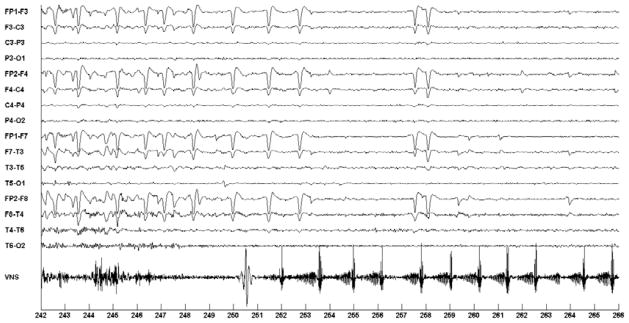
False detection and activation of on-demand VNS following detection of eye-flutter not associated with a seizure in the EEG of patient A.
It is important to note that the abovementioned specificity results apply only to EEG signals recorded during periods of rest and limited mobility by the patient. During anticipated periods of ambulation around the research floor or other vigorous movement, study staff allowed the system to continue to log detections but did not allow these detections to activate on-demand VNS since these activities trigger false activations. If these periods are taken into account, the the EEG-based detector’s false detection rate becomes 2.5 false detections per hour.
Review of the ECG-based seizure detector logs revealed that the detection rule described in Sec. 2.3 has poor specificity. False detections logged by the ECG-based detector were caused primarily by short runs of heart rate acceleration that satisfied the ECG detector’s criteria. Over the 81.13 hour testing period, the ECG-based seizure detector logged a false detections rate of 21 false detections per hour.
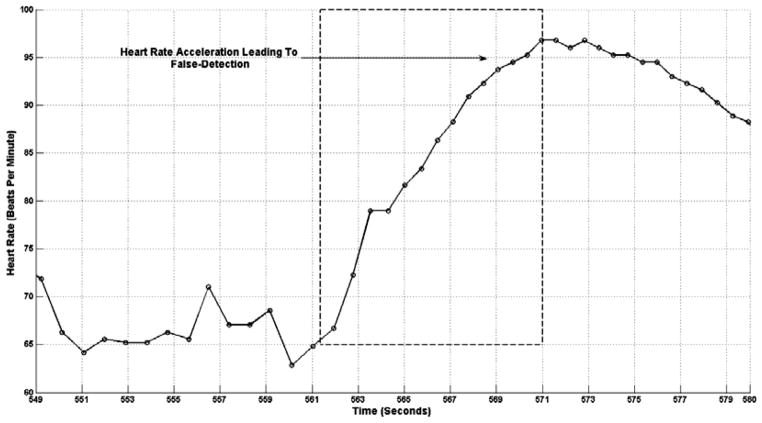
Figure 9 shows heart rate acceleration occurring while the patient was asleep; the heart rate acceleration was not associated with the appearance of seizure discharges in the scalp EEG and was therefore counted as a false detection generated by the ECG-based detector. It is possible that seizure activity was ongoing within a deep brain structure during the heart rate acceleration shown in Fig. 9, but due to the brain structure’s depth its activity was not reflected in scalp EEG.27,28
Fig. 9.
Heart rate acceleration experienced by patient A during sleep. Heart rate acceleration was not associated with ictal discharges that could be detected on scalp EEG, but was detected by the ECG-based seizure onset detector.
For patient A the specificity of the ECG-based event detector may have been improved if classifications of tachycardias were made in the context of the activity observed in the EEG signal, as was done for neonatal seizure onset detection.29 Consider that patient A’s seizure-associated tachycardia is coincident with seizure-associated eye-flutter as can be seen in Fig. 6 between the 1491–1492 second marks. Given this association between ECG and EEG activity, the computerized system could misclassify fewer activity-associated tachycardias by requiring that the sequence of heart rate changes described in Sec. 3.2 be accompanied by an eye-flutter event in the EEG.
