Abstract
Human preterm neonates are subjected to repetitive pain during neonatal intensive care. We hypothesized that exposure to repetitive neonatal pain may cause permanent or long-term changes because of the developmental plasticity of the immature brain. Neonatal rat pups were stimulated one, two, or four times each day from P0 to P7 with either needle prick (noxious groups N1, N2, N4) or cotton tip rub (tactile groups T1, T2, T4). In groups N2, N4, T2, T4 stimuli were applied to separate paws at hourly intervals; each paw was stimulated only once a day. Identical rearing occurred from P7 to P22 days. Pain thresholds were measured on P16, P22, and P65 (hot-plate test), and testing for defensive withdrawal, alcohol preference, air-puff startle, and social discrimination tests occurred during adulthood. Adult rats were exposed to a hot plate at 62°C for 20 s, then sacrificed and perfused at 0 and 30 min after exposure. Fos expression in the somatosensory cortex was measured by immunocytochemistry. Weight gain in the N2 group was greater than the T2 group on P16 (p < 0.05) and P22 (p < 0.005); no differences occurred in the other groups. Decreased pain latencies were noted in the N4 group [5.0 ± 1.0 s vs. 6.2 ± 1.4 s on P16 (p < 0.05); 3.9 ± 0.5 s vs. 5.5 ±1.6 s on P22 (p < 0.005)], indicating effects of repetitive neonatal pain on subsequent development of the pain system. As adults, N4 group rats showed an increased preference for alcohol (55 ± 18% vs. 32 ± 21%; p < 0.004); increased latency in exploratory and defensive withdrawal behavior (p < 0.05); and a prolonged chemosensory memory in the social discrimination test (p < 0.05). No significant differences occurred in corticosterone and ACTH levels following air-puff startle or in pain thresholds at P65 between N4 and T4 groups. Fos expression at 30 min after hot-plate exposure was significantly greater in all areas of the somatosensory cortex in the T4 group compared with the N4 group (p < 0.05), whereas no differences occurred just after exposure. These data suggest that repetitive pain in neonatal rat pups may lead to an altered development of the pain system associated with decreased pain thresholds during development. Increased plasticity of the neonatal brain may allow these and other changes in brain development to increase their vulnerability to stress disorders and anxiety-mediated adult behavior. Similar behavioral changes have been observed during the later childhood of expreterm neonates who were exposed to prolonged periods of neonatal intensive care.
Keywords: Behavioral effects, Repetitive pain, Rat pups
Premature infants are often exposed to repetitive invasive procedures causing acute pain during the course of neonatal intensive care (4). These experiences occur during a critical window of increased plasticity in the developing brain (32,48). Effects of repetitive pain are coupled with maternal separation, nonsocial physical handling, and stimulation from intense light and sound in the Neonatal Intensive Care Unit (NICU). These adverse psychophysiologic conditions, taken together, may exert permanent untoward effects on the developing brain. Furthermore, preterm neonates frequently do not receive adequate analgesia or sedation during their exposure to invasive, technology-driven intensive care (28).
Human infants and neonatal rat pups display a variety of behavioral and physiologic responses in response to acute pain. These responses have highly conserved developmental or age-specific patterns (19,26). At birth, the neurological maturity of neonatal rat pups is similar to that of human preterm neonates at 24 weeks of gestation, and follows remarkably similar milestones in the development of the pain system (9). For example, exaggerated cutaneous reflexes, large receptive fields of dorsal horn cells, and prolonged excitation in the dorsal horn occur in both species following noxious cutaneous stimulation (10,19,25).
The plasticity of the developing brain during the first post-natal week in newborn rat pups also corresponds with that of the human premature infant from 24 to 36 weeks gestation (31,58). We hypothesized that this developmental plasticity predisposes newborns to long-term or permanent alterations following exposure to repetitive painful stimuli during this critical period. A model of repetitive acute pain in neonatal rat pups, designed to simulate the experience of preterm infants in the NICU, was used to test this hypothesis.
Our experimental model was designed to mimic the experience of preterm neonates who receive repetitive heel sticks for blood sampling. Heel sticks are the commonest invasive procedures performed in the NICU (4,28) (the heel is warmed, a small incision is made through the skin and subcutaneous tissue along the edges of the plantar aspect of the heel, the heel is squeezed and the incision site is touched repeatedly with a capillary tube to obtain blood for clinical monitoring). We decided to mimic this procedure by inserting a 25-gauge needle through the paw of a neonatal rat and repeated this stimulus at hourly intervals, using a separate paw for each subsequent stimulus. The control group was stimulated in a similar manner and at similar intervals using tactile stimuli.
MATERIALS AND METHODS
Animals and Experimental Design
Following transport to our facility on Day 13 of gestation (E13), timed-pregnant Sprague–Dawley rats (Harlan Labs, Birmingham, AL) were housed individually in polycarbonate cages (46 × 25 × 20 cm) containing 3.5 cm high wood chip bedding. Animals were maintained on a 12:12-h light:dark cycle, with food and water freely available. Litters were standardized to eight pups per dam, and rat pups were randomly distributed between the dams just after birth.
Six groups of neonatal rat pups received daily stimulation from P0 (day of birth) to P7 (7 days of age). The paws of rat pups were stimulated once, twice, or four times a day at hourly intervals with tactile (groups, T1, T2, and T4) or noxious (groups, N1, N2, and N4) stimuli. Tactile stimuli consisted of four strokes on the paw with a cotton-tipped swab, and noxious stimuli consisted of a 25-gauge needle inserted rapidly through the paw. Rat pups were returned to the dams between consecutive stimuli after control of bleeding in the noxious stimulation groups. Stimulation occurred between 1400 and 1800 h (Table 1). All rats were reared identically from P7 to P22, weaned on P23, and housed in sex-matched cages until further testing.
TABLE 1.
STIMULATION SCHEDULE
| Data Group | Day of stimulation
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| M/F | P0 | P1 | P2 | P3 | P4 | P5 | P6 | P7 | |
| N1 | 5/8 | Right | Left | Right | Left | Right | Left | Right | Left |
| T1 | 5/6 | forepaw | forepaw | hindpaw | hindpaw | forepaw | forepaw | hindpaw | hindpaw |
| N2 | 2/10 | Right, | Right, | Right, | Right, | Right, | Right, | Right, | Right, |
| T2 | 2/10 | Left | Left | Left | Left | Left | Left | Left | Left |
| forepaw | hindpaw | forepaw | hindpaw | forepaw | hindpaw | forepaw | hindpaw | ||
| N4 | 6/6 | R/L/R/L | R/L/R/L | R/L/R/L | R/L/R/L | R/L/R/L | R/L/R/L | R/L/R/L | R/L/R/L |
| T4 | 5/6 | forepaw | hindpaw | forepaw | hindpaw | forepaw | hindpaw | forepaw | hindpaw |
Observers who were blinded to the group assignment for neonatal rats actually performed the behavioral testing and other measurements. Weight gain was measured with an electronic scale (sensitivity 0.1 g) on P8, P15, and P21. Pain thresholds were measured using a modified hot-plate test on P16 and P22, and a standard hot-plate test on P65. Adult rat behavior was also evaluated using the alcohol preference test, defensive withdrawal test, social discrimination, and air-puff startle tests. Adult rats from groups N4 and T4 were sacrificed at 0 or 30 min after a 20-s exposure to the hot plate at 62°C.
