Abstract
Genomic technologies have revolutionized our understanding of complex Mendelian diseases and cancer. Solid tumors present several challenges for genomic analyses, such as tumor heterogeneity and tumor contamination with surrounding stroma and infiltrating lymphocytes. We developed a protocol to (i) select tissues of high cellular purity on the basis of histological analyses of immediately flanking sections and (ii) simultaneously extract genomic DNA (gDNA), messenger RNA (mRNA), noncoding RNA (ncRNA; enriched in microRNA (miRNA)) and protein from the same tissues. After tissue selection, about 12–16 extractions of DNA/RNA/protein can be obtained per day. Compared with other similar approaches, this fast and reliable methodology allowed us to identify mutations in tumors with remarkable sensitivity and to perform integrative analyses of whole-genome and exome data sets, DNA copy numbers (by single-nucleotide polymorphism (SNP) arrays), gene expression data (by transcriptome profiling and quantitative PCR (qPCR)) and protein levels (by western blotting and immunohistochemical analysis) from the same samples. Although we focused on renal cell carcinoma, this protocol may be adapted with minor changes to any human or animal tissue to obtain high-quality and high-yield nucleic acids and proteins.
Keywords: Extraction, co-extraction, co-isolation, purification, small RNAs, cancer genomics, molecular genetics, BAP1, PBRM1
INTRODUCTION
Whole-genome and exome sequencing have revolutionized our understanding of complex Mendelian diseases1 and cancer2, 3. Blood cancer cells (from leukemias) have traditionally been more successfully used in genomic analysis because the circulating tumor cells can be separated with high accuracy using flow cytometry and fluorescence-activated cell sorting (FACS) on the basis of immunolabeling4.
Solid tumors may show high heterogeneity5. Even adjacent areas of the tumor can harbor different mutations and/or alterations5. However, DNA and RNA are often isolated separately from different samples that may not correspond to the same affected area. Thus, correlations of DNA with RNA in downstream analyses may be compromised. In addition, tumor cellularity is often estimated on the basis of a sample that may not be representative of the sample used for nucleic acid extraction. Solid tumors are also surrounded by stromal cells and may be infiltrated with lymphocytes. These ‘contaminating’ cells contain diploid normal DNA, normal RNA and normal levels of proteins, diluting the contribution of tumor cells. Some very sensitive techniques, such as SNP arrays can withstand a large degree of DNA dilution and are still able to detect chromosomal imbalances. However, other techniques, including DNA sequencing, are substantially affected by dilution with normal DNA. Laser-capture microdissection allows highly pure cell populations to be obtained through direct microscopic visualization6. However, it has several limitations. Obtaining enough cells for downstream applications is very time-consuming, it requires an expert pathologist or cytologist, and the quality of the nucleic acid and protein may be affected by sample processing and the time involved in the microdissection6. Although some techniques, such as DNA copy-number analysis and whole-genome sequencing, can be performed on single cells7, 8, this methodology requires whole-genome amplification, which may result in the generation of artifacts during the amplification process. Tumor cells from solid tumors may instead be enriched by generating early-passage cell lines to remove contaminating fibroblasts9, although additional genomic alterations may be acquired by growing the cells on plastic under nonphysiological conditions. Alternatively, human tumors implanted in immunocompromised mice (tumorgrafts) may be used, in which stroma is replaced by the host10, providing nearly pure human tumor populations. Tumorgrafts have mouse stroma contamination, but this does not interfere with human-specific Sanger sequencing, gene expression microarrays, and SNP arrays (for copy-number analyses)11. Therefore, tumorgrafts offer the possibility of studying highly-pure tumor populations from solid tumors11. Furthermore, tumorgrafts reproduce the mutations, gene expression, and treatment responsiveness of the corresponding tumors in patients and thus represent faithful models12. However, although tumorgrafts are helpful, when tumor samples are carefully selected, high sensitivities for mutation detection may be obtained directly from patient tumors11.
This protocol describes steps for extracting nucleic acids and proteins from carefully selected tissues on the basis of their immediate histology using a modification of two commercially available kits. We were successful at obtaining high-quality and high-yield gDNA, RNA and proteins11. By using this approach, we achieved a very high sensitivity of mutation detection. For instance, we observed nonsilent somatic mutations in the most characteristic tumor suppressor gene in clear-cell renal cell carcinoma (ccRCC), VHL, in 79% of the tumors. Similar results were obtained by a recent study13 and compares favorably with other large-scale sequencing studies14–19 (Table 1). The mutation rate is very close to the highest reported in the literature to date (82%)20. This study used endonuclease scanning, Sanger sequencing, and subcloning to maximize mutation detection20.
TABLE 1.
Comparison of mutation detection rates among different genomic studies in ccRCC.
| Incidence of Somatic Mutations |
||||||
|---|---|---|---|---|---|---|
| Study | n | VHL | PBRM1 | BAP1 | DNA extraction | Sequencing method |
| Peña-Llopis et al. 201211 | 176 | 139 (79%)* | 92 (52%) | 24 (14%) | This protocol | Sanger sequencing |
| Sato et al. 201313 | 240 | 194 (81%)* | 98 (41%) | 25 (10%) | Not indicated | Deep sequencing |
| KIRC TCGA14,15 | 421 | 237 (56%) | 141 (33%) | 41 (10%) | AllPrep (Qiagen) | Genome/Exome sequencing |
| Sanger Institute16,17 | 348 | 169 (49%) | 119 (34%) | Not indicated | Sanger sequencing | |
| Hakimi et al. 201314,18 | 185 | 91 (49%) | 54 (29%) | 11 (6%) | DNeasy (Qiagen) | Sanger sequencing |
| Guo et al. 201219 | 98 | 27 (28%) | 21 (21%) | 8 (8%) | QIAamp (Qiagen) | Exome sequencing |
| Nickerson et al. 200820 | 205 | 169 (82%)* | Proteinase K digestion and phenol extraction | Endonuclease scanning, Sanger sequencing and subcloning | ||
Only somatic but not germline mutations were considered except for the Nickerson et al.20 study, where somatic and germline mutations could not be distinguished. For instance, the total incidence of patients with nonsilent VHL mutations in Peña-Llopis et al.11 is 142 (81%), and in Sato et al.13 it is 197 (82%).
The sensitivity of mutation detection can be affected by several factors, including (i) tumor purity and contamination by normal cells, (ii) tissue specificity (samples with other histologies may dilute mutation rates; for instance, VHL is rarely mutated in renal tumors except ccRCC), (iii) tissue quality (high-quality DNA is difficult to obtain from poorly preserved tissues), (iv) tissue homogenization method (too vigorous homogenization may result in DNA shearing), (v) DNA extraction procedure (DNA degradation should be prevented), (vi) DNA quality (mutations are difficult to detect if there is significant noise), (vii) sequencing method (for instance, exome sequencing involves capturing reagents and retrieval is not uniform), (viii) depth of coverage, (ix) mutation detection algorithms (current algorithms are suboptimal for the detection of small insertions and deletions) and (x) reference comparator (some pathogenic mutations are included in dbSNP (http://www.ncbi.nlm.nih.gov/SNP/) or other databases and may be filtered out).
A reliable methodology for the selection of samples with high tumor content is likely to increase the sensitivity of mutation detection. A high sensitivity enabled us to discover that mutations in BRCA1-associated protein 1 (BAP1) and polybromo 1 (PBRM1) are mutually exclusive in ccRCC11. These results have been later validated by meta-analyses of data pooled from several other studies21, and were reproduced by a recent study using deep sequencing13.
Overview of the procedure
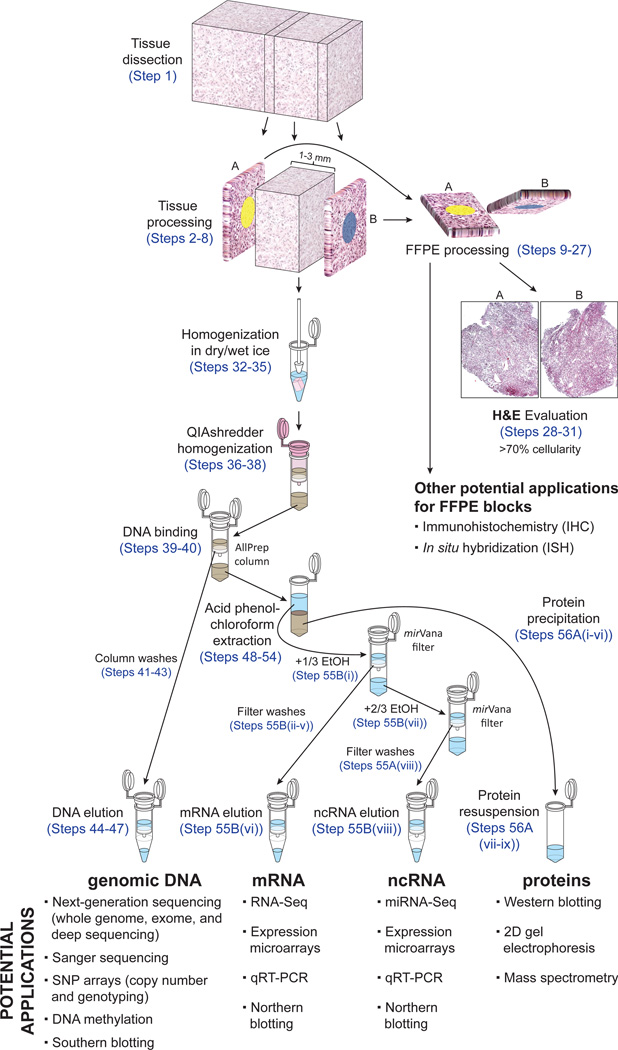
Essentially, the procedure described here consists of two parts: (i) tissue selection on the basis of the histology of flanking sections and (ii) simultaneous extraction of nucleic acids and proteins. An outline of our protocol is shown in Figure 1, quality controls are provided in Figures 2 and 3 and examples of its applications are displayed in Figures 4 and 5.
Figure 1.
Schematic workflow for obtaining immediate flanking sections and simultaneous extraction of nucleic acids and proteins from the same samples.
