Abstract
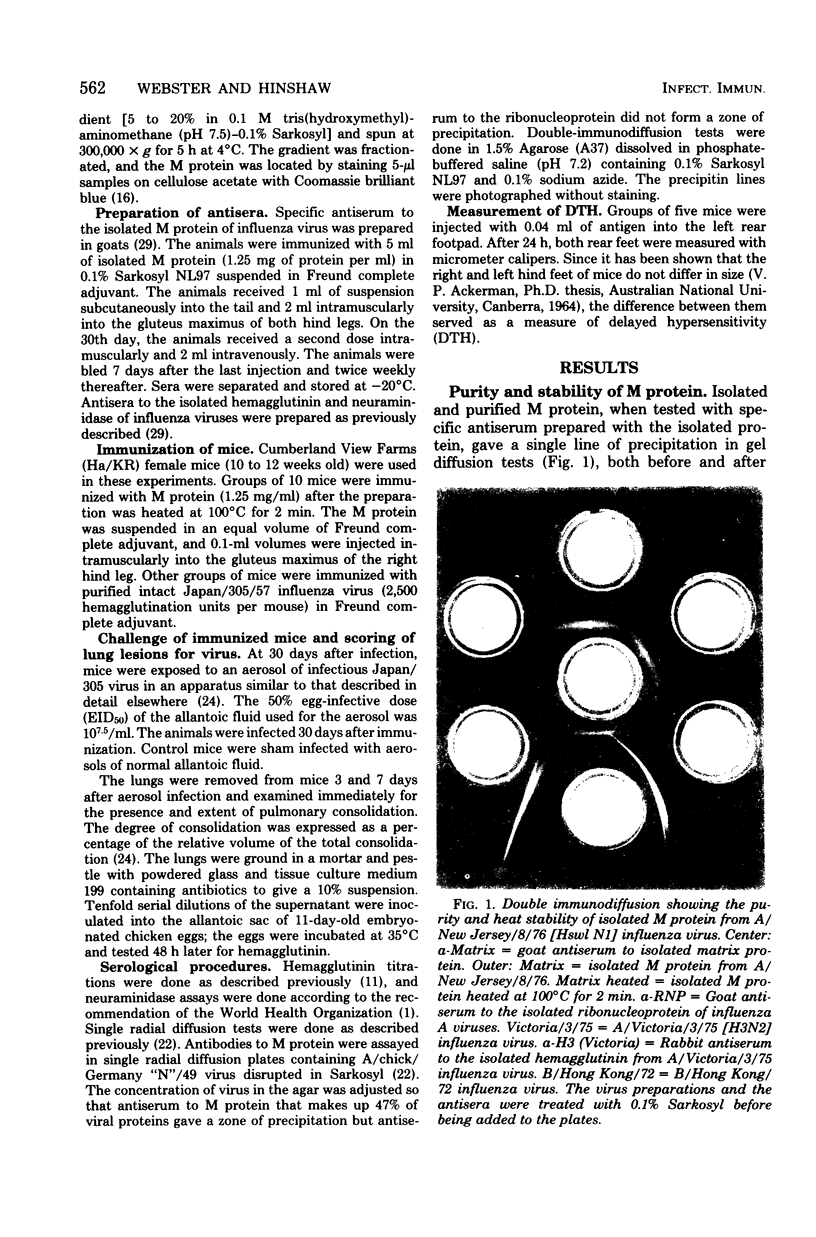
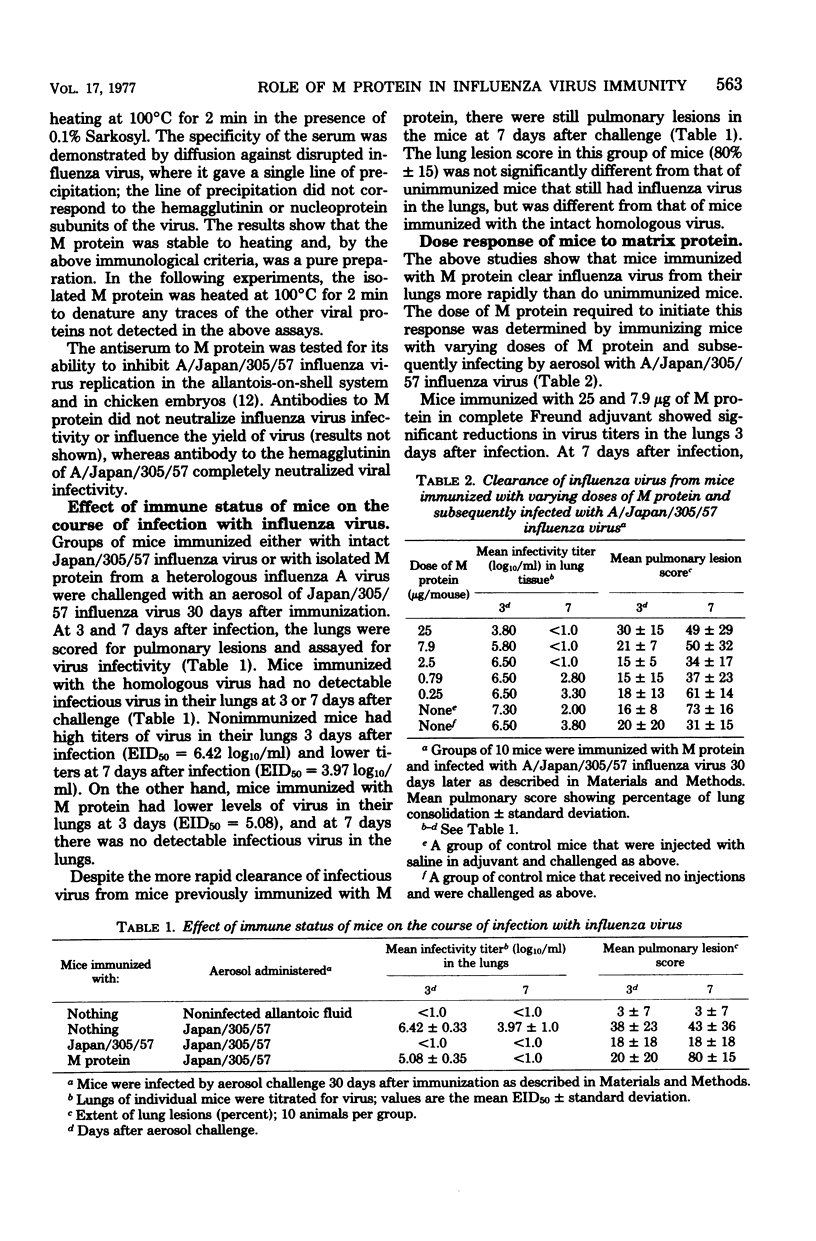
The matrix (M) protein of influenza A virus, one of the two group-specific internal proteins of the virion, was isolated in a pure form, and its immunogenicity was stable to heating at 100 degrees C for 2 min. Mice immunized with isolated M protein in complete Freund adjuvant and subsequently infected with influenza virus cleared virus more rapidly from their lungs than did unimmunized mice. Despite the rapid clearance of virus, the mice developed pneumonia that was at least as severe as in unimmunized mice. Preliminary studies suggest that the rapid clearance of influenza virus from the lungs of mice immunized with M protein may be initiated by a cell-mediated rather than a humoral response. The mechanism by which a cross-reactive internal virion protein can initiate clearance of the different subtypes of influenza is not clear. Perhaps the M protein is exposed on the surface of the virus-infected cell and is responsible for the cross-reactivity at the cytotoxic T-cell level recently detected between influenza A virus subtypes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aymard-Henry M., Coleman M. T., Dowdle W. R., Laver W. G., Schild G. C., Webster R. G. Influenzavirus neuraminidase and neuraminidase-inhibition test procedures. Bull World Health Organ. 1973;48(2):199–202. [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V. Mechanisms of recovery from a generalized viral infection: mousepox. I. The effects of anti-thymocyte serum. J Exp Med. 1970 Nov;132(5):1035–1054. doi: 10.1084/jem.132.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V. T cell response to viral and bacterial infection. Transplant Rev. 1974;19(0):56–88. doi: 10.1111/j.1600-065x.1974.tb00128.x. [DOI] [PubMed] [Google Scholar]
- Cambridge G., Mackenzie J. S., Keast D. Cell-mediated immune response to influenza virus infections in mice. Infect Immun. 1976 Jan;13(1):36–43. doi: 10.1128/iai.13.1.36-43.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cate T. R., Mold N. G. Increased influenza pneumonia mortality of mice adoptively immunized with node and spleen cells sensitized by inactivated but not live virus. Infect Immun. 1975 May;11(5):908–914. doi: 10.1128/iai.11.5.908-914.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W., Klenk H. D., Caliguiri L. A., Choppin P. W. Influenza virus proteins. I. Analysis of polypeptides of the virion and identification of spike glycoproteins. Virology. 1970 Dec;42(4):880–889. doi: 10.1016/0042-6822(70)90337-5. [DOI] [PubMed] [Google Scholar]