Behavioral Tests
Hot-plate (HP) test for pain threshold
A hot-plate (HP) test for pain threshold was performed with an Omnitech Analgesiometer (Omnitech Electronics, Columbus, OH). The apparatus consists of 25 × 25-cm metal hot-plate surface set at 52°C, a Plexiglas cage that fits over the hot plate, and a foot-switch operated timer. Pain thresholds were measured by the latency to nociceptive responses (limb shaking or paw lick) with a maximum cutoff time at 30 s (latency was averaged from three trials with 15-min intervals between each trial).
Modified HP testing
Infant rats placed on the hot plate did not exhibit the pain behaviors typical of adult rats. Therefore, infant rats were placed in the center of the hot-plate set at 25 or 52°C and contiguous with an extended area (cardboard) at room temperature. The latency to withdrawal from the hot plate was taken as a measure of pain threshold in infant rats. The test was performed at P16 and P22, when the eyes are not fused and the pup was capable of free locomotion. Two training and three testing sessions occurred with intervals of 15 min between each exposure.
Alcohol preference testing
Alcohol preference testing was performed by the simultaneous presentation of two fluid sources: 2% sucrose–water solution, and 5% alcohol in 2% sucrose solution. Alcohol intake was calculated as a proportion of the total intake for each 24-h period, with one training exposure and three testing periods. After each 24-h period, both bottles were weighed and refilled for the next day. Bottle positions were randomized for each test.
Defensive withdrawal testing
This was performed to measure anxiety behavior (57) in an open field (75 ± 75 ± 75 cm) under 300 lx diffuse illumination. Rats were exposed to the air-puff startle as an emotional stressor before the test and then transferred to a dark PVC tube (10 cm diameter × 30 cm long) closed at one end. The tube was placed in one corner of the open field, and behavioral patterns were observed for 10 min via a video camera (Model SS3-M370; Sony Corp., Tokyo, Japan). Each open-field entry was defined as all four paws in the open field, and (a) latency for the first open-field entry, (b) total time spent by the rats in the PVC tube, and (c) number of entries into the open field were measured.
Social discrimination testing
This enabled the assessment of juvenile recognition and discrimination abilities of adult rats (7). It was performed in quiet room during the dark phase of the cycle (2000–2359 h). A juvenile rat (20–30 days old) was introduced into the home cage of an adult male rat (N4 or T4 group) for 4 min. Adult investigation behaviors such as licking, sniffing, and chewing the fur of the juvenile rat were observed and timed with a dedicated computer program. The juvenile was then removed and kept individually in its cage. After a 30-min interexposure time (IET), the original juvenile was presented again simultaneously with a novel juvenile (same age/sex) to the adult rat for a second 4-min period. Investigation behaviors of the adult rat for both juveniles were observed and timed. On a separate night, the same procedure was repeated with a 120 min IET between the first exposure (single juvenile) and the second exposure (two juveniles). Juvenile rats were kept in individual cages with food and water freely available during the test and handled with separate rubber gloves. To allow the observer to distinguish between the two juveniles, the novel juvenile was marked at 2 h before testing, and a mirror was placed behind the test cage.
Cannulation
Between P60–P66, each rat was anesthetized by injecting an acepromazine–ketamine–xylazine mixture (150 μL/100 g b.wt.). This contained 1.0 mL Acepromazine Maleate (10 mg/ mL solution; Fermenta Animal Health Co., Kansas City, MO); 2.5 mL Ketamine (Ketalar® 100 mg/mL solution; Aveco Co. Inc., Fort Dodge, IA); 2.5 mL Xylazine (Rompun® 20 mg/mL solution; Miles Laboratories Inc., Shawnee, KS) in 4 mL water. Under aseptic conditions, a sterile polyethylene catheter with a 3-cm silastic tip was inserted into the right jugular vein until blood return occurred easily, then ligated to the vessel with 3–0 silk and tunneled subcutaneously to emerge between the scapulae. The wound was closed with metal clips and the cannula was filled with sterile heparinized (25 IU/mL) 0.9% saline containing Gentamicin (2.5 μg/100 gm b.wt.). The cannula was flushed with this solution at daily intervals to insure patency. Following surgery, animals were placed in an incubator until fully ambulatory, and then they were placed in a quiet room in polyethylene buckets (28 cm diameter × 37 cm high) with food and water available. The rats were allowed a recovery period for 1 week before further testing.
Air-Puff Startle and Blood Sampling
Air-puff startle was used to evaluate HPA axis responsiveness in adult rats from the N4 and T4 groups, presented as an episode of three blocks of air puffs at 1-min intervals directed toward the side of the head. Each block consisted of three 3 s-long air blasts from a pressurized can (Dust-Off; Fisher Scientific, Atlanta, GA) separated by a 1-s interval. Correct application of the air puff was indicated by the typical startle response (8).
On the morning of testing, extension lines were attached to the jugular catheters at 0800 h and rats were permitted a 90-min stabilization period. During this time, rats tended to go back to sleep. Baseline blood samples (0.3 mL) were obtained for RIA. Blood samples (0.3 mL) were obtained at 5 min before, and 3, 6, 9, 12, and 18 min after presentation of the air-puff startle for measurement of ACTH and corticosterone responses. All blood samples were replaced with an equal volume of sterile 0.9% saline to avoid hypovolemia or hemorrhagic stress. Samples were placed in polyethylene tubes containing cold EDTA solution (60 mg/mL EDTA), and kept on ice until centrifugation (15,000 rpm, 10 min, 4°C), plasma separation, and storage at −20°C.
Radioimmunoassays
Plasma ACTH was determined using a commercially available radioimmunoassay kit, Allegro® HS-ACTH (Nichols Institute, San Juan Capistrano, CA). ACTH was assayed in 100-μL plasma samples using 50 μL of iodinated tracer and one avidin-coated bead per tube as previously described (46). Sensitivity was 3 pg/tube, with an EC50 of 17 pg and a working range of 3–1500 pg/mL. Plasma corticosterone was measured by the commercial ImmuChem Double Antibody kit (ICN Biomedicals, Costa Mesa, CA). The volume of tracer and antibody added to each assay tube was reduced by 50% in the CORT assay, with a working range of 5–1000 ng/mL. The intra- and interassay coefficients of variation were less than 8% for both assays.
Immunocytochemistry (ICC)
Adult Sprague–Dawley rats were anesthetized with sodium pentobarbital (400–500 μL, intraperitoneal) and transcardially perfused with cold 0.9% saline followed with cold 4% paraformaldehyde fixative. Brains were removed and cryoprotected in 20% sucrose at 4°C. Free-floating 25-μm coronal sections were cut on a freezing-sliding microtome, collected in cold cryoprotectant solution, and stored at −20°C until staining. Sections from the experimental and control groups were run together in each ICC assay. While floating on a rotary shaker (40–50 rpm/min), the sections were: rinsed three times for 15 min in 50 mM potassium phosphate-buffered saline pH 7.2 (KPBS); preblocked in KPBS containing 4% normal goat serum (NGS), 0.4% Triton X-100 (TX) and 1% bovine serum albumin (BSA) for 20 min; and then incubated for 72 h at 4°C in a 1:2000 dilution of Fos Ab (Oncogene c-fos Ab-2) in KPBS containing 1% NGS, 0.4% TX, and 1% BSA. After 10 KPBS rinses at room temperature, sections were incubated for 60 min in 1:750 biotinylated goat antirabbit IgG (Vector Labs). Fos immunoreactivity was visualized by the avidin–biotin complex method (ABC, Vector Labs); the sections were rinsed with KPBS, and developed in 0.03% diaminobenzidine in acetate imidazole buffer (175 mM acetate and 10 mM imidazole, pH 7.2) and 0.003% H2O2 following Nickel peroxidase intensification. Finally, the sections were rinsed several times in KPBS, mounted on subbed slides, air dried, dehydrated in alcohols, defatted in xylene, and coverslipped.