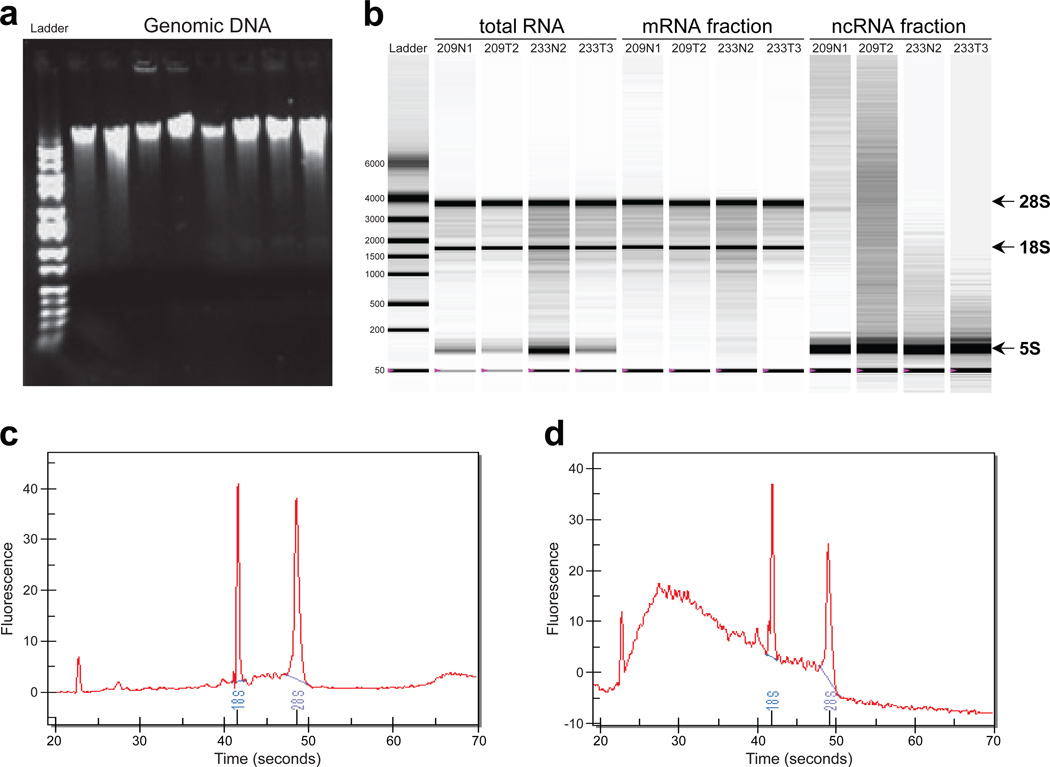
Figure 2.
Examples for DNA and RNA quality, and integrity from isolated samples. (a) Agarose gel electrophoresis of genomic DNA to verify that there is no DNA degradation resulting in a DNA ladder. (b) Representative electropherograms run with Bio-Rad Experion of RNA samples isolated as total RNA, high-molecular-weight RNA (enriched for mRNA) and low-molecular-weight RNA (enriched for ncRNA like miRNA). (c) Representative electropherogram for a good-quality RNA. (d) Representative electropherogram for a highly-degraded RNA. Abundant human ribosomal RNAs of 5S, 18S, and 28S are indicated.
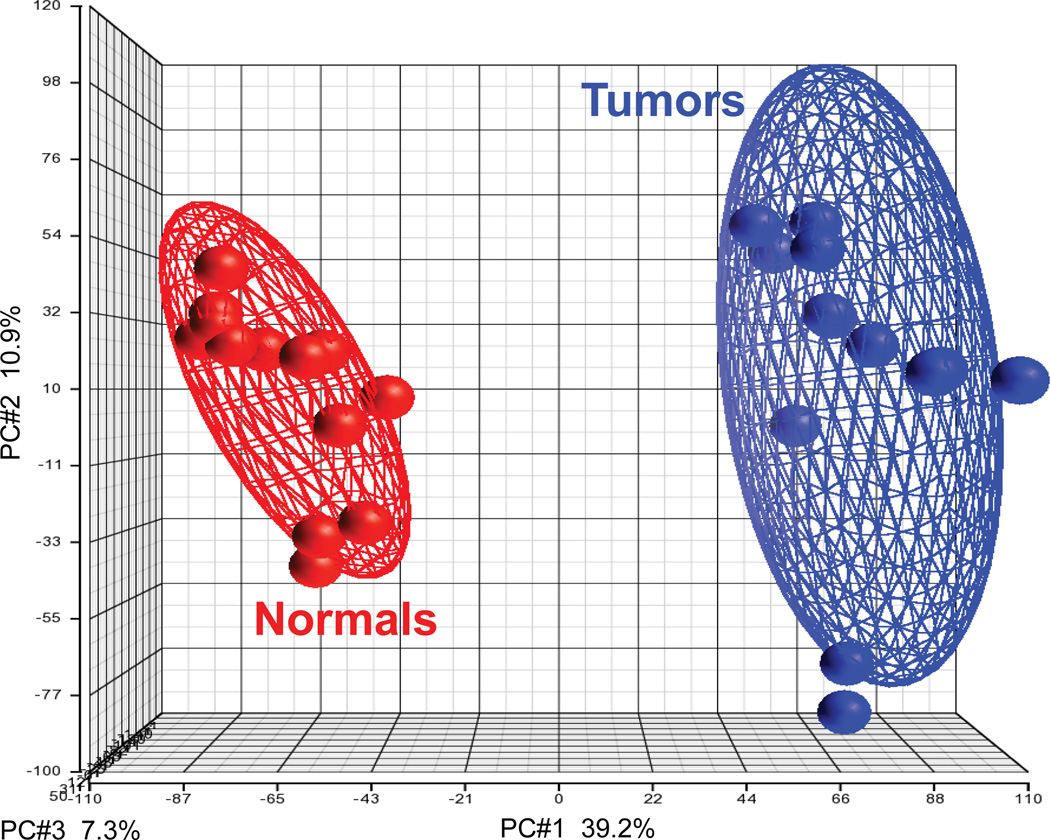
Figure 3.
Gene expression profiling distinguishes tumors from normal samples with high accuracy. Gene expression microarrays were obtained from samples hybridized in 2007 by Peña-Llopis et al.11, available at Gene Expression Omnibus (GEO) Data Sets (http://www.ncbi.nlm.nih.gov/gds) under accession number GSE36895. Partek Genomics Suite 6.5 was used to normalize the CEL files with the RMA algorithm and to perform the Principal Component Analysis (PCA; see PC#1, PC#2, PC#3) of tumor and normal samples for all probe sets available in the Affymetrix HG-U133 Plus 2.0 array (54,675) using a covariance matrix. PC#1 is the main principal component, which accounts for 39.2% of the variance (the variability in the data). Ellipsoids delineate the 95% confidence limit for each group and show that tumor samples cluster together and are clearly different from normal samples.
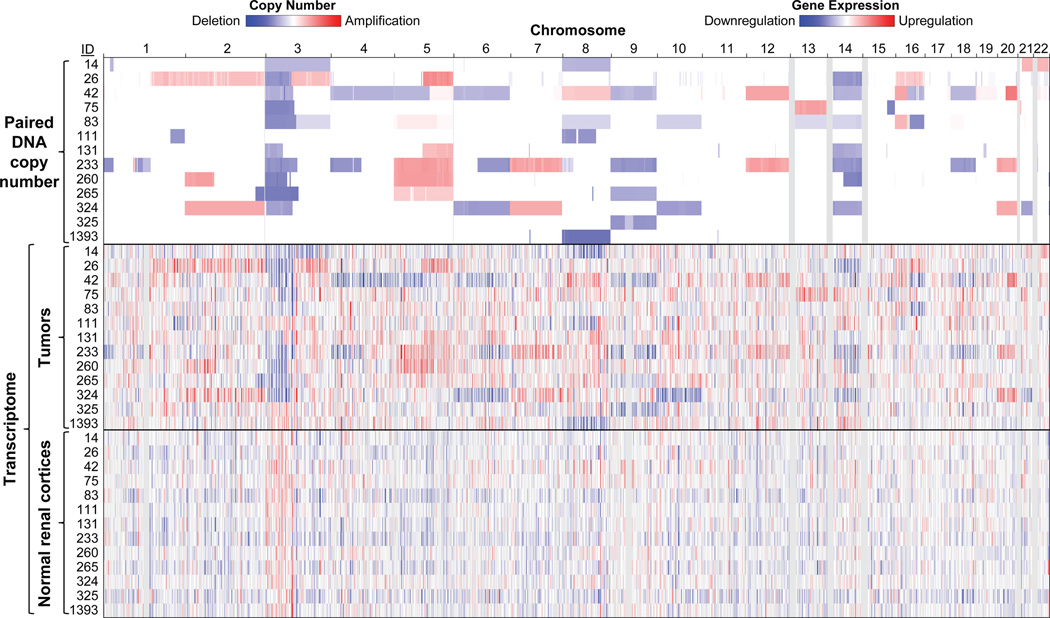
Figure 4.
Genome-wide correlation of DNA copy number alterations with gene expression profiling in tumors. DNA SNP and gene expression arrays were obtained from samples hybridized in 2007 by Peña-Llopis et al.11, available at GEO Data Sets (http://www.ncbi.nlm.nih.gov/gds) under accession numbers GSE25540 and GSE36895, respectively. Paired DNA copy numbers were segmented as previously described11 and displayed in the Integrative Genomics Viewer (IGV, http://www.broadinstitute.org/igv) along with transcriptomes. Simultaneous isolation of gDNA and RNA allows the correlation of these nucleic acids by displaying mRNA downregulation in chromosomal regions of deletion and mRNA upregulation in areas of amplification in tumor samples but not normal samples.
Figure 5.