3.2. Patient B: Medical history
Patient B is a 41-year-old woman with a long history of simple partial, complex partial and secondarily generalized seizures. At the time of her admission to our study, she was experiencing 5–6 seizures per month. Patient B’s secondarily generalized seizures consist of a tonic phase followed by a clonic phase, and finally a post-ictal phase. The tonic-clonic phase lasts for 1 minute. The post-ictal phase, which is characterized by generalized EEG slowing, lasts for 30 minutes. According to patient B’s caregiver, initiating VNS early in the ictal phase results in reduced anxiety and confusion during the post-ictal phase.
3.2.1. Patient B: Automatic, EEG-based activation of on-demand VNS
During the study period, patient B experienced two secondarily generalized seizures. The first was used to train the computerized system, and the second to investigate the impact of initiating on-demand VNS early in the course of patient B’s seizure.
Figure 11 illustrates an EEG trace of the first seizure. The seizure begins following the 1340 second mark with an electrodecrement most prominent on the occipital channels {P3-O1; P4-O2}. Next, at the 1348 second mark, a 3–5 Hz occipital rhythm emerges from the electrodecrement and rapidly generalizes. By the 1360 second mark, muscle artifact associated with the tonic-clonic phase of the seizure becomes visible. The tonic-clonic phase of the first seizure lasted for 55 seconds and the post-ictal phase lasted for 29 minutes. During the post-ictal phase, the patient was unaware of her surroundings and very anxious. The computerized system was trained to recognize the occipital rhythm and generalized activity associated with the tonic-clonic phase of the seizure.
Fig. 11.
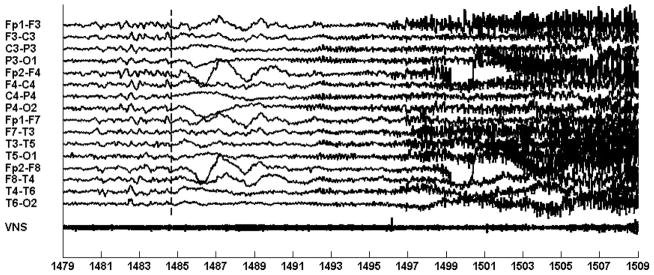
Automatic initiation of VNS following automatic detection of ictal discharges in the EEG of patient B. Seizure starts at the 1485 second mark; seizure is detected at the 1505 second mark. Evidence of on-demand VNS activation is more clearly seen in Fig. 12.
Figure 12 shows the seizure that triggered the system to initiate on-demand VNS. The seizure is similar to the training seizure in evolution; it begins with an electrodecrement at the 1485 second mark. The computerized system failed to detect the onset of the focal occipital rhythm at the 1490 second mark; instead it detected the tonic-clonic phase and initiated VNS at the 1501 second mark (16 second delay). Evidence of initiating on-demand VNS cannot be seen immediately after seizure detection because the VNS channel is obscured by muscle artifact. However, at the conclusion of the ictal phase and disappearance of muscle artifact, evidence of VNS generator activity can be seen on the VNS channel as shown in Fig. 13.
Fig. 12.
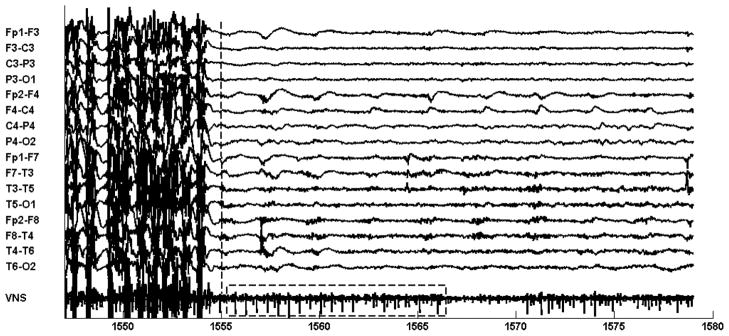
Within the boxed interval of the VNS channel (1555–1565 seconds) the VNS channel shows a spike-train associated with on-demand activity of the VNS generator. The generator was triggered at 1505 seconds (see Fig. 11) following computerized detection of the tonic-clonic phase of patient B’s seizure.