Cell Counting
The Paxinos atlas helped to define the sections from areas of interest in the somatosensory cortex and Fos-like immunoreactivity was compared between the treatment groups (N4 vs. T4). Observers blinded to the treatment group allocation of these rats performed all steps of the image capture and cell counting. Six coronal sections were matched for corresponding neuroanatomical levels in the treatment and control groups. Three frames were captured from the superior, intermediate, and inferior parts of each hemisphere of the somatosensory cortex under 10× magnification with a CCD camera (MTI CCD 72; Dage) mounted on a Nikon Microphot 2A microscope. To keep the capture conditions constant (i.e., microscope and camera settings) for all sections from each ICC assay, image capture was completed in one session and all images were saved on magnetoptical disks until analysis with the NIH Image analysis program (version 1.60).
Background noise was first subtracted from the original capture files to provide a sharp black/gain signal level and a clear background, thereby reducing the signal-to-noise ratio for the densitometry steps. The staining of Fos in the cell nucleus produced a dense black appearance, and the “density slice” function was used to label positive cells according to the nuclear blackness density. Specific criteria for negative cells (×6 pixels, no overlay, nonnuclear morphology) were used to determine the density threshold so that only positive cells were labeled. Round-shaped particles in labeled cells were counted by the “analyze particles” function to obtain the total number of particles from each image in this computer counting routine. To verify the results, original image capture files and computer-labeled files were superimposed. Manual corrections to the computer cell counts were made in case of disagreement between the original capture and computer-labeled image files (e.g., two positive nuclei located close together may be labeled as one, or differential staining within one nucleus may be counted as two nuclei). Manually corrected cell counts were used for statistical analysis.
Statistical Analyses
Descriptive analyses on these data sets defined whether they were normally distributed or not. Parametric tests (ANOVA, paired and unpaired t-tests) were used for normally distributed data and other data sets were analyzed by nonparametric tests (Mann–Whitney U-test). Repeated-measures ANOVA was used to compare the Fos labeling between the two treatment groups. An α-error of less than 5% was accepted as significant.
RESULTS
Weight Gain and Pain Thresholds During Development
Rat pups were weighed on P8, P15, and P21 to assess the effect of repetitive stimulation on infant growth (Table 2). Rat pups in the N2 group had significantly lower body weights on P8 (p < 0.05) and P15 (p < 0.005) compared to the T2 group. Rat pups receiving noxious or tactile stimulation once (T1, N1) or four times daily (T4, N4) had no differences in weight gain.
TABLE 2.
BODY WEIGHTS AND PAIN THRESHOLDS IN INFANT AND ADULT RATS
| Data Groups | Weight on P8 | Weight on P15 | Weight on P21 | P16 Hot Plate | P16 Cold Plate | P22 Hot Plate | P22 Cold Plate | P65 Hot Plate |
|---|---|---|---|---|---|---|---|---|
| N1 (n = 13) | 17.4 (1.2) | 32.3 (1.5) | 50.5 (3.5) | 5.9 (1.2) | 19.8 (5.2) | 3.3 (0.7) | 11.3 (4.8) | 12.6 (3.8) |
| T1 (n = 11) | 17.6 (1.9) | 32.7 (1.1) | 50.5 (2.4) | 5.6 (1.0) | 16.2 (4.8) | 3.6 (0.9) | 13.9 (7.7) | 13.4 (4.6) |
| N2 (n = 12) | 15.3 (1.5)* | 30.0 (2.1)† | 45.9 (2.8) | 6.0 (1.7) | 15.6 (4.0) | 3.6 (0.7) | 12.2 (5.3) | 13.1 (3.7) |
| T2 (n = 12) | 16.4 (1.1) | 32.8 (1.9) | 51.3 (4.9) | 6.1 (1.0) | 18.5 (4.8) | 4.3 (0.9) | 9.7 (7.0) | 13.0 (4.1) |
| N4 (n = 12) | 18.3 (2.0) | 35.3 (2.5) | 55.2 (4.5) | 5.0 (1.0)* | 15.8 (3.2) | 3.9 (0.5)† | 15.1 (4.3) | 10.0 (4.9) |
| T4 (n = 11) | 18.5 (1.1) | 34.1 (1.4) | 54.4 (3.6) | 6.2 (1.4) | 15.4 (4.0) | 5.5 (1.6) | 16.7 (3.8) | 12.5 (5.3) |
Weight measured in grams, HP threshold measured in seconds.
p < 0.05,
p < 0.005.
Exposure to the hot plate at 25°C was associated with greater latency than exposure at 52°C (p < 0.001) in all groups of infant rats. Noxious and tactile groups receiving one, two, or four stimuli per day were compared (Table 2). Hot-plate latencies at P16 (p < 0.05) and P22 (p < 0.005) in the N4 rats were significantly decreased as compared to the T4 group.
Behavioral Tests During Adulthood
Adult rats receiving four painful or tactile stimuli per day as neonates were subjected to all behavioral tests, whereas adult rats in the N1, N2, T1, and T2 groups were sacrificed without further testing. Previous experiments noted the absence of behavioral differences between adult rats subjected to neonatal stimulation (painful versus tactile) once or twice a day.
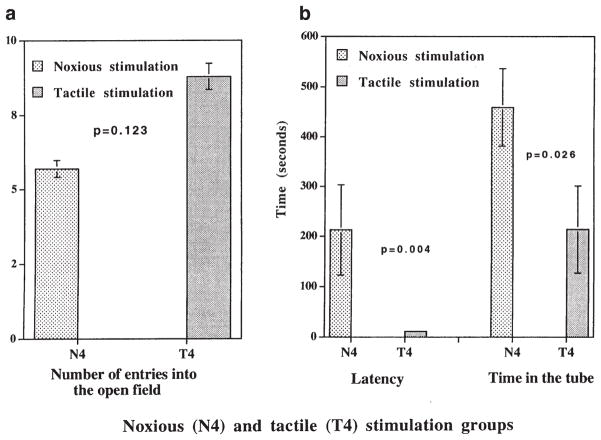
No significant differences occurred in the hot plate latencies of adult rats at P65. The N4 group displayed an increased preference for alcohol, with a significantly higher proportion of alcohol intake [55(18)% intake/24 h] compared to the tactile stimulation group [32(21)% intake/24 h, p = 0.004, unpaired t-test]. These differences were particularly striking between female rats in the N4 group [61(19)% intake/24 h] and T4 group [31(11)% intake/24 h, p = 0.024] (Fig. 1). The N4 group had increased latency for exploration of an open field (p < 0.05), and spent more time in the PVC tube compared to the T4 group (p < 0.05) (Fig. 2). The number of entries into the open field were similar in both groups (N4 group: 5.7 ± 2.7, T4 group: 8.8 ± 2.6, p = 0.123). No significant differences occurred between the corticosterone and ACTH responses of rats in the N4 and T4 groups to the air-puff startle (Table 3).
FIG. 1.
Alcohol preference was tested by the proportion of total fluid intake by the adult rat given a choice between: 2% sucrose or 5% alcohol in 2% sucrose. Each rat was exposed to three testing periods (each period = 24 h) and average intake was calculated for each rat. Data were compared between noxious and tactile stimulation groups using the Mann–Whitney U-test.