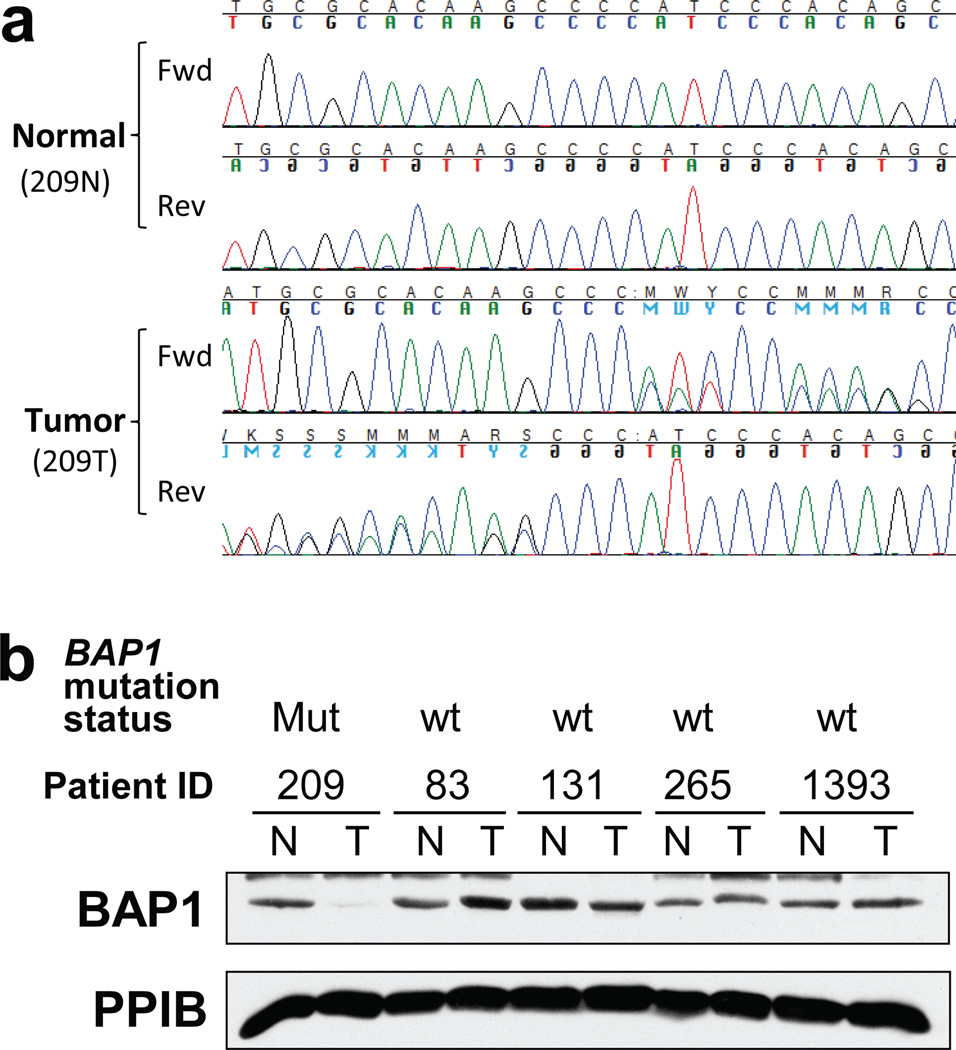
Correlation of mutations in DNA with loss of protein expression. (a) Representative chromatogram of bidirectional Sanger sequencing for a single-nucleotide deletion in a tumor and matching normal DNA. (b) Western blot for BAP1 and, as loading control, Cyclophilin B (PPIB) of tumor (T) and normal (N) samples with BAP1 mutation status. Mut, mutant; wt, wild type. Chromatogram shows that tumor ID 209T had a single-nucleotide deletion in BAP1, resulting in a frame-shift deletion and early truncation of the protein, which showed a loss of protein expression by western blotting. Other tumors without BAP1 mutations showed normal protein levels of BAP1. Figure 5b was reprinted from ref. 11.
The tissue selection consists of the processing of the tissues as indicated in Figure 1. Tissues are cut with a blade on dry ice and preserved as frozen sections. The two immediate flanking sections of the largest tissue area are stained with pathology dyes, cut, placed in a cassette and fixed in formalin. Depending on the morphology of the tissue, additional flanking sections might be obtained, although two are enough as long as the distance between them is not >3 mm. Flanking sections further apart may not be representative of the tissue in the middle used for nucleic acid extraction. The formalin-fixed tissues are subsequently dehydrated, embedded in paraffin and stained with hematoxylin and eosin (H&E) to show the two direct flanking sections. The conditions explained here for performing the histology may be different in other laboratories and/or adapted to different tissues. The H&E slides are recommended to be reviewed by an expert pathologist to select the best tissues for nucleic acid extraction. In this protocol, the pathologist only reviews the slides of the flanking sections already cut and stained with H&E. Thus, an expert pathologist can review about 100–150 slides per hour. Alternative methods such as laser capture microdissection may require the pathologist for the whole process, which is substantially more time-consuming (about 6–8 samples per hour6).
Methods for nucleic acids extraction have been extensively developed. Until the recent introduction of silica-based spin columns22, the standard method for obtaining high-quality gDNA from mammalian tissues involved proteinase K treatment, phenol extraction and ethanol precipitation23, 24. The method of Chomczynski and Sacchi25 of tissue homogenization with guanidinium isothiocyanate and extraction in acid-phenol:chloroform remains the procedure of choice for many investigators when isolating RNA26. A modification of this procedure allowed the simultaneous extraction of DNA, RNA and proteins27. Several companies commercialize this product as TRIzol (Life Technologies) or TRI Reagent (Molecular Research Center). However, many of these techniques are time-consuming, require optimization and may be performed differently in different laboratories. Kits provide a fast, simple and reliable way to execute these methods. For instance, the Ambion mirVana kit involves a modification of the guanidinium thiocyanate-phenol-chloroform isolation method25 by further purifying RNA over a glass-fiber filter instead of precipitating RNA with isopropanol. Traditional isopropanol precipitation of RNA25 may not be efficient to recover low-molecular-weight RNAs. In addition, inexperienced researchers may have problems dealing with small pellets and/or overdrying RNA pellets, thereby resulting in variable RNA recovery. Thus, the use of columns not only allows the extraction of a fraction enriched in small RNAs, but also ensures reproducibility and consistency of RNA recovery. However, the mirVana kit does not provide a way to extract gDNA. In fact, several companies provide kits for extracting nucleic acids from tissues, but not many offer the possibility of isolating gDNA, RNA, miRNA and proteins from the same samples. Qiagen allows this option; however, although the kit works well for cell lines, we have been unsuccessful in obtaining high-quality and high-yield RNA from tissues in a consistent manner. Most likely, the unexpectedly low RNA yield and quality in some samples was caused by the clogging of the RNeasy column (even with the recommended starting material), because of the fibrous and/or lipid nature of the tissue and/or the presence of trace amounts of histology dyes.
We therefore developed adaptations of two commercially available kits (Qiagen’s AllPrep and Ambion’s mirVana) and integrated them into a highly successful working pipeline to easily and efficiently extract nucleic acids and proteins from the same sample. First, freshly frozen tissues are homogenized in alternating dry and wet ice with an RNase-free pestle with Ambion mirVana’s lysis/binding buffer, which is compatible with low temperatures. The alternation of dry and wet ice facilitates the total disaggregation of the tissue, because when the tissue starts to freeze it is easier to disaggregate the tissue with a pestle. Sample homogenization using a rotor-stator homogenizer or other mechanical instruments is not recommended because of extensive DNA shearing, generation of free radicals by sample oxygenation, and potential cross-contamination. The tissue is then further homogenized by centrifuging the lysate twice in a QIAshredder column, which retains any fragment not disaggregated and any insoluble material goes to the pellet. Thus a clear lysate is loaded into a Qiagen AllPrep column to isolate DNA. The DNA is retained, bound to the membrane, and the flow-through, which contains RNA and proteins, can be subjected to an acid-phenol:chloroform extraction followed by RNA retention on glass-fiber columns with the Ambion mirVana kit. RNA can be extracted as total RNA or be further purified into a large-molecular-weight RNA fraction, enriched in protein-coding mRNAs (suitable for gene expression analyses), and another fraction of small-molecular-weight RNAs, enriched in ncRNAs, such as miRNAs (involved in translational regulation of gene expression28), small nuclear RNAs (snRNA; involved in splicing mRNA28), small nucleolar RNAs (snoRNAs; involved in ribosomal RNA (rRNA) modification28) and PIWI-interacting small RNAs (piRNAs; involved in the silencing of transposable elements in the germline of many animal species29). The proteins in the remaining lysate are then precipitated with acetone and resuspended in a protein lysis buffer supplemented with protease and phosphatase inhibitors.
The modifications made to the two commercial kits for extracting nucleic acids are summarized as follows: (i) tissue homogenization (with an RNase-free pestle) that alternates dry and wet ice (Step 34); (ii) homogenization performed using Ambion mirVana’s lysis/binding buffer instead of Qiagen buffer RLT (Step 33); (iii) two homogenization steps in the same QIAshredder column to ensure a clear lysate for DNA extraction (Steps 37 and 38); (iv) additional centrifugation step with the Qiagen AllPrep column after the washing steps to minimize ethanol carryover (Step 43); (v) DNA elutions with 50 µl for 2–5 min instead of 100 µl for 2 min (Steps 44–46); (vi) DNA isolation before the acid-phenol:chloroform extraction of RNA (Steps 41–47); (vii) loading the Ambion filter twice for the mRNA extraction to minimize the carryover of large-molecular-weight RNA into the flow-through (Step 55B(iv)); (viii) simultaneous extraction of mRNA and ncRNA with alternating spinning (Step 55B(viii)); (ix) use of a multidispenser pipette to dispense liquids more efficiently; (x) use of a Liquid Aspiration System under the fume hood to minimize phenol exposure (Step 55A(iii)); and (xi) acetone precipitation of proteins (Steps 56A(i-ix)).
The present protocol has several limitations. (i) The isolation of DNA is limited by the binding capacity of the AllPrep column. This protocol is suitable for maximizing nucleic acid yield for very valuable small tissue samples (5–30 mg). Larger tissue samples may not be representative of the flanking sections, and in any case they may overload the column. (ii) The extraction of RNA uses phenol, which is highly toxic. Thus, recommendations in this protocol should be followed to minimize exposure to phenol. (iii) The use of two commercially available kits is more expensive than homemade solutions, although it provides a faster, more convenient and reliable procedure. If obtaining a fraction enriched in ncRNA is not necessary, the method of Chomczynski27 may be used. Nonetheless, the costs of some of the downstream applications make the expense in nucleic acid extraction negligible. (iv) Tissue homogenization with guanidine thiocyanate strongly denatures proteins. Although this is essential to prevent the degradation of nucleic acids by DNases and RNases, it may not be suitable for specific protein downstream applications, such as native gel electrophoresis. In this case, an alternative protocol is proposed to lyse intact tissues directly with the Protein Lysis Buffer (Step 56B).