- DE ST GROTH S. F., WITHELL J., LAFFERTY K. J. An improved assay method for neutralizing antibodies against influenza viruses. J Hyg (Lond) 1958 Sep;56(3):415–426. doi: 10.1017/s0022172400037906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C., Zinkernagel R. M. T-cell-mediated immunopathology in viral infections. Transplant Rev. 1974;19(0):89–120. doi: 10.1111/j.1600-065x.1974.tb00129.x. [DOI] [PubMed] [Google Scholar]
- Fazekas de St Groth, Webster R. G. Disquisitions of Original Antigenic Sin. I. Evidence in man. J Exp Med. 1966 Sep 1;124(3):331–345. doi: 10.1084/jem.124.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam E. A., Hampson A. W., Radiskevics I., White D. O. The polypeptides of influenza virus. 3. Identification of the hemagglutinin, neuraminidase and nucleocapsid proteins. Virology. 1970 Nov;42(3):566–575. doi: 10.1016/0042-6822(70)90303-x. [DOI] [PubMed] [Google Scholar]
- ISAACS A., HITCHCOCK G. Role of interferon in recovery from virus infections. Lancet. 1960 Jul 9;2(7141):69–71. doi: 10.1016/s0140-6736(60)91215-0. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Webster R. G. Preparation and immunogenicity of an influenza virus hemagglutinin and neuraminidase subunit vaccine. Virology. 1976 Feb;69(2):511–522. doi: 10.1016/0042-6822(76)90481-5. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Webster R. G. Studies on the origin of pandemic influenza. II. Peptide maps of the light and heavy polypeptide chains from the hemagglutinin subunits of A 2 influenza viruses isolated before and after the appearance of Hong Kong influenza. Virology. 1972 May;48(2):445–455. doi: 10.1016/0042-6822(72)90055-4. [DOI] [PubMed] [Google Scholar]
- McLaren C., Potter C. W. Immunity to influenza in ferrets. VII. Effect of previous infection with heterotypic and heterologous influenza viruses on the response of ferrets to inactivated influenza virus vaccines. J Hyg (Lond) 1974 Feb;72(1):91–100. doi: 10.1017/s0022172400023251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostow S. R., Schild G. C., Dowdle W. R., Wood R. J. Application of the single radial diffusion test for assay of antibody to influenza type A viruses. J Clin Microbiol. 1975 Dec;2(6):531–540. doi: 10.1128/jcm.2.6.531-540.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford J. S., Schild G. C. Immunological and physicochemical studies of influenza matrix (M) polypeptides. Virology. 1976 Oct 15;74(2):394–402. doi: 10.1016/0042-6822(76)90345-7. [DOI] [PubMed] [Google Scholar]
