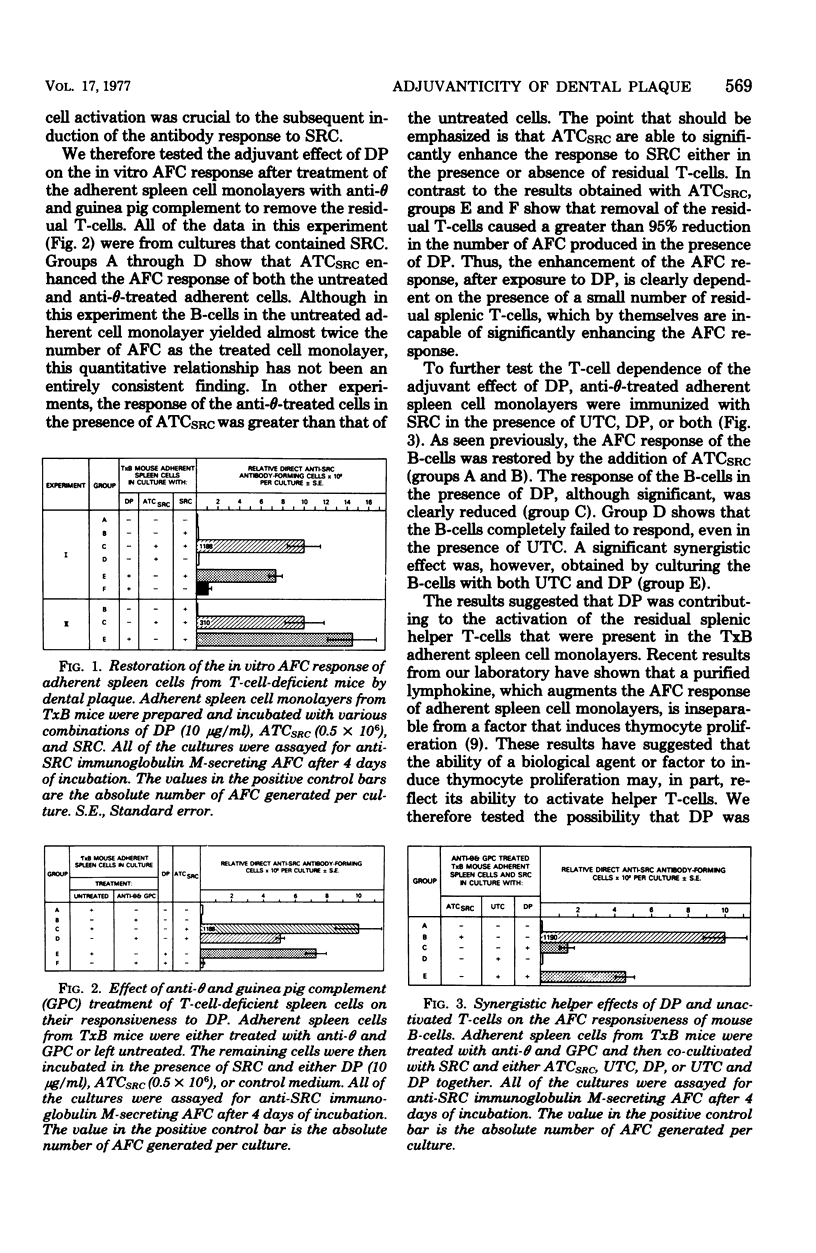
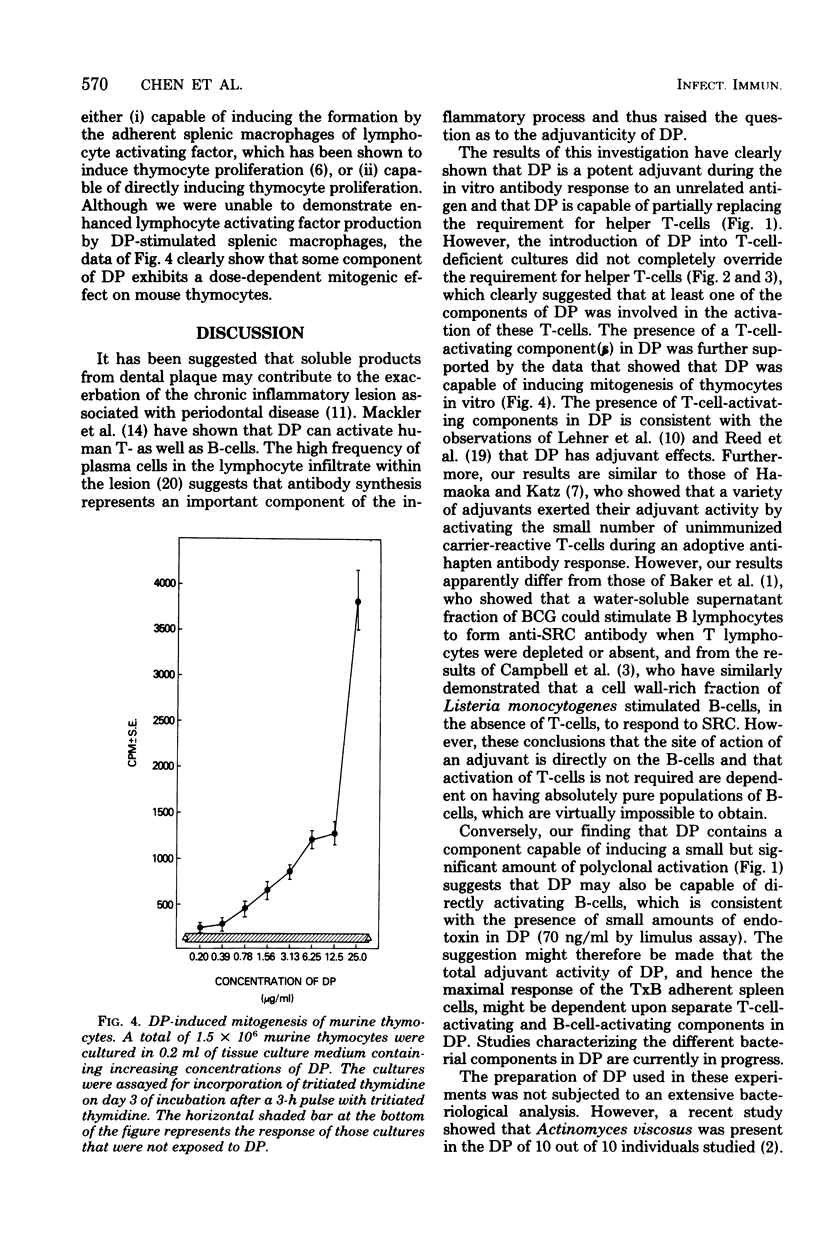
Abstract
The immunoenhancing activity of a water-soluble extract of dental plaque (DP), which contains a mixture of microbial antigens, has been investigated. DP was tested for its capacity to augment the in vitro antibody-forming cell (AFC) response to sheep erythrocyte by adherent spleen cells from thymectomized, lethally irradiated, and bone marrow-transplanted (TxB) mice. Although DP was found to induce a small polyclonal AFC response, most of the increase in AFC induced by DP was antigen dependent. The latter enhancing effect is an indicator of the adjuvanticity of DP. This adjuvant activity of DP was T-cell-dependent, since removal of the residual prethymic and/or thymic-derived lymphocytes (T-cells) by anti-T-cell serum (anti-theta) and guinea pig complement abrogated the capacity of DP to augment the in vitro AFC response. This view was further supported by the synergistic restorative effect obtained by culturing anti-theta-treated adherent spleen cells with both DP and a population of unactivated T-cells that by themselves were unable to significantly enhance AFC responsiveness. Moreover, DP was found to be mitogenic for thymocytes. The cumulative results suggest that the adjuvant activity of DP is dependent on both the T- and B-cell-activating components present in DP.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker L. A., Sharpton T., Minden P., Campbell P. A. Adjuvant and mitogenic properties of a supernatant fraction of sonically treated Myobacterium bovis (BCG). Infect Immun. 1976 Jul;14(1):83–87. doi: 10.1128/iai.14.1.83-87.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden G. H., Hardie J. M., Slack G. L. Microbial variations in approximal dental plaque. Caries Res. 1975;9(4):253–277. doi: 10.1159/000260162. [DOI] [PubMed] [Google Scholar]