The main advantages of this protocol are as follows: (i) This protocol allows for careful identification and selection of the desired tissues by performing H&E staining on immediate flanking sections to ensure tissues of high purity. (ii) It allows for comprehensive but gentle tissue homogenization with an RNase-free pestle alternating dry and wet ice followed by passing the lysate through a QIAshredder column (Steps 32–38). This procedure facilitates the fast and reliable extraction of nucleic acids described in this protocol. In addition, simply by changing the lysis buffer, this homogenization methodology may be suitable for other applications, such as protein analysis (using Protein Lysis Buffer, Step 56B), immunoprecipitation (using immunoprecipitation buffer)11, or histone purification (using 0.4 M HCl)11. (iii) It allows for the possibility of performing reliable integrative analyses of different nucleic acids and proteins from the same samples (Figs. 4 and 5). Downstream applications can be performed with the purified nucleic acids and proteins and can be integrated into multi-ome studies, such as whole-genome/exome sequencing projects, investigations of whole-genome copy-number alterations, as well as analyses of the transcriptome, miRNAome, methylome and proteome from the same samples.
MATERIALS
REAGENTS
Tissues of human or animal origin. Δ CRITICAL All experiments that use human or animal tissues should comply with all relevant institutional and governmental guidelines and regulations. Informed consent must be obtained from all human subjects. ! CAUTION Human tissues might contain viruses such as HIV and hepatitis, which may infect the researcher. Therefore, the use of protective equipment such as gloves, a lab coat and goggles is recommended.
Dry ice
Blue and yellow pathology dyes (StatLab Medical Products, cat. nos. SL662BL and SL662YL)
Formalin, 10% (wt/vol) buffered (Fisher, cat. no. SF100-4) ! CAUTION Formalin is considered toxic if swallowed, inhaled or absorbed through the skin. Use it under a fume hood and wear protective gear such as gloves and a lab coat.
Ethanol, 100%, for histology (Koptec, cat. no. V1001) and for nucleic acid extraction (Koptec, cat. no. 1016)
Xylene (StatLab, cat. no. 8400-1)
Paraffin (Paraplast Plus tissue embedding medium, McCormick Scientific, cat. no. 39502004)
Hematoxylin (StatLab, cat. no. SL95-16)
High Def (StatLab, cat. no. SL103)
Bluing reagent (Thermo Scientific, cat. no. 7301)
Eosin Y (StatLab, cat. no. SL98-16)
Cytoseal 60 (Thermo Scientific, cat. no. 8310-4)
QIAshredder (Qiagen, cat. no. 79654)
AllPrep DNA/RNA mini kit (Qiagen, cat. no. 80204)
mirVana miRNA isolation kit (Life Technologies (Ambion), cat. no. AM1560)
Nuclease-free water (Life Technologies (Ambion), cat. no. AM9937)
Tris base (Research Products International, cat. no. T60040-5000)
HCl (Fisher, cat. no. A144-500)
NaCl (Research Products International, cat. no. S23020-5000)
Igepal (Sigma, cat. no. I8896)
Complete protease inhibitor cocktail (Roche, cat. no. 11873580001)
Aprotinin (Research Products International, cat. no. A20550)
Leupeptin (Research Products International, cat. no. L22035)
Pepstatin A (Research Products International, cat. no. P30100)
Benzamidine (Sigma, cat. no. 12072)
Phenylmethanesulfonyl fluoride (PMSF; Research Products International, cat. no. P20270)
NaF (Sigma, cat. no. S7920)
Imidazole (Sigma, cat. no. I5513)
Sodium molybdate (Sigma, cat. no. 243655)
Sodium orthovanadate (Sigma, cat. no. S6508)
Microcystin (Millipore, cat. no. 475815)
Acetone (Millipore, cat. no. AX0120-8)
Bio-Rad Protein Assay (Bio-Rad, cat. no. 500-0002)
EQUIPMENT
Benchtop centrifuges: regular and refrigerated (Eppendorf, cat. nos. 5415D and 5415R, respectively)
Metal (aluminum cooling) rack (LabScientific, cat. no. 2073)
Bucket (VWR, cat. no. 89198-970)
RNase-free tubes, 1.5 and 2 ml (Life Technologies (Ambion), cat. nos. AM12400 and AM12475, respectively)
Nylon printer, IDXPERT v3.0 handheld labeler (Brady, cat. no. XPERT-ABC)
Vial top label for 1.5-ml tubes (Labxpert, cat. no. X-83-499)
Solvent-resistant marker (Fisher, cat. no. 14-905-30)
Analytical balance
Petri dishes (Fisher, cat. no. 08-757-12)
Dissecting forceps (VWR, cat. no. 82027-406)
Scalpel (Cincinnati Surgical, cat. no. 074S)
Scalpel blades (Cincinnati Surgical, cat. no. 01SM24)
Sterile alcohol prep pads (PDI, cat. no. B60307)
Tissue-Loc HistoScreen cassettes (Thermo Scientific, cat. no. C-1000-AQ)
Fume hood
Beaker
Biohazard waste and sharp disposal container (Kendall, cat. no. 31353603)
Pipettes for volumes 0.5 – 1,000 µl
Pipette filter tips for volumes 0.5 – 1,000 µl
Microm STP 120-3 spin tissue processor (Thermo Scientific, cat. no. 813160)
Disposable base molds (Thermo Scientific, cat. no. 41741)
HistoStar embedding workstation (Thermo Scientific, cat. no. A81000002)
Rotary microtome (Leica Biosystems, cat. no. RM2235)
Microtome blade (Thermo Scientific, cat. no. MX35)
Flotation bath (Fisher Scientific, cat. no.15-464-80)
Microscope slides (Fisher Scientific, cat. no. 12-550-17)
Oven (Fisher Scientific, cat. no. 13-247-625G)
Tissue-Tek II manual slide staining set (Sakura, cat. no. 4451)
Coverslips (Statlab, cat. no. SL102450)
Microscope (Olympus, cat. no. BX-41)
Heat block (Denville Scientific, cat. no. I0520)
RNase-free pestle (VWR, cat. no. 47747-358)
Distriman repetitive pipette (Gilson, cat. no. F164001)
Sterilized Distritips for repetitive pipette (Gilson, cat. no. F164150)
NanoDrop ND-1000 spectrophotometer (Thermo Scientific, cat. no. ND-1000) or a UV spectrophotometer
Experion system (Bio-Rad, cat. no. 700-7001) or Agilent 2100 Bioanalyzer (Agilent Technologies, cat. no. G2943CA)
REAGENT SETUP
Protein Lysis Buffer Prepare a solution of 50 mM Tris-HCl (pH 7.4), 250 mM NaCl and 0.5% (vol/vol) Igepal. You can prepare the following stock solutions: 1 M Tris-HCl (pH 7.4), 5 M NaCl, and 10% (vol/vol) Igepal. Mix 25 ml of each and bring the volume to 500 ml. Keep the solution at 4 °C indefinitely. Before use, add protease and phosphatase inhibitors, which can be obtained from Roche (or other companies) or can be prepared by mixing the following solutions to the indicated final concentrations30: 0.1 µM aprotinin, 0.02 mM leupeptin, 0.01 mM pepstatin A, 0.5 mM benzamidine, 0.5 mM PMSF, 0.01 M NaF, 2 mM imidazole, 1.15 mM sodium molybdate, 1 mM sodium orthovanadate and 5 nM microcystin.
EQUIPMENT SETUP
Preparation for tissue processing Fill a bucket with dry ice and place a metal rack on top. Label Eppendorf tubes on the lid with a Nylon printer and on the side with a pencil or a solvent-resistant marker. As the tube weight varies slightly from tube to tube, it is recommended to weigh the 1.5-ml RNase-free tubes in an analytical balance with at least 0.1-mg readability before placing the tissues inside. Label cassettes with a pencil or a solvent-resistant marker with the same names as used on the tubes. Fill a beaker with formalin under the fume hood and cover with aluminum foil to minimize its exposure.
Preparation for paraffin embedding of tissues, microtome sectioning and H&E staining for histology Fill the flotation bath with distilled water and heat it to 37 °C. Fill a bucket with ice to keep the paraffin blocks cold. Label slides with a solvent-resistant marker.
Preparation for tissue homogenization Fill a bucket with dry ice and place a metal rack on top of it.
Preparation for DNA extraction Add the appropriate volume of 100% ethanol to the washing solutions AW1 and AW2 from the AllPrep DNA/RNA mini kit as indicated on the bottle before using for the first time. It is recommended to label the tubes on the lid with a Nylon printer and on the side with a pencil or a solvent-resistant marker.
Preparation for RNA extraction Add the appropriate volume of 100% ethanol to miRNA wash solutions 1 and 2/3 from the mirVana miRNA isolation kit as indicated on the bottle before using for the first time. Arrange a benchtop centrifuge and a Liquid Aspiration System inside the fume hood. It is recommended to label the tubes on the lid with a Nylon printer and on the side with a pencil or a solvent-resistant marker. Place a 1.5-ml tube filled with RNAase-free H2O on a heat block at 95 °C.
Liquid Aspiration System setup Connect the side of a 1-liter vacuum (Büchner) flask to one side of a 0.45-µm membrane filter using a 20-cm rubber tube. Connect with rubber tube the other side of the membrane filter to a vacuum unit (in-house or water assisted). Close the vacuum flask with a drilled rubber stopper that has a glass Pasteur pipette across the hole. Connect the Pasteur pipette with a 30- cm rubber tube to another glass Pasteur pipette, which will be used to aspirate liquids using a 200-µl pipette tip.