Fig. 13.
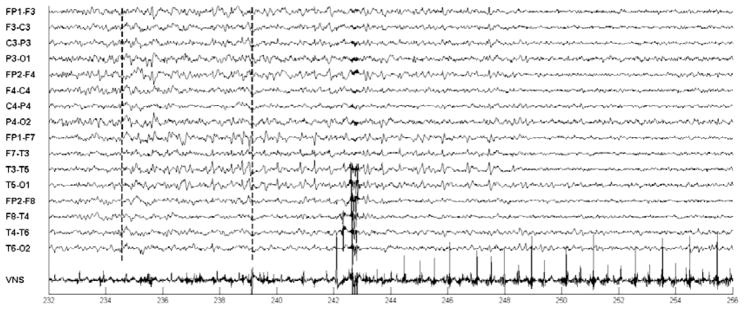
Automatic initiation of VNS following automatic detection of an epileptiform discharge in the EEG of patient B. Discharge starts at the 234 second mark; discharge is detected at 239; VNS is initiated at 244.
Similar to the first seizure, the tonic-clonic phase of the second seizure lasted for 65 seconds and the post-ictal phase for 30 minutes. We believe that the length and character of these phases was unaltered because the computerized system initiated on-demand VNS during the generalized portion of the seizure rather than at the focal onset stage of the seizure. However, behaviorally, relative to the first seizure, patient B was significantly less anxious and became oriented to her surroundings much sooner following the end of the tonic-clonic phase of the second seizure. Our observation that applying on-demand VNS during the seizure improves post-ictal recovery from the seizure is consistent with the experience of patient B’s caregiver.
During the 26 hours that the computerized system was used to monitor patient B, no false detections were declared. While the system exhibited excellent specificity, its detection latency was poor. We hypothesize that the increased latency was due to the subtlety of the EEG changes associated with patient B’s seizure onset pattern and the similarity of this pattern to non-seizure activity seen during periods of drowsiness. We expect that with additional training seizures the computerized system’s detection latency will improve.
3.3. Patient C: Automatic, EEG-based activation of on-demand VNS
For patient C no ictal events were recorded during the study period, but an epileptiform discharge of variable duration occurred frequently during sleep. During a 6 hour period containing 34 epileptiform discharges lasting more than 4 seconds in duration, the computerized system detected 31/34 of these discharges, and declared zeros false detections. Figure 13 illustrates the automatic activation of on-demand VNS following the detection of an epileptiform discharge. The discharge begins at the dotted line following the 234 second mark and consists of a train of sharp waves appearing most prominently on the left-hemispheric EEG channels {FP1-F7; F7-T3; T3-T5; T5-O1} between the 234–238 second marks. The system detected the ongoing discharge following the 239 second mark (4 second detection delay), and then initiated on-demand Vagus nerve stimulation by energizing the electromagnet for a period of 3 seconds. On-demand Vagus nerve stimulation begins following the 244 second mark (10 second delay).
3.4. Patient D: Automatic, EEG-based activation of on-demand VNS
For Patient D no ictal events occurred during the study period, but three different types of epileptiform discharges were noted while patient D was awake. The three types of epileptiform discharges all exhibited a spike-and-slow-wave morphology, but differed in their spatial localization. The epileptiform discharge either involved left-hemispheric EEG channels, right-hemispheric EEG channels, or both right and left-hemispheric EEG channels. The computerized system was trained to detect each type of epileptiform discharge independently. This allowed research staff to select which of the three types of epileptiform discharges would lead the system to initiate on-demand VNS.