FIG. 2.
Defensive withdrawal behavior was tested by placing the rat in a dark PVC tube that was located in a corner of the open field. Behavior was monitored using a video camera (Model SS3-M370; Sony Corp., Tokyo, Japan) and (a) number of entries into the open field, (b) latency for the first open-field entry, and (c) total time spent by the rats in the PVC tube were compared between N4 and T4 groups using the Mann–Whitney U-test. Significantly greater latency and total time spent in the tube by rats in the N4 group suggest increased anxiety following noxious stimulation in the neonatal period.
TABLE 3.
PLASMA CORTICOSTERONEa AND ACTHb LEVELS FOLLOWING AIR-PUFF STARTLE IN ADULT RATS
| Groups | −5 min | + 3 min | + 6 min | + 9 min | + 12 min | + 18 min |
|---|---|---|---|---|---|---|
| N4 (n = 9)a | 51 (63) | 98 (135) | 163 (161) | 213 (203) | 196 (139) | 226 (160) |
| T4 (n = 7)a | 23 (16) | 62 (62) | 157 (84) | 192 (97) | 203 (83) | 240 (128) |
| Corticosterone, *p = | 0.269 | 0.531 | 0.933 | 0.808 | 0.920 | 0.855 |
| N4 (n = 9)b | 15.8 (9.6) | 63.7 (52.7) | 65.7 (57.4) | 64.1 (46.7) | 75.5 (52.8) | 148 (243) |
| T4(n = 7)b | 13.8 (3.6) | 87.6 (42.2) | 93.7 (52) | 109.5 (84) | 128 (145) | 150 (183) |
| ACTH, *p = | 0.593 | 0.343 | 0.331 | 0.190 | 0.332 | 0.989 |
All values = Mean (S.D.);
two-tailed, unpaired t test for comparison between groups.
In the social discrimination test, both groups spent significantly more time investigating the novel juvenile after an inter-exposure time (IET) of 30 min [N4 group: novel juvenile 72.2(16.4) s versus same juvenile 48.7(13.2) s, p < 0.013; T4 group: novel juvenile 69.8(9.1) s versus same juvenile 40.6(17.5) s, p < 0.008], indicating an intact chemosensory memory after initial exposure of the same juvenile (Fig. 3). Following an IET of 120 min, the T4 group rats spent similar times investigating the two juveniles [63.2(19.0) s and 68.4(19.5) s, indicating a loss of chemosensory memory from the initial exposure. In the N4 group this memory was retained even after an IET of 120 min, indicated by a significantly greater time spent investigating the novel juvenile [78.5(11.3) s] compared with the same juvenile [54.8(18.6) s, p < 0.021, t-test) (Figure 3).
FIG. 3.
Social discrimination tested the juvenile recognition and discrimination abilities of adult rats (see text for details of the test procedure). In the T4 group, significantly greater (p = 0.008) investigation of the novel juvenile after an IET (interexposure time) of 30 min implies a chemosensory memory for the previously exposed juvenile, and this memory was lost following an IET of 120 min (no difference between novel and same juvenile, p = 0.421). In the N4 group, chemosensory memory for the previously exposed juvenile was retained after an IET of 30 min (p = 0.013) as well as 120 min (p = 0.021), implying greater vigilance for intruders following noxious stimulation during the neonatal period.
Expression of Fos in the Somatosensory Cortex
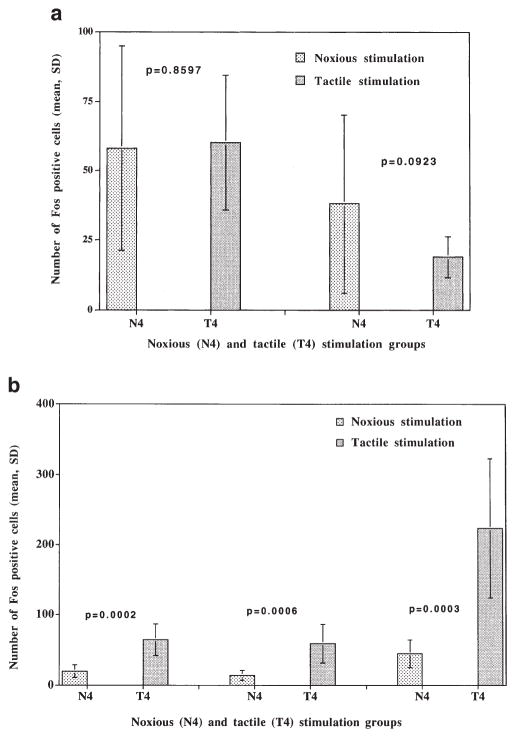
Similar expression of nuclear Fos-like immunoreactivity occurred in sections from the somatosensory cortex of rats in the N4 and T4 groups sacrificed immediately after hot-plate exposure (Fig. 8a). In rats sacrificed at 30 min after hot-plate exposure, the number of somatosensory neurons with Fos expression were significantly increased in the T4 compared to the N4 rats receiving similar treatment (p < 0.0001, repeated-measures ANOVA) (Fig. 8b). Images captured from corresponding somatosensory cortical areas in the T4 and N4 groups are illustrated in Figs. 4–7 as examples of the specific nuclear immunostaining that was noted. Quantitative data obtained from counting the number of Fos-stained cells in the medial, intermediate, and lateral regions of the somatosensory cortex are summarized in Fig. 8.
FIG. 8.
The number of Fos-immunoreactive cells were counted in the somatosensory cortex according to procedures detailed in the text. Rats from the N4 and T4 groups were paired based on gender and body weight. From each pair (one N4 rat and one T4 rat), six repre- sentative sections from the somatosensory cortex were matched for the anatomical level and stained in the same immunocytochemistry assay. Anatomically matched sections included in the same ICC assay from matched N4 and T4 rats were used for cell counting. (a) This shows data from rats killed immediately after exposure to the hot plate. No significant differences occurred in the number of Fos-positive cells between N4 and T4 rats. (b) This shows data from rats killed at 30 min after exposure to the hot plate. Significantly greater numbers of Fos-positive cells were counted in the somatosensory cortex of rats from the T4 group compared to the N4 group (p < 0.0001, repeated measures ANOVA). These data indicate that the constitutive level of Fos expression was similar at baseline for both groups, and that the noxious stimulation activated a significantly greater number of neurons in the somatosensory cortex of the T4 group compared to the N4 group.
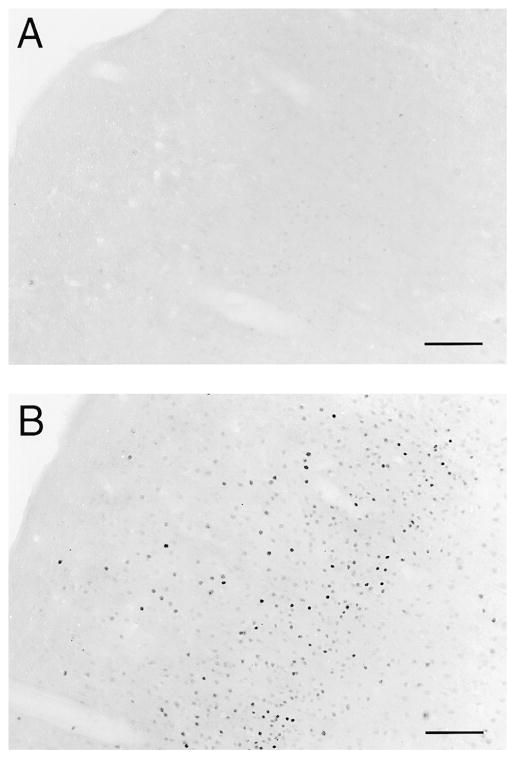
FIG. 4.