Sample handling recommendations RNA is very sensitive to degradation. As the skin contains RNases, wear disposable gloves all the time and replace them regularly. Clean the area you will process the tissues including pipettes and centrifuges by spraying with 70% (vol/vol) ethanol (other solutions might be used, such as Ambion RNaseZap solution). Spray 70% (vol/vol) ethanol over your gloves every time you touch anything that has not been cleansed. Although solutions and reusable glassware and plasticware can be autoclaved to be sterile, this protocol uses RNase-free solutions and disposable plasticware, which are more convenient. RNase-free 1.5- and 2-ml tubes are supplied open. To minimize contamination, take one tube at a time from the bag with tweezers or forceps wiped with 70% (vol/vol) ethanol, close the lid and place them in a closed container. Otherwise, the RNase-free tubes might no longer will be free of RNases. Use RNase-free filter tips to handle solutions, and do not reuse them. Pipetting for many samples can be expedited by using a repetitive pipette and sterile syringes. Do not leave solutions open if they are not in use, because RNases can be introduced.
PROCEDURE
Tissue dissection and processing for obtaining flanking sections • TIMING 1 h for 24 samples
Δ CRITICAL You must handle samples throughout the PROCEDURE as detailed in sample handling recommendations in the EQUIPMENT SETUP section to avoid degradation by RNases.
-
1
Dissect the tissue of choice according to your institution’s regulations, and place it in a 1.5-ml RNase-free tube. Freeze tissues in liquid nitrogen as quickly as possible after their excision, and then transfer them to a −80 °C freezer for indefinite storage. Alternatively, tissues can be stabilized by immersion in RNAlater (Ambion) or Allprotect tissue reagent (Qiagen) as recommended by the manufacturers. If you are removing a solid tumor, make sure that you remove the most characteristic and homogeneous areas. If you are dissecting a normal sample from an excised organ, try to get several samples from the furthest distance available to the solid tumor to prevent tumor contamination. Generally, to maximize the chances of obtaining good material is desirable to fill at least four RNase-free Eppendorf tubes with representative samples of each tissue type (e.g., four tumors and four normal samples of sizes about 5 × 5 × 20 mm).
Δ CRITICAL STEP Do not let the tissue thaw at any point during this protocol, which would result in RNA degradation.
-
2
Put a tissue sample on a clean Petri dish on top of a metal rack on dry ice.
Δ CRITICAL STEP The metal rack should be placed on dry ice at least 5 min before adding the samples to keep them frozen.
-
3
Hold the tissue with dissecting forceps, keeping it on the Petri dish and ink one side with blue pathology dye using a pipette tip as indicated in Figure 1.
Δ CRITICAL STEP The pathology dyes dry out over time, so pour just one or two drops of dye on a different Petri dish at room temperature (20–25°C).
? TROUBLESHOOTING
-
4
By using a scalpel blade, cut off a thin (2–4 mm) piece from the blue end of the tissue, and place it in a pre-labeled tissue cassette on top of the metal rack.
! CAUTION Blades are very sharp and there is a risk of laceration when using them, which is a potential source of infection by viruses.
-
5
Cut off a 1–3-mm piece of tissue from the same end previously cut using the scalpel blade, and place it in a labeled and pre-weighed 1.5-ml RNAse-free tube kept on the metal rack on dry ice.
Δ CRITICAL STEP Bigger pieces can be cut, but these might not be representative of the flanking sections.
-
6
Ink the recently cut end of the remaining tissue with the yellow pathology dye, which is the section flanking the tissue placed in the tube. Cut a thin (2–4 mm) slice and place it in the same cassette containing the blue-inked tissue (from Step 4).
Δ CRITICAL STEP The yellow dye is for orientation purposes, and thus it is not necessary to ink the whole surface, just a sufficient amount to identify it (Fig. 1).
? TROUBLESHOOTING
-
7
Put the cassette in the formalin beaker overnight (at least 12 h), but not for more than 24 h.
! CAUTION The formalin beaker should be covered with aluminum foil as much as possible to minimize the exposure to this chemical.
Δ CRITICAL STEP Fixation in formalin for over 48 h produces too many cross-links and results in more degraded nucleic acids and proteins, compromising some downstream applications such as immunohistochemical analysis or in situ hybridization. It is recommended to process the tissues in Step 9 up to 24 h after formalin fixation, or transfer the cassettes into a 50% (vol/vol) ethanol solution for extended storage times.
-
8
Repeat Steps 3–7 until the whole tissue is processed using a new sample name for each tissue cassette (containing the blue- and yellow-end slices) and corresponding tubes (containing the tissue for potential downstream genomic applications). Name successive sets of tubes and cassettes preferentially with consecutive numbers or letters after the sample ID. Dispose of the blade into a sharp disposal container. Use a new Petri dish and a new scalpel blade for each sample and wipe the forceps carefully with alcohol prep pads.
■ PAUSE POINT Weigh and store the tubes of processed tissues indefinitely in a −80 °C freezer. When weighing the tubes, wipe them with a Kimwipe to remove external ice that would increase the weight of the tissue.
Δ CRITICAL STEP Try to minimize the amount of time the frozen tissue is in the analytical balance to prevent thawing.
Paraffin embedding of tissues, microtome sectioning, and H&E staining for histology † TIMING 2 d (about 3 h of hands-on time) for 24 samples
-
9
Remove cassettes from formalin (or 50% (vol/vol) ethanol) from Step 7 and place them in a loading basket of a Microm STP 120 Spin Tissue Processor or a similar tissue processor. Make sure that the basket is over station 1.
-
10
Run a program at 45 min per station for the following stations: 50% (vol/vol) ethanol, 70% (vol/vol) ethanol, 80% (vol/vol) ethanol, 2 × 95% (vol/vol) ethanol, 2 × 100% (vol/vol) ethanol, 3 × xylene and 2 × melted paraffin. If no tissue processor is available, cassettes can be transferred into the different solutions manually. At this point, tissues are dehydrated (by exposure to increasing amounts of alcohol) and infiltrated with paraffin.
Δ CRITICAL STEP Do not leave the tissues in the tissue processor for extended periods of time. It is recommended to program it to start during the night and finish early in the morning.
-
11
Remove the cassettes from the basket when the program is finished, and then place them in an embedding workstation.
-
12
Discard the lid of the cassette.
-
13
Place the tissue pieces without touching each other in a plastic mold, facing the blue dye on top and the yellow dye on the bottom, so when inverted they will look as in Figure 1.
-
14
Pour melted paraffin to cover the mold using preferentially an embedding workstation.
-
15
Place the cassette on top of the mold with hollow side facing up.
-
16
Cover the hollow area with melted paraffin and place it on a cooling rack from the embedding workstation to cool.
■ PAUSE POINT Paraffin blocks can be stored indefinitely at 4 °C or room temperature. Do not leave the cooling rack on overnight or for extended periods of time as it will cause problems for the embedding workstation.
-
17
Once the paraffin block has cooled for at least 30 min, remove the mold and shave off the excess paraffin. Now, the yellow dye part is on top and the blue part is not visible; these parts represent the two immediate flanking areas of the tissue piece that is stored in the −80 °C freezer. Keep the paraffin blocks on ice to keep them cold.
-
18
Place the cassette in a microtome apparatus and insert a microtome blade.
! CAUTION Be careful to completely secure the microtome and apply all brakes on all rotating parts of the machine before inserting the blade or the block.
-
19
Adjust the orientation so that the paraffin block is flush against the blade.
-
20
Release the lock and turn the wheel clockwise to shave off the first few layers of paraffin. Set the microtome at a 5-µm thickness for H&E or at a 3-µm thickness for immunohistochemical analysis.
-
21
Once the tissue is visible in the cut layers, wet the block with water-soaked gauze and begin cutting several sections (about 3–6). For the tissue inked with yellow dye, discard the first sections containing the yellow dye until the tissue is visible.
? TROUBLESHOOTING
-
22
Place the sections in a flotation bath at 37 °C with a small brush.
Δ CRITICAL STEP The temperature of the water should be ~30–40 °C to flatten out the paraffin as it comes in contact with water. Do not let the temperature get too hot (>40 °C), as the paraffin will melt.
? TROUBLESHOOTING
-
23
Label the slides and carefully place the cut sections onto slides. Although just one section is necessary, up to four sections are recommended to be placed per slide.
-
24
Place the slides in an oven at 45 °C for 40 min or overnight on a drying rack.
■ PAUSE POINT Keep the slides that are not going to be immediately stained at −20 °C indefinitely to obtain better results for immunohistochemical analysis and in situ hybridization.
-
25
Stain slides according to the H&E recommended staining guidelines in Box 1.
-
26
Coverslip slides by placing three drops of slide glue (Cytoseal 60) on each slide, and then carefully placing a coverslip on top. Press the coverslip to remove bubbles.
-
27
Allow the slides to dry overnight or at least for several hours.
BOX 1 | H&E: recommended staining guidelines.
Immerse slides in a slide staining set filled with the following solutions for the indicated time under a fume hood:
3 × 3 min - xylene
3 × 1 min - 100% ethanol
2 × 1 min - 95% (vol/vol) ethanol
30 s - distilled water
2 min - hematoxylin
Rinse with running water until water is clear
10 s - High Def
1 min - running water
10 s - bluing reagent
1 min - running water
5–10 dips - eosin Y
2 × 1 min - 95% (vol/vol) ethanol
3 × 1 min - 100% ethanol
× 1 min - xylene
Δ CRITICAL STEP Incubations in xylene and ethanol are recommended to be performed in sequential staining dishes. Solutions can be reused for other slides until too much contamination is visible.
Tissue H&E slide evaluation by an expert † TIMING ~30 s per slide
-
28
Collect, organize and review the H&E slides with a microscope for cellularity.
-
29
Arrange with an expert to review the H&E slides. For solid tumors, an expert pathologist is essential for identifying the tumor type, the percentage of tumor or normal/benign cells, the tumor grade, the amount of necrosis and other histological characteristics. Tumor cellularity may be calculated using surface areas occupied by tumor and nontumor cells. However, this assumes that tumor and nontumor cells are similar in size. If the tumor is infiltrated by normal cells that are much smaller (such as lymphocytes), the ratio of contaminating DNA would be underestimated by using a surface area assessment. In this situation, cellularity may be estimated on the basis of ratios of tumor and normal cell nuclei in the specimen.