Figure 14 illustrates the activation of on-demand VNS following the detection of an epileptiform discharge involving the left-hemispheric EEG channels. The discharge coincides with the 468 second mark and involves the EEG channels {FP1-F3; F3-C3; C3-P3; P3-O1; FP1-F7; F7-T3; T3-T5; T5-O1}. The computerized system recognized the discharge following the analysis of a two-second window spanning the 467–469 second marks. On-demand VNS stimulation begins following the 473 second mark.
Fig. 14.
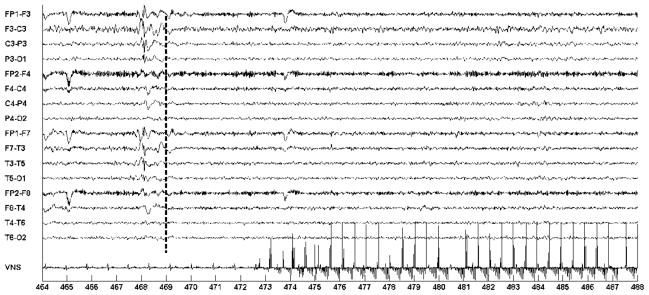
Automatic initiation of VNS following automatic detection of a left-sided epileptiform discharge in the EEG of patient C. Discharge starts at the 468 second mark; VNS is initiated at 473.
Figure 15 illustrates the activation of on-demand VNS following the detection of an epileptiform discharge involving the right-hemispheric EEG channels. The discharge coincides with the 1400 second mark and involves the EEG channels {FP2-F4; F4-C4; C4-P4; P4-O3; FP2-F8; F8-T4; T4-T6; T6-O2}. The computerized system recognized the discharge following the analysis of a two-second window spanning the 1399–1401 second marks. On-demand VNS begins following the 1406 second mark.
Fig. 15.
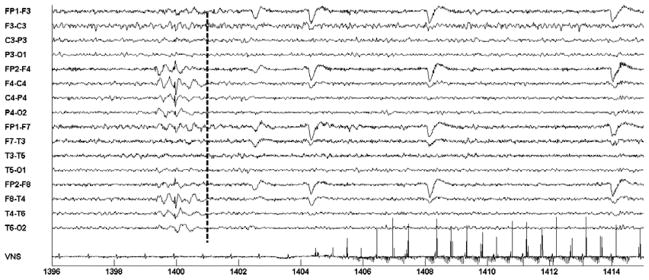
Automatic initiation of VNS following automatic detection of a right-sided epileptiform discharge in the EEG of patient C. Discharge starts at the 1400 second mark; VNS is initiated at 1406.
Figure 16 shows an epileptiform discharge involving the right-hemispheric EEG channels. When this epileptiform discharge occurred, the computerized system did not initiate on-demand VNS since at the time it was set to do so only upon the occurrence of an epileptiform discharge involving both the right and left-hemispheric channels; note the absence of a VNS spike train similar to that seen in Figs. 14–15. This demonstrates how the EEG-based detector utilizes both spectral and spatial information in order to determine whether or not an EEG observation is a member of the target epileptic EEG event.
Fig. 16.
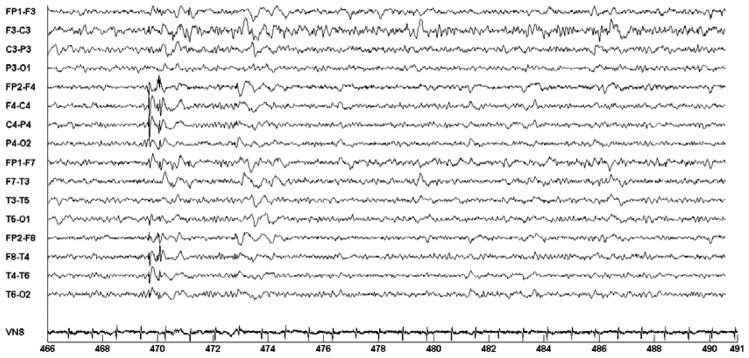
A right-sided epileptiform discharge in the EEG of Patient C that did not trigger initiation of on-demand VNS. At the time, the system was set to detect discharges involving both the right and left hemispheres.