- Campbell P. A., Schuffler C., Rodriguez G. E. Listeria cell wall fraction: a B cell adjuvant. J Immunol. 1976 Mar;116(3):590–594. [PubMed] [Google Scholar]
- Farrar J. J., Fuller-Bonar J. The xenogeneic effect. II. Requirement for unactivated murine T cells during restoration of immune responsiveness with xenogeneic reconstitution factor. J Immunol. 1976 Jul;117(1):274–282. [PubMed] [Google Scholar]
- Farrar J. J., Loughman B. E., Nordin A. A. Lymphopoietic potential of bone marrow cells from aged mice: comparison of the cellular constituents of bone marrow from young and aged mice. J Immunol. 1974 Mar;112(3):1244–1249. [PubMed] [Google Scholar]
- Gery I., Waksman B. H. Potentiation of the T-lymphocyte response to mitogens. II. The cellular source of potentiating mediator(s). J Exp Med. 1972 Jul 1;136(1):143–155. doi: 10.1084/jem.136.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaoka T., Katz D. H. Cellular site of action of various adjuvants in antibody responses to hapten-carrier conjugates. J Immunol. 1973 Nov;111(5):1554–1563. [PubMed] [Google Scholar]
- Jerne N. K., Nordin A. A. Plaque Formation in Agar by Single Antibody-Producing Cells. Science. 1963 Apr 26;140(3565):405–405. doi: 10.1126/science.140.3565.405. [DOI] [PubMed] [Google Scholar]
- Koopman W. J., Farrar J. J., Oppenheim J. J., Fuller-Bonar J., Dougherty S. Association of a low molecular weight helper factor(s) with thymocyte proliferative activity. J Immunol. 1977 Jul;119(1):55–60. [PubMed] [Google Scholar]
- LOE H., THEILADE E., JENSEN S. B. EXPERIMENTAL GINGIVITIS IN MAN. J Periodontol. 1965 May-Jun;36:177–187. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehner T., Wilton J. M., Challacombe S. J., Ivanyi L. Sequential cell-mediated immune responses in experimental gingivitis in man. Clin Exp Immunol. 1974 Mar;16(3):481–492. [PMC free article] [PubMed] [Google Scholar]