Δ CRITICAL STEP Reviewing H&E slides without an expert is very risky. Cells might not be identified properly and then might be mistaken for others, thereby affecting the ultimate results when making comparisons. Tumor cells might be mixed with infiltrating lymphocytes and/or normal stromal cells, thus diluting the tumor content.
-
30
Create a database (for instance, in Microsoft Excel) that includes the names of the samples, the weight of the tubes and the histological information collected. Calculate the average tissue cellularity of both flanking sections.
-
31
Select the best sample from each subject and histology for further nucleic acid extraction on the basis of the following criteria. Ensure that all samples correspond to the same histology of choice, and select, for each individual, the tissue portion with the highest average tissue cellularity on both flanking sections. For solid tumors, an average tumor cellularity from both flanking sections equal or >70% is recommended. Thus, one flanking section might be 100% (all tumor cells) and the other one 50%, being 75% on average and suitable for further analysis. Tumors with <70% cellularity are not recommended for downstream genomic applications, although tumors with more than 60% cellularity might be considered if the number of subjects is limited. Discard tumors with more than 20% necrosis. Large amounts of stroma, extensive infiltrating lymphocytes or vast necrosis may reflect intrinsic properties of the tumor, and the systematic elimination of such samples may introduce an unavoidable selection bias. Discard normal-appearing samples with any percentage of tumor cells. For DNA analysis, any source of normal samples, such as peripheral blood mononuclear cells, is fine. However, for gene expression, select normal samples with >70% of the same type of normal cells that give rise to the tumor of choice compared with other histologies. Some macroscopic heterogeneous areas might be removed from the tissues with a blade on dry ice after observing the corresponding H&E slides. However, it is recommended to remove them at the time of getting the flanking sections to assure the remaining cells are the desired ones.
Tissue homogenization † TIMING 1 h for 8 samples
-
32
Keep the selected samples in 1.5-ml RNase-free tubes on a metal rack on dry ice.
Δ CRITICAL STEP Tissues should weigh about 5–30 mg. If tissues are >40 mg, it is recommended to cut them with the blade on dry ice and divide them into several tubes to prevent overloading and clogging the AllPrep column. Alternatively, the tissue can be homogenized using twice the amounts described below and loaded into two AllPrep columns.
-
33
Add 400 µl of ice-cold Ambion lysis/binding buffer (supplied in the Ambion kit).
Δ CRITICAL STEP Qiagen RLT Plus buffer should not be used cold, because it cannot protect from RNA degradation at low temperatures, and thus it is preferable to homogenize the tissue with Ambion lysis/binding buffer.
-
34
Homogenize the tissue gently but thoroughly with an RNase-free pestle on dry and wet ice by transferring the sample from a metal rack on dry ice onto a metal rack on wet ice and vice versa until total disaggregation. This typically requires 2–4 transitions.
Δ CRITICAL STEP Whereas the tissue may not be completely disaggregated on wet ice, it can be achieved by freezing partially the tissue on dry ice and crushing it gently with a pestle. The use of a pestle is preferable to mechanical disruption to minimize gDNA shearing and cross-contamination of the samples.
-
35
Wash the material off the pestle with 200 µl of Ambion lysis/binding buffer. Use one pestle per sample and discard it in a biohazard waste container.
-
36
Pipette the lysate into a QIAshredder spin column, and then homogenize it further by centrifuging for 3 min at full speed (16,000g) at 4 °C.
Δ CRITICAL STEP Do not add more than 700 µl of lysate per column to avoid overloading the collecting tube.
-
37
Discard the pellet and/or the fatty top layer (if any), and then load the supernatant into the same QIAshredder column but with a new 2-ml collection tube.
-
38
Centrifuge the lysate again for 2 min at maximum speed (16,000g) at 4 °C.
-
39
Discard any pellet and transfer the lysate to an AllPrep DNA spin column (supplied in the AllPrep Qiagen kit) placed in an RNase-free 2-ml tube instead of the collection tube.
Δ CRITICAL STEP Do not use a 1.5-ml tube, which will not be enough to collect all the flow-through. This tube may be labeled ‘RNA+Prot’.
-
40
Centrifuge the sample for 1 min at 10,000g at room temperature. As the lids of the 2-ml tubes cannot be closed, leave at least two empty spots between the tubes in the benchtop centrifuge, so the lids can move freely during centrifugation. At this point, DNA is retained in the AllPrep column, whereas RNA and protein are in the flow-through.
Δ CRITICAL STEP Do not add >700 µl of lysate to avoid overloading the collecting tube.
■ PAUSE POINT If only a few samples are being processed to extract DNA and RNA on the same day, then it is recommended to isolate first the RNA in Step 48 and meanwhile store at 4 °C the AllPrep column in its own 2-ml collection tube for later DNA purification in Step 41. Do not freeze or leave the AllPrep DNA spin column at room temperature for several hours. If many samples are being processed, then it is recommended to continue the protocol with the DNA extraction (Step 41), freeze on dry ice the flow-through tubes from Step 40 (the tubes labeled ‘RNA+Prot’), and then store them at −80 °C indefinitely until extracting the RNA in Step 48.
DNA extraction † TIMING 30 min for 8 samples
-
41
Add 500 µl of buffer AW1 (supplied in the AllPrep Qiagen kit) to the AllPrep column from Step 40, and centrifuge the column at 10,000g and room temperature for 30 s to wash the column membrane. Discard the flow-through.
Δ CRITICAL STEP Make sure that ethanol has been added to buffer AW1.
-
42
Add 500 µl of buffer AW2 (supplied in the AllPrep Qiagen kit) to the AllPrep column, and centrifuge the column at maximum speed (16,000g) at room temperature for 2 min.
Δ CRITICAL STEP Make sure that ethanol has been added to buffer AW2.
-
43
Place the AllPrep column into a new tube and centrifuge the column for 1 min at full speed (16,000g) at room temperature.
Δ CRITICAL STEP This step ensures that no ethanol is carried over during the DNA elution, which might interfere with downstream reactions.
-
44
Place the AllPrep column into a new 1.5-ml tube labeled as ‘DNA’. Add 50 µl of buffer EB (supplied in the AllPrep Qiagen kit) directly to the membrane of the column without damaging it. Let the column sit at room temperature for 2–5 min, and then centrifuge it at 10,000g for 1 min to recover the DNA.
-
45
Repeat Step 44 to recover additional DNA in the same tube, leaving the tube on ice during the incubation.
-
46
Repeat Steps 44 and 45 to elute additional DNA in a new 1.5-ml tube.
-
47
Measure the DNA concentration in 1 µl of eluates with a NanoDrop spectrophotometer, using an extinction coefficient (constant) of 50. Expect an A260/A280 ratio of ~1.8 for highly pure DNA. Expect a yield of 5–80 µg, depending on the tissue and amount of starting material. DNA quality can be further checked by running the samples on a 0.8% (wt/vol) agarose gel, as shown in Figure 2a. High-quality DNA should be of high molecular weight and no DNA ladder should be present.
? TROUBLESHOOTING
■ PAUSE POINT Samples can be stored indefinitely at −20 or −80 °C until further processing.
RNA isolation (organic extraction) • TIMING 30 min for 8 samples
Δ CRITICAL RNA extraction should be carried out under RNase-free conditions - see sample handling recommendations in the EQUIPMENT SETUP section.
-
48
Add 60 µl (1/10 of the volume) of miRNA Homogenate Additive (supplied in the Ambion kit) to the flow-through from Step 40, and mix them thoroughly by vortexing at the highest speed.
-
49
Allow the mixture to sit on ice for 10 min.
-
50
Add 600 µl (1 volume) of acid-phenol:chloroform (supplied in the Ambion kit).
! CAUTION Phenol is highly toxic when inhaled. Carry out all the following steps under a fume hood to minimize exposure to phenol.
Δ CRITICAL STEP Pipette from the lower phase in the bottle of acid-phenol:chloroform (the upper phase consists of an aqueous buffer).
-
51
Vortex the mixture at the highest speed for 30 s.
-
52
Centrifuge the mixture at maximum speed (16,000g) for 5 min at room temperature to separate the aqueous and organic phases. The aqueous (upper) phase contains RNA, the interphase contains small amounts of DNA not retained in the AllPrep column and the organic (lower) phase contains proteins.
Δ CRITICAL STEP Centrifuge the sample again if the interphase after spinning is not compact.
-
53
Transfer carefully 500 µl of the upper aqueous phase into a new RNase-free tube.
Δ CRITICAL STEP Do not transfer the interphase or the organic (lower) phase, which would result in the contamination of the sample with DNA or phenol, respectively. If a different volume of the upper phase is taken, follow the volumes indicated in brackets in the following steps.
-
54
Keep the organic phase from Step 52 on ice for protein extraction in Step 56.
-
55
RNA can be isolated from Step 53 as total RNA or be split into two fractions enriched in RNA of different molecular weights. Follow option A for the extraction of total RNA; follow option B to obtain a fraction with high-molecular-weight RNA (enriched for mRNA) and to obtain a fraction with low-molecular-weight RNA (enriched for RNAs smaller than 200 bases, largely ncRNAs).
-
Total RNA extraction † TIMING 30 min for 8 samples
Add 625 µl of 100% ethanol (1.25 volumes) at room temperature to the aqueous (upper) phase collected from the organic extraction in Step 53. Vortex the mixture vigorously.
-
Place a filter cartridge into one of the collection tubes (supplied both in the Ambion kit) and pipette 700 µl of the mixture of the flow-through with ethanol from the previous step.
Δ CRITICAL STEP To avoid overloading the collecting tube, do not add more than 700 µl of mixture.
-
Centrifuge the tube for 30 s at 10,000g at room temperature, and then discard the flow-through with a Liquid Aspiration System.
! CAUTION Use the Liquid Aspiration System to remove the flow-throughs in the following steps (Step 55A(iii-viii)) to minimize exposure to phenol.
Δ CRITICAL STEP The filter might be damaged by centrifuging faster.
Repeat Step 55A(ii-iii) until all the liquid has passed through the same filter.
-
Add 700 µl of miRNA wash solution 1 (supplied in the Ambion kit) to the filter and centrifuge the mixture for 30 s at 10,000g at room temperature.