4. Discussion and Conclusion
The proof-of-principle system described in this report is an example of a closed-loop, neurostimulator that delivers potentially anti-seizure therapy contingent upon the detection of a target seizure onset pattern in non-invasive physiologic signals (EEG/ECG). Other detect-and-treat systems described in the literature deliver therapy based on the analysis of invasive physiologic signals (IEEG).30–32 In addition to being used to initiate Vagus nerve stimulation following seizure onset, the system we developed could be used to trigger other extracranial, non-invasive brain stimulation modalities such as repetitive transcranial magnetic stimulation (rTMS), transcranial direct current stimulation (tDCS), and Trigeminal nerve stimulation (TNS).33,34
The clinical evaluation of our proof-of-principle system demonstrated that the system can be set to initiate on-demand VNS on a target seizure (Sec. 3.1.1 and 3.2.1) or target epileptiform discharges (Sec. 3.2–3.3) with good sensitivity, specificity, and short delay. However, enhancements to the EEG and ECG-based event detection algorithms are necessary prior to using our system to initiate on-demand VNS in the ambulatory, outpatient setting. An artifact processing or rejection stage35 should be added to the EEG-based seizure detector so that the system can correctly classify or discard EEG artifacts induced by movement. Similarly, a more sophisticated approach to ECG-based seizure detection is necessary to differentiate between seizure-associated tachycardia and activity-associated tachycardia. This may involve the fusion of EEG and ECG information (see Sec. 3.1.4); the application of data-mining techniques to discover sequential heart rate and morphology patterns in the ECG that are unique to seizure onset36; or the analysis of heart rate in the context of information from other sensors, for example, an accelerometer may be used to suggest that heart rate acceleration is due to patient movement.
An ambulatory version of the proposed closed-loop, VNS system can be implemented using existing signal acquisition, signal processing, and communication technology. The EEG required by the system can be captured using a few dry EEG electrodes37 positioned over the region of the head demonstrating the earliest ictal discharges. The captured EEG can then be wirelessly transmitted to a digital signal processor (DSP) that implements an EEG-event detection algorithm.38,39 Finally, detection of seizure activity by the digital signal processor will trigger the VNS generator through a dedicated DSP-VNS radio-link.
Data recorded by an ambulatory version of the proposed closed-loop, VNS system may be of benefit to neurologists as well as patients. The computerized system can maintain an “electronic seizure diary” that documents the total number of seizures as well as whether or not initiation of on-demand VNS affects the course of a seizure. Neurologists can periodically review the electronic diary in order to determine whether a set of VNS stimulation parameters (both automatic-mode VNS as well on-demand mode VNS) are therapeutic for a given patient. Currently, neurologists determine whether VNS settings are therapeutic based on seizure diaries maintained by the patient or their cargiver. While informative these accounts have been shown to be inaccurate.40
Future clinical evaluations of our closed-loop VNS system are planned. These evaluations will shed light on the therapeutic benefit of on-demand VNS.
Fig. 10.
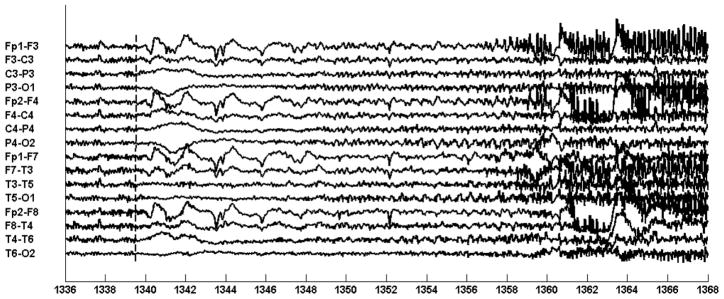
EEG trace illustrating electrographic signature associated with patient B’s seizures.