Photomicrographs of matched coronal sections from the (A) N4 and (B) T4 groups located in the rostral part of the left somatosensory cortex (Paxinos Rat Brain Atlas: corresponding to Fig. #16; stereotaxic parameters: bregma 0.48 mm, interaural 9.48 mm). Sections were stained with a 1:2000 dilution of Fos antibody, and Fos-like immunoreactivity was visualized by the avidin–biotin complex method using Nickel peroxidase intensification. The darkly stained nuclei represent the nuclear expression of Fos-like immunoreactivity at 30 min following exposure to a hot plate shown at 5× magnification (scale = 400 μm). Greater cortical expression of Fos-like immunoreactivity occurred at 30 min after hot plate exposure in the T4 group (B) compared with the N4 group (A).
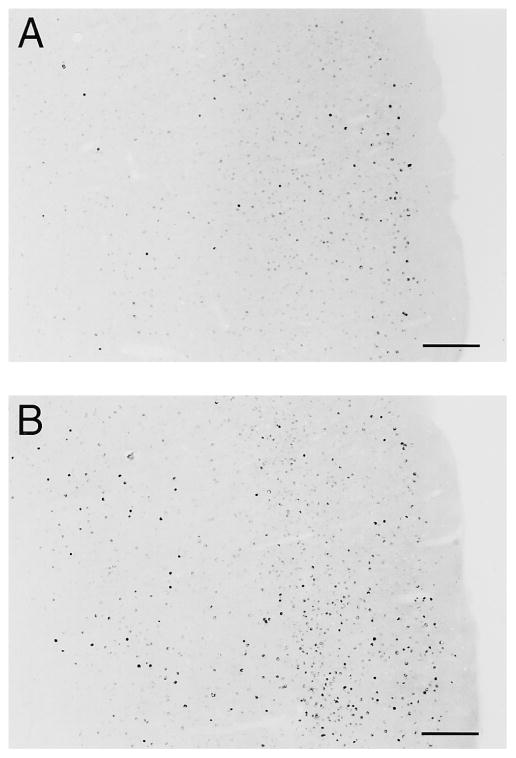
FIG. 7.
Photomicrographs of matched coronal sections from the (A) N4 and (B) T4 groups located in the caudal part of the right somatosensory cortex shown at 20× magnification (scale = 100 μm) (Paxinos Rat Brain Atlas: corresponding to Fig. #33; stereotaxic locations: bregma 3.80 mm, interaural 5.20 mm). The cortical expression of Fos-like immunoreactivity at 30 min following exposure to a hot plate is much greater in the T4 group (B) compared with the N4 group (A).
DISCUSSION
We have found decreased pain thresholds during development in rats who were exposed to repetitive neonatal pain. During adulthood, these rats showed an increased preference for alcohol, increased anxiety and defensive withdrawal behavior, and a prolonged chemosensory memory suggestive of social hypervigilance. During adult life, these rats demonstrated unaltered pain thresholds and HPA responses to an emotional stressor (air-puff startle). Expression of nuclear Fos in the somatosensory cortex following exposure to a hot plate was lower in the adult rats exposed to repetitive neonatal pain compared to rats receiving tactile stimulation. We propose that repetitive neonatal pain may be associated with lower pain thresholds during development and lead to stress vulnerability and anxiety-mediated adult behavior.
Recent data from human preterm infants also suggest that exposure to repetitive pain may alter their responses to subsequent painful or stressful experiences. Johnston and Stevens found a pattern of increased physiological and decreased behavioral responses to a heelstick in preterm neonates exposed to 4 weeks of NICU care. This response pattern, suggestive of learned helplessness, was strongly correlated with the number of invasive procedures experienced since birth (27). Pain experienced during neonatal circumcision was associated with increased behavioral responsiveness to vaccination pain at 4–6 months of age (56). Stressful conditions at birth were associated with increased salivary cortisol responses to vaccination pain at 4 and 6 months of age (49). Reported relationships between exposure to neonatal pain and the physiological correlates of infant temperament and responsiveness (18,54,55) further suggest a widespread distribution of neurobiological changes following neonatal pain/stress.
Based on parent report, expreterm neonates at 18 months corrected age were less reactive to everyday pain than exfull-term toddlers (16). Compared with socially similar exfull-term peers, abnormally increased somatization noted in 4-year-old expreterm children was best predicted by the duration of their NICU stay (15). At age 8–10 years, expreterm children rated pictures of medical events as more painful relative to psychosocial painful events, unlike their peers who were born at full term (17). Again, the duration of their NICU stay was correlated with higher ratings of affective distress to pictures of nonmedical pain events (17). These clinical data suggest a neuropsychological complex of altered pain thresholds and abnormal pain-related behaviors during early childhood (16,20). Correlations with the duration of NICU stay (15,17) and the total number of invasive procedures experienced (27) suggest a closer investigation of the underlying mechanisms in experimental models of repetitive neonatal pain.
Because of ethical considerations, these experiments were designed to detect the minimum number of painful stimuli in neonatal rats associated with changes in adult behavior. The choice of behavioral tests for adult rats was based on the long-term effects of neonatal stress noted from previous experimental studies (40,45,52) and from the behavioral characteristics noted in follow-up studies of expremature (15–17) or exfull-term (15,18,54–56) neonates. Data from the behavioral tests performed in this study suggest that four stimuli at hourly intervals from P0 to P7 will produce some long-term effects. Other behavioral tests may detect long-term changes resulting from fewer stimuli or fewer days of stimulation in the neonatal period, although we are not aware of any such published data. The finding of increased weight gain following noxious stimulation twice a day was not confirmed in subsequent experiments.
Following noxious stimulation in the neonatal period, juvenile rats on P16 and P22 had decreased pain thresholds compared to the tactile stimulation group. These were associated with similar trends in the pain threshold measured during adulthood on P65. These findings were consistent with the increased responsiveness to immunization pain in 4–6-month-old infants, who were previously exposed to unanesthetized neonatal circumcision (56). In contrast, preterm neonates who were exposed to more frequent painful stimulation over prolonged periods of time were noted to have a decreased behavioral responsiveness to acute pain during intensive care (27), after hospital discharge (16), and during later childhood (20).
Mechanistic explanations of decreased pain thresholds in the N4 group may be based on peripheral and/or central mechanisms. Peripheral mechanisms include the hyperinnervation and low pain thresholds noted following skin wounds in the neonatal period compared to similar wounds applied at older ages (P7, P14) or during adult life (50). Noxious stimulation in this study occurred during a critical period of postnatal development in the dorsal horn of the spinal cord (44). Repetitive excitation may promote the persistence of developmental connections between A-β fibers and substantia gelatinosa (SG) neurons. Normally, these fibers are retracted after the first 2 weeks postnatal life when C-fiber connections in the SG have been formed (11). Excitotoxic damage from repetitive noxious stimulation may leave areas of deafferentation in the spinal cord and arrested somatodendritic development (12). Nearby intact sensory neurons would then send collateral sprouts into the deafferented area to form new synaptic connections. This may lead to inappropriate innervation in an area in the dorsal horn that corresponds to sensory information from the stimulated paws (24). Altered connections in the thalamus and somatosensory cortex were also observed in neonatal animal models following afferent neuronal injury (29,33). Further experiments may help to identify an altered development of the peripheral or central pain system components that play a role in the decreased pain thresholds.