Δ CRITICAL STEP Make sure that ethanol has been added to miRNA wash solution 1.
Discard the flow-through with the Liquid Aspiration System.
-
Wash the filter with 500 µl of wash solution 2/3 (supplied in the Ambion kit), centrifuge for 30 s at 10,000g at room temperature and discard the flow-through.
Δ CRITICAL STEP Make sure that ethanol has been added to wash solution 2/3.
Repeat the previous step to wash again with another 500 µl of wash solution 2/3 and discard the flow-through.
Centrifuge the filter in the empty collection tube for 1 min at 10,000g at room temperature to remove any remaining liquid from the filter.
Place the filter into a new collection tube labeled as ‘Total RNA’. Elute with 50 µl of nuclease-free water preheated at 95 °C. Allow the tube to sit at room temperature for 1 min, and then centrifuge it for 1 min at 10,000g at room temperature to collect the total RNA.
Repeat the previous step to elute further RNA in a new RNase-free 1.5-ml tube.
-
Measure RNA concentration in 1 µl of eluates with a NanoDrop spectrophotometer, using an extinction coefficient (constant) of 40. Expect an A260/A280 ratio of 1.8–2.1 for highly pure RNA. Expect a yield of 2–70 µg depending on the tissue and amount of starting material. If NanoDrop is not available, dilute samples with 10 mM Tris-HCl (pH 7.5) instead of RNase-free H2O, and measure A260 and A280 in a UV spectrophotometer. RNA quality can be further examined by running the samples on a Bio-Rad Experion or an Agilent Bioanalyzer, as depicted in Figure 2b-d.
? TROUBLESHOOTING
■ PAUSE POINT Samples should be stored indefinitely at −80 °C until further processing.
-
mRNA and ncRNA extraction † TIMING 1 h for 8 samples
Δ CRITICAL mRNA and ncRNA extraction steps can be performed simultaneously, so while centrifuging one group of samples the others can be washed.
-
mRNA extraction. Add 167 µl of 100% ethanol at room temperature (1/3 of the initial volume) to the aqueous (upper) phase collected from the organic extraction in Step 53. Vortex the mixture vigorously.
Δ CRITICAL STEP Make sure that you do not exceed the ethanol concentration, because higher amounts of ethanol can make mRNA and ncRNA to bind both to the column.
-
Place a filter cartridge into one of the collection tubes (supplied both in the Ambion kit) and pipette up to 700 µl of the mixture of the flow-through with ethanol from the previous step.
Δ CRITICAL STEP To prevent overloading the collecting tube, do not add more than 700 µl.
-
Centrifuge the tube for 30 s at 10,000g and room temperature to draw the mixture through the filter. The filter retains high-molecular-weight RNA, whereas small RNAs are not bound because of the low ethanol content.
Δ CRITICAL STEP The filter might be damaged if it is centrifuged faster than 10,000g.
-
Load the flow-through again through the same filter cartridge, and then centrifuge it for 30 s at 10,000g and room temperature.
Δ CRITICAL STEP This step minimizes the carryover of high-molecular weight RNA into the flow-through.
Recover the flow-through, transfer it to a new tube and keep it on ice until Step 55B(vii). Repeat Step 55B(ii-v) until all of the liquid passes through the filter, pooling all the collected flow-throughs.
-
Repeat Steps 55A(v-xii) to elute mRNA from the filter, labeling the collection tube ‘mRNA’. Eluted RNA should be >200 nt.
? TROUBLESHOOTING
■ PAUSE POINT Samples should be stored indefinitely at −80 °C until further processing.
ncRNA extraction. Add 430 µl of 100% ethanol (2/3 of the initial volume) at room temperature to the flow-through from Step 55B(v) and mix thoroughly. This allows small RNAs to bind to the column.
-
Repeat Steps 55A(ii-xii), eluting small RNAs into a new collection tube labeled as ‘ncRNA’ or ‘miRNA’. Use an extinction coefficient of 33 when measuring small RNAs concentration. Expect an A260/A280 ratio of 1.8–2.1 for a highly pure small RNAs fraction and a yield of 0.5–10 µg, depending on the tissue and amount of starting material. Small RNA quality can be further examined by running the samples on a Bio-Rad Experion or an Agilent Bioanalyzer, as shown in Figure 2b-d.
? TROUBLESHOOTING
■ PAUSE POINT Samples should be stored indefinitely at −80 °C until further processing.
-
-
Protein extraction
-
56
Proteins can be extracted using two methods. Follow option A to precipitate proteins from Step 54 with acetone. Owing to the strong denaturation conditions of the nucleic acid extraction method, precipitated proteins might not be suitable for certain applications, such as native gel electrophoresis. In that case, follow option B to isolate proteins directly from tissues.
-
Protein precipitation with acetone † TIMING 1 h for 8 samples
Add 1.5 ml (2.5 volumes) of ice-cold acetone to the organic fraction from Step 54.
-
Incubate the mixture at −20 °C (or on ice) for 30 min.
■ PAUSE POINT Samples can be kept at −20 °C for extended periods of time.
Centrifuge the samples for 5 min at 10,000g at 4 °C. Discard the supernatant with a pipette.
Wash the pellet by pipetting up and down with 1 ml of ice-cold acetone, and then centrifuge for 5 min at 5,000g at 4 °C.
Vortex the pellet with 1 ml ice-cold ethanol and centrifuge it for 5 min at 5,000g at 4 °C.
-
Discard the supernatant with a pipette, and leave the tube open for 5 min to remove residual ethanol.
Δ CRITICAL STEP Do not let the pellet to dry out completely, which would result in higher difficulty to resuspend the pellet.
Resuspend the pellet with 200 µl of Protein Lysis Buffer, pipetting up and down and incubating the tubes in a heat block at 50 °C for about 5–10 min. Continue pipetting up and down and heating the samples until the pellet is resuspended.
Centrifuge the suspension for 1 min at full speed (16,000g) to remove insoluble material and transfer the supernatant into a 1.5-ml tube labeled ‘Proteins’.
-
Measure protein concentration with the Bio-Rad protein assay according to the manufacturer’s instructions. Expect a yield of 30–500 µg of protein, depending on the tissue and amount of starting material. Although it is not necessary, protein quality may be assessed by using Bio-Rad Experion Pro260 chips.
? TROUBLESHOOTING
■ PAUSE POINT Samples can be stored indefinitely at −20 °C or −80 °C until further processing.
-
Protein isolation directly from tissues † TIMING 1 h for 8 samples
-
Repeat Steps 32–36 homogenizing the tissue in 10–20 volumes of Protein Lysis Buffer, and using 50–100 µl of Protein Lysis Buffer to wash off the pestle. By changing the lysis buffer, this homogenization procedure may be used for a wide variety of applications.
Δ CRITICAL STEP It is recommended to homogenize the tissues with an amount of Protein Lysis Buffer proportional to their weight rather than a fixed one.
-
Measure protein concentration with the Bio-Rad protein assay according to the manufacturer’s instructions. Expect a yield of 0.05–2 mg of protein, depending on the tissue and amount of starting material (about 0.5–5 mg/ml). Although it is not necessary, protein quality may be assessed by using Bio-Rad Experion Pro260 chips.
? TROUBLESHOOTING
■ PAUSE POINT Samples can be stored indefinitely at −20 °C or −80 °C until further processing.
-
-
? TROUBLESHOOTING
Troubleshooting advice can be found in Table 2.
TABLE 2.
Troubleshooting guide.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 3, 6 | Tissue for nucleic acid extraction stained with dye | Staining during tissue processing | Try to remove as much dye as possible, although it should not interfere in the nucleic acid extraction protocol |
| 21 | Paraffin blocks are difficult to cut | Tissues are not properly dehydrated. Solutions from the tissue processor are too old or diluted from previous solutions | Replace the solutions from the tissue processor and soak the paraffin block with water to soften the tissue before cutting |
| 22 | Wrinkles and/or holes in paraffin sections | Microtome blade is not sharp enough | Shift the microtome blade a little bit to use a new area, and replace the blade with a new one when necessary |
| 47 | DNA fragmentation | Excessive homogenization in Step 34 or sample vortexing | Do not vortex DNA samples, homogenize gently and do not use mechanical disruption of tissues |
| 47, 55A(xii), 55B(vi,viii) | Low DNA and RNA yields | Incomplete tissue disruption/homogenization | Make sure to process about 5-30 mg of tissue and to completely disrupt it with the RNase-free pestle on dry/wet ice and pass through a QIAshredder column |
| Tissue of choice contains much lower levels of nucleic acids than expected | Increase up to 60 mg the amount of tissue to be homogenized; be careful not to clog the AllPrep column because of the fibrous nature of the tissue | ||
| Low DNA but high RNA yield | Overloading the AllPrep column | Homogenize less tissue (cells in some tumors are densely packed, resulting in high levels of nucleic acids) or load it into two AllPrep columns | |
| 55A(xii), 55B(vi,viii) | Low RNA A260/A280 ratio | Phenol contamination during organic extraction | Centrifuge again for 5 min at maximum speed (16,000g) at room temperature to separate the aqueous and organic phases, and do not remove any lower phase |
| RNA samples diluted with RNase-free water | If a NanoDrop spectrophotometer is not available, dilute the RNA samples with Tris-HCl (pH 7.5) and measure them in a regular spectrophotometer | ||
| RNA degradation | RNA degradation before sample processing | Freeze tissues immediately in liquid nitrogen and store at -80 °C (if samples cannot be frozen immediately, treat tissues with RNAlater or Allprotect Tissue Reagent) | |
| RNase contamination | Ensure the samples are handled in RNase-free conditions and no RNases have been introduced. Please follow the handling recommendations indicated in the Equipment Setup section | ||
| 56A(ix), 56B(ii) | Low protein yield but high nucleic acid yields | Incomplete protein solubilization | Dissolve the protein pellet with a stronger solubilization agent, such as 1% (wt/vol) SDS or 10 M urea |
† TIMING
Steps 1–8, Tissue dissection and processing for obtaining flanking sections: 1 h for 24 samples
Steps 9–27, Paraffin embedding of tissues, microtome sectioning and H&E staining for histology: 2 d (about 3 h of hands-on time) for 24 samples
Steps 28–31, Tissue H&E slides evaluation by an expert: ~30 sec per slide
Steps 32–40, Tissue homogenization: 1 h for 8 samples
Steps 41–47, DNA extraction: 30 min for 8 samples
Steps 48–54, RNA extraction isolation (organic extraction): 30 min for 8 samples
Step 55A, Total RNA extraction: 30 min for 8 samples
Step 55B, mRNA and ncRNA extraction: 1 h for 8 samples
Step 56A, Protein precipitation with acetone: 1 h for 8 samples
Step 56B, Protein isolation directly from tissues: 1 h for 8 samples
For large-scale genomic studies, you can increase the number of tissues to process every day (Steps 1–27). Thereafter, arrange with an expert to evaluate the histology in order to select the best individual tissues to use for isolating nucleic acids and proteins. The number of samples for tissue homogenization and DNA extraction can be scaled up per batch (up to 24 samples per batch). However, it is recommended to perform several batches a day of 8 samples for RNA extraction, rather than increasing the number of samples per batch, in order to minimize the time of each batch and thus minimize RNA degradation. The number of samples for protein isolation can be scaled up per batch. Although tissue processing and pathologic review may take some time, once the selected tissues are available, these procedures facilitate obtaining high-quality and high-yield nucleic acids and proteins for about 12–16 samples per day.