Acknowledgments
We would like to thank the individuals that participated in this research study as well as the nurses of the Beth Israel Deaconess Medical Center General Clinical Research Center for their dedication. The project described in this report was supported by Grant Number UL1 RR025758 and M01-RR-01032 Harvard Clinical and Translational Science Center, from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Contributor Information
ALI SHOEB, Email: ashoeb@mit.edu, Electrical Engineering and Computer Science Department, Massachusetts Institute of Technology, Cambridge, Massachusetts, USA.
TRUDY PANG, Email: tpang@bidmc.havard.edu, Neurology Department, Beth Israel Deaconess Medical Center and the Clinical Investigator Training Program: Beth Israel Deaconess Medical Center — Harvard/MIT Health Sciences and Technology, in collaboration with Pfizer Inc. and Merck & Co., Boston, Massachusetts, USA.
JOHN GUTTAG, Email: guttag@mit.edu, Electrical Engineering and Computer Science Department, Massachusetts Institute of Technology, Cambridge, Massachusetts, USA.
STEVEN SCHACHTER, Email: sschacht@bidmc.harvard.edu, Neurology Department, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
References
- 1.Schachter SC, Saper CB. Vagus nerve stimulation. Epilepsia. 1998;39(7):677–686. doi: 10.1111/j.1528-1157.1998.tb01151.x. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Menachem E. Vagus nerve stimulation for the treatment of epilepsy. Lancet Neurol. 2002;1(8):477–482. doi: 10.1016/s1474-4422(02)00220-x. [DOI] [PubMed] [Google Scholar]
- 3.Theodore WH, Fisher RS. Brain stimulation for epilepsy. Lancet Neurol. 2004;3(2):111–118. doi: 10.1016/s1474-4422(03)00664-1. [DOI] [PubMed] [Google Scholar]
- 4.Schuele SU, Lüders HO. Intractable epilepsy: management and therapeutic alternatives. Lancet Neurol. 2008;7(6):514–524. doi: 10.1016/S1474-4422(08)70108-X. [DOI] [PubMed] [Google Scholar]
- 5.Physician’s Manual for the VNS therapy pulse model 102 generator. Cyberonics, Inc; 2002. [Google Scholar]
- 6.The Vagus Nerve Stimulation Study Group. A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. Neurology. 1995;45(2):224–230. doi: 10.1212/wnl.45.2.224. [DOI] [PubMed] [Google Scholar]
- 7.Handforth A, DeGiorgio CM, Schachter SC, Uthman BM, Naritoku DK, Tecoma ES, et al. Vagus nerve stimulation therapy for partial onset seizures: a randomized active control trial. Neurology. 1998;51(1):48–55. doi: 10.1212/wnl.51.1.48. [DOI] [PubMed] [Google Scholar]
- 8.DeGiorgio CM, Schachter SC, Handforth A, Salinsky M, Thompson J, Uthman B, et al. Prospective long-term study of Vagus nerve stimulation for the treatment of refractory seizures. Epilepsia. 2000;41(9):1195–1200. doi: 10.1111/j.1528-1157.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 9.Hammond EJ, Uthman BM, Reid SA, Wilder BJ. Electrophysiological studies of cervical vagus nerve stimulation in humans. I. EEG effects. Epilepsia. 1992;33(6):1013–1020. doi: 10.1111/j.1528-1157.1992.tb01752.x. [DOI] [PubMed] [Google Scholar]
- 10.Morris GL., III A retrospective analysis of the effects of magnet-activated stimulation in conjuction with vagus nerve stimulation therapy. Epilepsy Behav. 2003;4(6):740–745. doi: 10.1016/j.yebeh.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 11.Labar D, Murphy J, Tecoma E. Vagus nerve stimulation for medication-resistant generalized epilepsy. E04 VNS Study Group. Neurology. 1999;52(7):1510–1512. doi: 10.1212/wnl.52.7.1510. [DOI] [PubMed] [Google Scholar]