Adult rats subjected to repetitive noxious stimulation in the neonatal period demonstrated several behavioral alterations compared to the rats subjected to similar handling and tactile stimulation. Similar patterns of behavioral differences were noted to those observed using other paradigms of neonatal stress [maternal separation (40,45), sepsis (52)], although specific sequelae have occurred from the long-term effects of repetitive neonatal pain (13). One specific effect was the increased preference for alcohol in the N4 group (Fig. 1). Greater alcohol preference was associated with an increased level of anxiety in previous studies of rats, and was correlated with baseline anxiety (6,51,53) as well as following changes in housing conditions (1).
Increased anxiety in the N4 group relative to the T4 group may be inferred from the defensive withdrawal behavior of N4 rats in this study (Fig. 2), with similar behavior noted from previous studies using daily maternal separation as a paradigm for neonatal stress (8). Female rats undergoing repetitive neonatal pain showed a greater susceptibility to the development of alcohol preference, which may be related to postpubertal changes as noted under stressed and nonstressed conditions (2,35) or to increased hepatic ethanol metabolism in female rats (41).
A myriad of mechanisms for increased alcohol preference exist but may include decreased dopaminergic and serotonergic projections to the nucleus accumbens from regions known to regulate reinforcement behavior (22): the ventral tegmental area, anterior and medial hypothalamus, and hippocampus (37,59), increased expression of δ- and μ-opioid receptors in these areas (14,23), or increased norepinephrine release in the paraventricular nucleus (47). Further involvement of the HPA axis was suggested by greater alcohol preference in rats with increased basal concentrations of plasma corticosterone (47) and the prevention of its development by adrenalectomy (34).
We have previously demonstrated long-term changes in HPA axis regulation in rats exposed to neonatal stress, including increased glucocorticoid responses to emotional stressors and impaired negative feedback control mechanisms in the hypothalamus (40,45). In contrast, in the present study, no significant differences occurred in corticosterone and ACTH responses to an air-puff startle (8), and plasma corticosterone levels at baseline were only slightly increased in the N4 group (Table 3). It is likely that the combination of repetitive pain and maternal separation in neonatal rats (as experienced by preterm neonates in the NICU) may lead to permanent alterations in HPA axis regulation.
Results from the social discrimination test, with a prolonged chemosensory memory in the N4 group, indicate the coexistence of anxiety and hypervigilance behavior in adult rats subjected to repetitive neonatal pain (Fig. 3). Bergvall and colleagues (5) have recently reported the lack of a correlation between aggressivity and increased alcohol drinking, although they used a social aggression paradigm (intermittent exposure to sexually active females) rather than the juvenile intruder paradigm used in this study. A large body of literature has correlated alcohol preference with anxiety and increased aggressiveness in humans, rats, and other species (21). During the social discrimination test, aggressive behaviors (biting, mounting, aggressive posture) were noted more frequently in male rats from the N4 group compared to the T4 group, and were particularly directed at the novel juvenile during the second exposure. An alternative explanation that aggressive behavior followed their stress reactions to novel environmental factors (Fig. 2) seems less likely (3).
Expression of neuronal Fos in the adult somatosensory cortex was measured as an index of neuronal activation and correlated with behavior following hot-plate exposure. Neuronal Fos expression is a nonspecific marker for neuronal activation, and may represent functional plasticity of the somatosensory cortex (30,39). Rats in both N4 and T4 groups killed just after hot-plate exposure had similar numbers of cortical cells expressing nuclear Fos (Fig. 8). At 30 min after hot-plate exposure Fos expression was significantly greater in the adult T4 group compared to the N4 group. Notes made at the time of hot plate exposure revealed mostly freezing and learned-helplessness behavior in the N4 group, whereas exploratory and escape behavior occurred commonly in the T4 group. High-stress behavior in the N4 group may be correlated with the increased anxiety noted in the defensive withdrawal test, and the increased preference for alcohol. Higher Fos expression noted in the T4 group may represent a more cortical response to inescapable nociception, because the cellular density of cortical areas in the two groups appeared qualitatively similar.
Evidence that environmental manipulations in newborn rat pups may lead to persistent changes in the endocrine, immune, and behavioral reactivity of adult rats is mounting (39,42,43,45). Neonatally stressed adult rats have decreased exploratory behavior in novel environments, a lower threshold for learned helplessness, and increased loss of hippocampal neurons associated with early onset cognitive defects compared to control groups of adult rats (36,39,45). Endocrine changes include increased HPA axis responses to emotional stressors and impaired negative feedback control mechanisms in the hypothalamus (40,52). These long-term changes have been attributed to plasticity in the neonatal hypothalamus, forebrain, prefrontal cortex, and hippocampus, and were related to the expression of glucocorticoid and other receptor systems involved in regulation of the HPA axis, the autonomic nervous system, and adult stress behavior (38,39). As large numbers of expreterm neonates progress through adolescence and adult life, a deeper understanding of the consequences of invasive neonatal intensive care will become possible. Such experimental models can also be used to test mechanisms and therapies that may be beneficial for the future health care of prematurely born individuals.
FIG. 5.
Photomicrographs of matched coronal sections from the (A) N4 and (B) T4 groups located in the rostral part of the left somatosensory cortex shown at 20× magnification (scale = 100 μm). Greater cortical expression of Fos-like immunoreactivity is evident in the T4 group (B) compared with the N4 group (A).
FIG. 6.
Photomicrographs of matched coronal sections from the (A) N4 and (B) T4 groups located in the caudal part of the right somatosensory cortex (Paxinos Rat Brain Atlas: corresponding to Fig. #33; stereotaxic locations: bregma 3.80 mm, interaural 5.20 mm). Sections were stained with a 1:2000 dilution of Fos antibody, and Fos-like immunoreactivity was visualized by the avidin–biotin complex method using Nickel peroxidase intensification. The darkly stained nuclei represent the nuclear expression of Fos-like immunoreactivity shown at 5× magnification (scale = 400 μm). The cortical expression of Fos-like immunoreactivity at 30 min following exposure to a hot plate is much greater in the T4 group (B) compared with the N4 group (A).
Acknowledgments
This work was supported by the National Institute for Child Health and Human Development (HD-01123-01A1), Emory-Egleston Children’s Research Center, and National Institute of Mental Health (MH-42088).