- Loughman B. E., Farrar J. J., Nordin A. A. Presence of antibody-forming cell precursors in the mouse adherent spleen cell population. J Immunol. 1974 Jan;112(1):430–432. [PubMed] [Google Scholar]
- Mackler B. F., Altman L. C., Wahl S., Rosenstreich D. L., Oppenheim J. J., Mergenhagen S. E. Blastogenesis and lymphokine synthesis by T and B lymphocytes from patients with periodontal disease. Infect Immun. 1974 Oct;10(4):844–850. doi: 10.1128/iai.10.4.844-850.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Cell to cell interaction in the immune response. I. Hemolysin-forming cells in neonatally thymectomized mice reconstituted with thymus or thoracic duct lymphocytes. J Exp Med. 1968 Oct 1;128(4):801–820. doi: 10.1084/jem.128.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E. A requirement for two cell types for antibody formation in vitro. Science. 1967 Dec 22;158(3808):1573–1575. doi: 10.1126/science.158.3808.1573. [DOI] [PubMed] [Google Scholar]
- Plotz P. H., Talal N., Asofsky R. Assignment of direct and facilitated hemolytic plaques in mice to specific immunoglobulin classes. J Immunol. 1968 Apr;100(4):744–751. [PubMed] [Google Scholar]
- Reed M. J., Neiders M. E., Genco R. J. Synergistic effects on DNA synthesis in peripheral blood lymphocytes of mitogens and dental plaque sonicates in man and macaque monkeys. Arch Oral Biol. 1976;21(7):441–445. doi: 10.1016/0003-9969(76)90009-1. [DOI] [PubMed] [Google Scholar]
- Schlossberg A., Ferrigno P. D. A histological study of the incidence of plasma cells and lymphocytes in human gingival tissues. J Oral Med. 1971 Dec;26(3):99–105. [PubMed] [Google Scholar]