- 12.Boon P, Vonck K, Van Walleghem P, et al. Programmed and magnet-induced vagus nerve stimulation for refractory epilepsy. J Clin Neurophysiol. 2001;18(5):402–407. doi: 10.1097/00004691-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Olejniczak PW, Fisch BJ, Carey M, Butter-baugh G, Happel L, Tardo C. The effect of vagus nerve stimulation on epileptiform activity recorded from hippocampal depth electrodes. Epilepsia. 2001;42(3):423–429. doi: 10.1046/j.1528-1157.2001.10900.x. [DOI] [PubMed] [Google Scholar]
- 14.Koo B. EEG changes with vagus nerve stimulation. J Clin Neurophysiol. 2001;18(5):434–441. doi: 10.1097/00004691-200109000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Zijlman M, Flanagan D, Gotman J. Heart rate changes and ECG abnormalities during epileptics seizures: prevalence and definition of an objective clinical sign. Epilepsia. 2002;43(8):847–854. doi: 10.1046/j.1528-1157.2002.37801.x. [DOI] [PubMed] [Google Scholar]
- 16.Di Gennaro G, Quarato PP, Sebastiano F, Esposito V, Onorati P, Grammaldo LG, et al. Ictal heart rate increases precedes EEG discharge in drug-resistant mesail temporal lobe seizures. Clinical Neurophysiol. 2004;115(5):1169–1177. doi: 10.1016/j.clinph.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Saab ME, Gotman J. A system to detect the onset of epileptic seizures in scalp EEG. Clin Neurophysiol. 2005;116(2):427–442. doi: 10.1016/j.clinph.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Meier R, Dittrich H, Schulze-Bonhage A, Aertsen A. Detecting epileptic seizures in long-term human EEG: a new approach to automatic online and real-time detection and classification of polymorphic seizure patterns. J Clin Neurophysiol. 2008;25(3):119–131. doi: 10.1097/WNP.0b013e3181775993. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh-Dastidar S, Adeli H, Dadmehr N. Mixed-band wavelet-chaos-neural network methodology for epilepsy and epileptic seizure detection. IEEE Trans Biomed Eng. 2007;54(2):1545–1551. doi: 10.1109/TBME.2007.891945. [DOI] [PubMed] [Google Scholar]
- 20.Wilson SB, Scheuer ML, Emerson RG, Gabor AJ. Seizure detection: evaluation of the reveal algorithm. Clin Neurophysiol. 2004;115(10):2280–2291. doi: 10.1016/j.clinph.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 21.Qu H, Gotman J. Improvement in seizure detection performance by automatic adaptation to the EEG of each patient. Electroencephalogr Clin Neurophysiol. 1993;86(2):79–87. doi: 10.1016/0013-4694(93)90079-b. [DOI] [PubMed] [Google Scholar]
- 22.Qu H, Gotman J. A patient-specific algorithm for the detection of seizure onset in long-term EEG monitering: Possible use as a warning device. IEEE Trans Biomed Eng. 1997;44(2):115–122. doi: 10.1109/10.552241. [DOI] [PubMed] [Google Scholar]
- 23.Shoeb A, Bourgeois B, Treves S, Schachter SC, Guttag J. Impact of patient-specificity on seizure onset detection performance. Conf Proc IEEE Eng Med Biol Soc. 2007:4110–4114. doi: 10.1109/IEMBS.2007.4353240. [DOI] [PubMed] [Google Scholar]
- 24.Gotman J, Ives JR, Gloor P. Frequency content of EEG and EMG at seizure onset: Possibility of removal of EMG artifact by digital filtering. Electroencephalography Clin Neurophysiol. 1981;52(6):626–639. doi: 10.1016/0013-4694(81)91437-1. [DOI] [PubMed] [Google Scholar]
- 25.Shawe-Taylor J, Cristianini N. Support vector machines and other kernel-based learning methods. Cambridge University Press; 2000. [Google Scholar]