References
- 1.Adams N, Oldham TD. Seminatural housing increases subsequent ethanol intake in male Maudsley Reactive rats. J Stud Alcohol. 1996;57:349–351. doi: 10.15288/jsa.1996.57.349. [DOI] [PubMed] [Google Scholar]
- 2.Adams N. Sex differences and the effects of tail pinch on ethanol drinking in Maudsley rats. Alcohol. 1995;12:463–468. doi: 10.1016/0741-8329(95)00032-m. [DOI] [PubMed] [Google Scholar]
- 3.Badishtov BA, Overstreet DH, Kashevskaya OP, et al. To drink or not to drink: Open field behavior in alcohol-preferring and nonpreferring rat strains. Physiol Behav. 1995;57:585–589. doi: 10.1016/0031-9384(94)00299-k. [DOI] [PubMed] [Google Scholar]
- 4.Barker DP, Rutter N. Exposure to invasive procedures in neonatal intensive care unit admissions. Arch Dis Childhood. 1995;72:F47–F48. doi: 10.1136/fn.72.1.f47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergvall AH, Fahike CJ, Aonsson L, Hansen S. In quest for a possible association between heightened social aggression and excessive alcohol drinking in the rat. Physiol Behav. 1996;59:807–812. doi: 10.1016/0031-9384(95)02156-6. [DOI] [PubMed] [Google Scholar]
- 6.Colombo G, Agabio R, Lobina C, Reali R, Zocchi A, Fadda F, et al. Sardinian alcohol preferring rats: A genetic animal model of anxiety. Physiol Behav. 1995;57:1181–1185. doi: 10.1016/0031-9384(94)00382-f. [DOI] [PubMed] [Google Scholar]
- 7.Engelmann M, Wotjak CT, Landgraf R. Social discrimination procedure: An alternative method to investigate juvenile recognition abilities in rats. Physiol Behav. 1995;58:315–321. doi: 10.1016/0031-9384(95)00053-l. [DOI] [PubMed] [Google Scholar]
- 8.Engelmann M, Thrivikraman KV, Su Y, Nemeroff CB, Montkowski A, Landgraf R, et al. Endocrine and behavioral effects of airpuff-startle in rats. Psychoneuroendocrinology. 1996;21:391–400. doi: 10.1016/0306-4530(96)00006-6. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald M, Anand KJS. The developmental neuroanantomy and neurophysiology of pain. In: Schechter N, Berde C, Yaster M, editors. Pain management in infants, children and adolescents. Baltimore, MD: Williams & Williams; 1993. pp. 11–32. [Google Scholar]
- 10.Fitzgerald M, Millard C, McIntosh N. Cutaneous hypersensitivity following peripheral tissue damage in newborn infants and its reversal with topical anaesthesia. Pain. 1989;39:31–36. doi: 10.1016/0304-3959(89)90172-3. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald M, Butcher T, Shortland P. Developmental changes in the laminar termination of A fibre cutaneous sensory afferents in the rat spinal cord dorsal horn. J Comp Neurol. 1994;348:225–233. doi: 10.1002/cne.903480205. [DOI] [PubMed] [Google Scholar]
- 12.Fitzerald M, Shortland P. The effect of neonatal peripheral nerve section on the somatodendritic growth of sensory projection cells in the rat spinal cord. Dev Brain Res. 1988;42:129–133. doi: 10.1016/0165-3806(88)90208-8. [DOI] [PubMed] [Google Scholar]
- 13.Francis D, Diorio J, LaPlante P, Weaver S, Seckl JR, Meaney MJ. The role of early environmental events in regulating neuroendocrine development. Ann NY Acad Sci. 1996;794:136–152. doi: 10.1111/j.1749-6632.1996.tb32517.x. [DOI] [PubMed] [Google Scholar]
- 14.Gianoulakis C. Implications of endogenous opioids and dopamine in alcoholism: Human and basic science studies. Alcohol Alcohol. 1996;31(Suppl 1):33–42. [PubMed] [Google Scholar]
- 15.Grunau RVE, Whitfield MF, Petrie JH, Fryer EL. Early pain experience, child and family factors as precursors of somatization: A prospective study of extremely premature and fullterm children. Pain. 1994;56:353–359. doi: 10.1016/0304-3959(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 16.Grunau RVE, Whitfield MF, Petrie JH. Pain sensitivity and temperament in extremely-low-birthweight premature toddlers and preterm and full-term controls. Pain. 1994;58:341–346. doi: 10.1016/0304-3959(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 17.Grunau RVE, Whitfield MF, Petrie JH. Children’s judgements about pain at age 8–10 years: Do extremely low birth-weight (<1000 gm) children differ from full birthweight peers? J Child Psychol Psychiatry. 1998;39:587–594. [PubMed] [Google Scholar]
- 18.Gunnar MR, Porter FL, Wolf CM, Rigatuso J, Larson MC. Neonatal stress reactivity: Predictions to later emotional temperament. Pediatrics. 1995;66:1–13. doi: 10.1111/j.1467-8624.1995.tb00851.x. [DOI] [PubMed] [Google Scholar]
- 19.Guy ER, Abbott FV. The behavioral response to formalin in preweanling rats. Pain. 1992;51:81–90. doi: 10.1016/0304-3959(92)90012-Z. [DOI] [PubMed] [Google Scholar]
- 20.Herzog JM. The neonatal intensive care syndrome: A pain complex involving neuroplasticity and psychic trauma. In: Call JD, Galenson E, Tyson RL, editors. Frontiers of infant psychiatry. New York: Basic Books; 1983. pp. 291–300. [Google Scholar]
- 21.Higley JD, Linnoila M. A nonhuman primate model of excessive alcohol intake: Personality and neurobiological parallels of type I- and type II-like alcoholism. Rec Dev Alcohol. 1997;13:191–219. [PubMed] [Google Scholar]
- 22.Hodge CW, Slawecki CJ, Aiken AS. Norepinephrine and serotonin receptors in the paraventricular nucleus interactively modulate ethanol consumption. Alcohol Clin Exp Res. 1996;20:1669–1674. doi: 10.1111/j.1530-0277.1996.tb01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honkanen A, Vilamo L, Wegelius K, Sarviharju M, HyytiAa P, Korpi ER. Alcohol drinking is reduced by mu 1-but not by a delta-opioid receptor antagonist in alcohol-preferring rats. Eur J Pharmacol. 1996;304:7–13. doi: 10.1016/0014-2999(96)00118-5. [DOI] [PubMed] [Google Scholar]
- 24.Jacquin MF, Renehan WE, Klein BG, Mooney RD, Rhoades RW. Functional consequences of neonatal infraorbital nerve section in rat trigeminal ganglion. J Neurosci. 1986;6:3706–3711. doi: 10.1523/JNEUROSCI.06-12-03706.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jennings E, Fitzgerald M. c-fos can be induced in the neonatal rat spinal cord by both noxious and innocuous peripheral stimulation. Pain. 1996;68:301–306. doi: 10.1016/s0304-3959(96)03194-6. [DOI] [PubMed] [Google Scholar]
- 26.Johnston CC, Stevens B, Craig KD, Grunau RVE. Developmental changes in pain expression in premature, full-term, two- and four-month-old infants. Pain. 1993;52:201–208. doi: 10.1016/0304-3959(93)90132-9. [DOI] [PubMed] [Google Scholar]
- 27.Johnston CC, Stevens BJ. Experience in a neonatal intensive care unit affects pain response. Pediatrics. 1996;98:925–930. [PubMed] [Google Scholar]
- 28.Johnston CC, Collinge JM, Henderson SJ, Anand KJS. A cross-sectional survey of pain and pharmacological analgesia in Canadian neonatal intensive care units. Clin J Pain. 1997;13:308–312. doi: 10.1097/00002508-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Kaas JH, Merzenich MM, Killackey HP. The reorganization of somatosensory cortex following peripheral damage in adult and developing animals. Annu Rev Neurosci. 1983;6:325–356. doi: 10.1146/annurev.ne.06.030183.001545. [DOI] [PubMed] [Google Scholar]