- Palese P., Ritchey M. B., Schulman J. L., Kilbourne E. D. Genetic composition of a high-yielding influenza A virus recombinant: a vaccine strain against "Swine" influenza. Science. 1976 Oct 15;194(4262):334–335. doi: 10.1126/science.968486. [DOI] [PubMed] [Google Scholar]
- SCHULMAN J. L., KILBOURNE E. D. EXPERIMENTAL TRANSMISSION OF INFLUENZA VIRUS INFECTION IN MICE. I. THE PERIOD OF TRANSMISSIBILITY. J Exp Med. 1963 Aug 1;118:257–266. doi: 10.1084/jem.118.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULMAN J. L., KILBOURNE E. D. INDUCTION OF PARTIAL SPECIFIC HETEROTYPIC IMMUNITY IN MICE BY A SINGLE INFECTION WITH INFLUENZA A VIRUS. J Bacteriol. 1965 Jan;89:170–174. doi: 10.1128/jb.89.1.170-174.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild G. C. Evidence for a new type-specific structural antigen of the influenza virus particle. J Gen Virol. 1972 Apr;15(1):99–103. doi: 10.1099/0022-1317-15-1-99. [DOI] [PubMed] [Google Scholar]
- Schulze I. T. The structure of influenza virus. II. A model based on the morphology and composition of subviral particles. Virology. 1972 Jan;47(1):181–196. doi: 10.1016/0042-6822(72)90251-6. [DOI] [PubMed] [Google Scholar]
- Skehel J. J. The characterization of subviral particles derived from influenza virus. Virology. 1971 May;44(2):409–417. doi: 10.1016/0042-6822(71)90271-6. [DOI] [PubMed] [Google Scholar]
- Stanley P., Haslam E. A. The polypeptides of influenza virus. V. Localization of polypeptides in the virion by iodination techniques. Virology. 1971 Dec;46(3):764–773. doi: 10.1016/0042-6822(71)90078-x. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Isachenko V. A., Carter M. A new avian influenza virus from feral birds in the USSR: recombination in nature? Bull World Health Organ. 1974;51(4):325–332. [PMC free article] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G. Studies on the origin of pandemic influenza. I. Antigenic analysis of A 2 influenza viruses isolated before and after the appearance of Hong Kong influenza using antisera to the isolated hemagglutinin subunits. Virology. 1972 May;48(2):433–444. doi: 10.1016/0042-6822(72)90054-2. [DOI] [PubMed] [Google Scholar]