- 26.Shoeb A, Edwards H, Connolly J, Bourgeois B, Treves T, Guttag J. Patient-specific seizure onset detection. Epilepsy Behav. 2004;5(4):483–498. doi: 10.1016/j.yebeh.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Weil S, Arnold S, Esensehr I, Noachtar S. Heart rate increase in otherwise subclinical seizures is different in temporal versus extratemporal seizure onset: support for temporal lobe autonomic influence. Epileptic Disord. 2005;7(3):199–204. [PubMed] [Google Scholar]
- 28.Pacia SV, Ebersole JS. Intracranial EEG substrates of scalp ictal patterns from temporal lobe foci. Epilepsia. 1997;38(6):642–654. doi: 10.1111/j.1528-1157.1997.tb01233.x. [DOI] [PubMed] [Google Scholar]
- 29.Greene BR, Boylan GB, Reilly RB, de Chazal P, Connolly S. Combination of EEG and ECG for improved automatic neonatal seizure detection. Clin Neurophysiol. 2007;118(6):1348–1359. doi: 10.1016/j.clinph.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Sun FT, Morell MJ, Wharen RE., Jr Responsive cortical stimulation for the treatment of epilepsy. Neurotherapeutics. 2008;5(1):68–74. doi: 10.1016/j.nurt.2007.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kossoff EH, Ritzl EK, Politsky JM, Murro AM, Smith JR, Duckrow RB, et al. Effect of an external responsive neurostimulator on seizures and electrographic discharges during sub-dural electrode monitoring. Epilepsia. 2004;45(12):1560–1567. doi: 10.1111/j.0013-9580.2004.26104.x. [DOI] [PubMed] [Google Scholar]
- 32.Peters TE, Bhavaraju NC, Frei MG, Osorio I. Network system for automated seizure detection and contingent delivery of therapy. J Clin Neurophysiol. 2001;18(6):545–549. doi: 10.1097/00004691-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Fregni F, Pascual-Leone A. Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol. 2007;3(7):383–393. doi: 10.1038/ncpneuro0530. [DOI] [PubMed] [Google Scholar]
- 34.DeGiorgio CM, Shewmon A, Murray D, Whitehurst T. Piolot study of trigeminal nerve stimulation (TNS) for epilepsy: a proof-of-concept trial. Epilepsia. 2006;47(7):1213–1215. doi: 10.1111/j.1528-1167.2006.00594.x. [DOI] [PubMed] [Google Scholar]
- 35.Le Van P, Urrestarazu E, Gotman J. A system for automatic artifact removal in ictal scalp EEG based on independent component analysis and Bayesian classification. Clin Neurophysiol. 2006;117(4):912–927. doi: 10.1016/j.clinph.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Syed Z, Guttag J, Stultz C. Clustering and symbolic analysis of cardiovascular signals: discovery and visualization of medically relevant patterns in long-term data using limited prior knowledge. Eurasip Journal on Advances in Signal Processing. 2007 [Google Scholar]
- 37.Taheri BA, Knight RT, Smith RL. A dry electrode for EEG recording. Electroencephalogr Clin Neurophysiol. 1994;90(5):376–383. doi: 10.1016/0013-4694(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 38.Shoeb A, Guttag J, Schachter S, Schomer D, Bourgeois B, Treves T. Detecting seizure onset in the ambulatory setting: demonstrating feasibility. Conf Proc IEEE Eng Med Biol Soc. 2005;4:3546–3550. doi: 10.1109/IEMBS.2005.1617245. [DOI] [PubMed] [Google Scholar]
- 39.Bhavaraju NC, Frei MG, Osorio I. Analog seizure detection and performance evaluation. IEEE Trans Biomed Eng. 2006;53(2):238–245. doi: 10.1109/TBME.2005.862532. [DOI] [PubMed] [Google Scholar]
- 40.Hopee C, Poepel A, Elger CE. Epilepsy: Accuracy of patient seizure counts. Arch Neurol. 2007;64(11):1595–1599. doi: 10.1001/archneur.64.11.1595. [DOI] [PubMed] [Google Scholar]