- 30.Kaminska B, et al. Elevated AP-1 transcription factor DNA binding activity at the onset of functional plasticity during development of rat sensory cortical areas. Brain Res Mol Brain Res. 1995;33:295–304. doi: 10.1016/0169-328x(95)00149-m. [DOI] [PubMed] [Google Scholar]
- 31.Kim JJ, et al. Behavioral stress modifies hippocampal plasticity through N-methyl-d-aspartate receptor activation. Proc Natl Acad Sci USA. 1996;93:4750–4753. doi: 10.1073/pnas.93.10.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knudsen EI. Capacity for plasticity in the adult owl auditory system expanded by juvenile experience. Science. 1998;279:1531–1533. doi: 10.1126/science.279.5356.1531. [DOI] [PubMed] [Google Scholar]
- 33.Koralek KA, Killackey HP. Callosal projections in rat somatosensory cortex are altered by early removal of afferent input. Proc Natl Acad Sci USA. 1990;87:1396–400. doi: 10.1073/pnas.87.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamblin F, De Witte P. Adrenalectomy prevents the development of alcohol preference in male rats. Alcohol. 1996;13:233–238. doi: 10.1016/0741-8329(95)02043-8. [DOI] [PubMed] [Google Scholar]
- 35.Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcohol Clin Exp Res. 1996;20:1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- 36.Landfield PW, McEwan BS, Sapolsky RM, Meaney MJ. Hippocampal cell death. Science. 1996;272:1249–1251. [PubMed] [Google Scholar]
- 37.McBride WJ, Bodart B, Lumeng L, Li TK. Association between low contents of dopamine and serotonin in the nucleus accumbens and high alcohol preference. Alcohol Clin Exp Res. 1995;19:1420–1422. doi: 10.1111/j.1530-0277.1995.tb01001.x. [DOI] [PubMed] [Google Scholar]
- 38.McEwen BS. The plasticity of the hippocampus is the reason for its vulnerability. Neuroscience. 1994;6:239–246. [Google Scholar]
- 39.Meaney MJ, Sharma S, Sarrieau S, Plotsky PM. Postnatal development and environmental regulation of hippocampal glucocorticoid and mineralocorticoid receptors in the rat. Dev Brain Res. 1988;43:158–162. doi: 10.1016/0165-3806(88)90162-9. [DOI] [PubMed] [Google Scholar]
- 40.Meaney MJ, Tannenbaum B, Bhatnagar S, Shanks N, Viau V, O’Donnel D. Early environmental programming of hypothalmic–pituitary–adrenal responses to stress. Neuroscience. 1994;6:247–259. [Google Scholar]
- 41.Nebbia C, Dacasto M, Ceppa L, Bosia S, Burdino E, Witkamp RF, et al. Gender differences in ethanol oxidation and cytochrome P4502E1 content and functions in hepatic microsomes from alcohol-referring and non-preferring rats. Xenobiotica. 1996;26:1121–1129. doi: 10.3109/00498259609050257. [DOI] [PubMed] [Google Scholar]
- 42.Neveu PJ, Deleplanque B, Puglisi–Allegra S, Amato FRD, Cabib S. Influence of early life events on immune reactivity in adult male mice. Dev Psychobiol. 1994;27:205–213. doi: 10.1002/dev.420270403. [DOI] [PubMed] [Google Scholar]
- 43.Pieretti S, d’Amore A, Loizzo A. Long-term changes induced by developmental handling on pain threshold: Effects of morphine and naloxone. Behav Neurosci. 1991;105:215–218. doi: 10.1037//0735-7044.105.1.215. [DOI] [PubMed] [Google Scholar]
- 44.Pignatelli D, Silva DA, Coimbra A. Postnatal maturation of primary afferent terminations in the substantia gelatinosa of the rat spinal cord. An electron microscope study. Brain Res. 1989;491:33–44. doi: 10.1016/0006-8993(89)90085-1. [DOI] [PubMed] [Google Scholar]
- 45.Plotsky PM, Meaney MJ. Early postnatal experience alters hypothalmic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 46.Plotsky PM, Thrivikraman KV, Watts GA, Hauger RL. Hypothalamic–pituitary–adrenal axis function in the Zucker obese rats. Endocrinology. 1992;130:1931–1941. doi: 10.1210/endo.130.4.1312431. [DOI] [PubMed] [Google Scholar]
- 47.Prasad C, Prasad A. A relationship between increased voluntary alcohol preference and basal hypercorticosteronemia associated with an attenuated rise in corticosterone output during stress. Alcohol. 1995;12:59–63. doi: 10.1016/0741-8329(94)00070-t. [DOI] [PubMed] [Google Scholar]
- 48.Rabinowicz T, de Courten–Myers GM, Petetot JM, Xi G, de los Reyes E. Human cortex development: Estimates of neuronal numbers indicate major loss late during gestation. J Neuropathol Exp Neurol. 1996;55:320–328. [PubMed] [Google Scholar]
- 49.Ramsay DS, Lewis M. The effects of birth condition on infants’ cortisol response to stress. Pediatrics. 1995;95:546–549. [PubMed] [Google Scholar]
- 50.Reynolds ML, Fitzgerald M. Long-term sensory hyperinnervation following neonatal skin wounds. J Comp Neurol. 1995;358:487–498. doi: 10.1002/cne.903580403. [DOI] [PubMed] [Google Scholar]
- 51.Sandbak T, Murison R. Voluntary alcohol consumption in rats: Relationships to defensive burying and stress gastric erosions. Physiol Behav. 1996;59:983–989. doi: 10.1016/0031-9384(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 52.Shanks N, Larocque S, Meaney MJ. Neonatal endotoxin exposure alters the development of the hypothalamic–pituitary–adrenal axis: Early illness and later responsivity to stress. J Neurosci. 1995;15:376–384. doi: 10.1523/JNEUROSCI.15-01-00376.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spanagel R, Montkowski A, Allingham K, StAohr T, Shoaib M, Holsboer F, et al. Anxiety: A potential predictor of vulnerability to the initiation of ethanol self-administration in rats. Psychopharmacology (Berlin) 1995;122:369–373. doi: 10.1007/BF02246268. [DOI] [PubMed] [Google Scholar]
- 54.Stifter C, Fox NA. Infant reactivity: Physiological correlates of newborn and 5-month temperament. Dev Psychol. 1990;26:582–588. [Google Scholar]
- 55.Stifter C, Jain A. Psychophysiological correlates of infant temperament: Stability of behavior and autonomic patterning from 5 to 18 months. Dev Psychobiol. 1996;29:379–384. doi: 10.1002/(SICI)1098-2302(199605)29:4<379::AID-DEV5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 56.Taddio A, Katz J, Ilersich AL, Koren G. Effect of neonatal circumcision on pain response during subsequent routine vaccination. Lancet. 1997;349:599–603. doi: 10.1016/S0140-6736(96)10316-0. [DOI] [PubMed] [Google Scholar]
- 57.Takahashi LK, Kalin NH, Van den Burgt JA, Sherman JE. Corticotrophin-releasing factor modulates defensive withdrawal and exploratory behavior in rats. Behav Neurosci. 1989;103:648–654. doi: 10.1037//0735-7044.103.3.648. [DOI] [PubMed] [Google Scholar]
- 58.Wilson DA. NMDA receptors mediate expression of one form of functional plasticity induced by olfactory deprivation. Brain Res. 1995;677:238–242. doi: 10.1016/0006-8993(95)00151-f. [DOI] [PubMed] [Google Scholar]
- 59.Zhou FC, Zhang JK, Lumeng L, Li TK. Mesolimbic dopamine system in alcohol-preferring rats. Alcohol. 1995;12:403–412. doi: 10.1016/0741-8329(95)00010-o. [DOI] [PubMed] [Google Scholar]