ANTICIPATED RESULTS
This protocol describes steps first to carefully select tumor specimens and then to isolate high-quality and high-yield nucleic acids and proteins, as summarized in Figure 1. Figure 2 shows examples of DNA and RNA quality verification. This protocol allowed a high sensitivity of mutation detection (Table 1), which may reflect higher sample quality. Figure 3 reveals the purity of tissue types selected by hybridizing the RNA samples to microarrays and comparing tumor with normal samples with principal component analysis.
Following on from this protocol, we have: used the gDNA for whole-genome and exome sequencing11, capillary (Sanger) sequencing11, 31 and DNA copy-number analyses11, 12; used the RNA for gene expression profiling11, 12 and quantitative reverse-transcribed PCR (qRT-PCR or qPCR)31; used the proteins for western blotting of human samples11, 31 and mouse livers32; and used the formalin-fixed, paraffin-embedded (FFPE) blocks for H&E staining and immunohistochemistry11, 31. Figure 4 shows the genome-wide correlation of paired DNA copy number with tumor transcriptomes. As expected, gene expression is upregulated in areas of amplication and downregulated in areas of deletion. This nice correlation may not be preserved if the gDNA and RNA samples were obtained independently from different regions of the tumor. Figure 5 shows other examples of downstream applications from this protocol. Additional potential downstream applications include: DNA methylation33, 34, Southern blotting35, RNA-seq36, northern blotting37, 38, miRNA microarrays39, 2D gel electrophoresis40, mass spectrometry41, and in situ hybridization of ncRNAs42.
ACKNOWLEDGEMENTS
We thank Dr. S. Vega-Rubín-de-Celis and A. Pavía-Jiménez for critically reviewing the manuscript. This work was supported by a Postdoctoral Fellowship of Excellence from Generalitat Valenciana (Spain) (no. BPOSTDOC06/004) to S.P.-L. and the following grants to J.B.: a grant from the Cancer Prevention and Research Institute of Texas (no. RP101075) and American Cancer Society Research Scholar grant (no. 55927). J.B. is a Virginia Murchison Linthicum Endowed Scholar in Medical Research. The tissue management shared resource was supported in part by the US National Cancer Institute (no. 1P30CA142543).
Footnotes
AUTHOR CONTRIBUTIONS
S.P.-L. developed the protocols and performed all the experiments. S.P.-L. and J.B. designed the experiments and wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare that they have no competing financial interests.
REFERENCES
- 1.Bamshad MJ, et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet. 2011;12:745–755. doi: 10.1038/nrg3031. [DOI] [PubMed] [Google Scholar]
- 2.Mwenifumbo JC, Marra MA. Cancer genome-sequencing study design. Nat Rev Genet. 2013;14:321–332. doi: 10.1038/nrg3445. [DOI] [PubMed] [Google Scholar]
- 3.Wheeler DA, Wang L. From human genome to cancer genome: The first decade. Genome Res. 2013;23:1054–1062. doi: 10.1101/gr.157602.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ley TJ, et al. DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature. 2008;456:66–72. doi: 10.1038/nature07485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerlinger M, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espina V, et al. Laser-capture microdissection. Nat Protoc. 2006;1:586–603. doi: 10.1038/nprot.2006.85. [DOI] [PubMed] [Google Scholar]
- 7.Baslan T, et al. Genome-wide copy number analysis of single cells. Nat Protoc. 2012;7:1024–1041. doi: 10.1038/nprot.2012.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navin N, et al. Tumour evolution inferred by single-cell sequencing. Nature. 2011;472:90–94. doi: 10.1038/nature09807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell PJ, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109–1113. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hahn SA, et al. Allelotype of pancreatic adenocarcinoma using xenograft enrichment. Cancer Res. 1995;55:4670–4675. [PubMed] [Google Scholar]
- 11.Peña-Llopis S, et al. BAP1 loss defines a new class of renal cell carcinoma. Nat Genet. 2012;44:751–759. doi: 10.1038/ng.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sivanand S, et al. A validated tumorgraft model reveals activity of dovitinib against renal cell carcinoma. Sci Transl Med. 2012;4:137ra75. doi: 10.1126/scitranslmed.3003643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato Y, et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat Genet. 2013;45:860–867. doi: 10.1038/ng.2699. [DOI] [PubMed] [Google Scholar]
- 14.Hakimi AA, et al. Adverse Outcomes in Clear Cell Renal Cell Carcinoma with Mutations of 3p21 Epigenetic Regulators BAP1 and SETD2: a Report by MSKCC and the KIRC TCGA Research Network. Clin Cancer Res. 2013;19:3259–3267. doi: 10.1158/1078-0432.CCR-12-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Creighton CJ, et al. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499:43–49. doi: 10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalgliesh GL, et al. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature. 2010;463:360–363. doi: 10.1038/nature08672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varela I, et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature. 2011;469:539–542. doi: 10.1038/nature09639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hakimi AA, et al. Clinical and pathologic impact of select chromatin-modulating tumor suppressors in clear cell renal cell carcinoma. Eur Urol. 2013;63:848–854. doi: 10.1016/j.eururo.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo G, et al. Frequent mutations of genes encoding ubiquitin-mediated proteolysis pathway components in clear cell renal cell carcinoma. Nat Genet. 2012;44:17–19. doi: 10.1038/ng.1014. [DOI] [PubMed] [Google Scholar]
- 20.Nickerson ML, et al. Improved identification of von Hippel-Lindau gene alterations in clear cell renal tumors. Clin Cancer Res. 2008;14:4726–4734. doi: 10.1158/1078-0432.CCR-07-4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peña-Llopis S, Christie A, Xie XJ, Brugarolas J. Cooperation and Antagonism among Cancer Genes: The Renal Cancer Paradigm. Cancer Res. 2013;73:4173–4179. doi: 10.1158/0008-5472.CAN-13-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boom R, et al. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gross-Bellard M, Oudet P, Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973;36:32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- 24.Strauss WM. Preparation of genomic DNA from mammalian tissue. Curr Protoc Mol Biol. 2001 doi: 10.1002/0471142727.mb0202s42. Chapter 2, Unit2 2. [DOI] [PubMed] [Google Scholar]
- 25.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 26.Chomczynski P, Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nat Protoc. 2006;1:581–585. doi: 10.1038/nprot.2006.83. [DOI] [PubMed] [Google Scholar]
- 27.Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques. 1993;15:532–534. 536–537. [PubMed] [Google Scholar]
- 28.Eddy SR. Non-coding RNA genes and the modern RNA world. Nat Rev Genet. 2001;2:919–929. doi: 10.1038/35103511. [DOI] [PubMed] [Google Scholar]
- 29.Siomi MC, Sato K, Pezic D, Aravin AA. PIWI-interacting small RNAs: the vanguard of genome defence. Nat Rev Mol Cell Biol. 2011;12:246–258. doi: 10.1038/nrm3089. [DOI] [PubMed] [Google Scholar]
- 30.Vega-Rubin-de-Celis S, et al. Structural analysis and functional implications of the negative mTORC1 regulator REDD1. Biochemistry. 2010;49:2491–2501. doi: 10.1021/bi902135e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kucejova B, et al. Interplay between pVHL and mTORC1 pathways in clear-cell renal cell carcinoma. Mol Cancer Res. 2011;9:1255–1265. doi: 10.1158/1541-7786.MCR-11-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peña-Llopis S, et al. Regulation of TFEB and V-ATPases by mTORC1. EMBO J. 2011;30:3242–3258. doi: 10.1038/emboj.2011.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark SJ, Statham A, Stirzaker C, Molloy PL, Frommer M. DNA methylation: bisulphite modification and analysis. Nat Protoc. 2006;1:2353–2364. doi: 10.1038/nprot.2006.324. [DOI] [PubMed] [Google Scholar]
- 34.Taiwo O, et al. Methylome analysis using MeDIP-seq with low DNA concentrations. Nat Protoc. 2012;7:617–636. doi: 10.1038/nprot.2012.012. [DOI] [PubMed] [Google Scholar]
- 35.Southern E. Southern blotting. Nat Protoc. 2006;1:518–525. doi: 10.1038/nprot.2006.73. [DOI] [PubMed] [Google Scholar]
- 36.Wilhelm BT, Marguerat S, Goodhead I, Bahler J. Defining transcribed regions using RNA-seq. Nat Protoc. 2010;5:255–266. doi: 10.1038/nprot.2009.229. [DOI] [PubMed] [Google Scholar]
- 37.Streit S, Michalski CW, Erkan M, Kleeff J, Friess H. Northern blot analysis for detection and quantification of RNA in pancreatic cancer cells and tissues. Nat Protoc. 2009;4:37–43. doi: 10.1038/nprot.2008.216. [DOI] [PubMed] [Google Scholar]
- 38.Pall GS, Hamilton AJ. Improved northern blot method for enhanced detection of small RNA. Nat Protoc. 2008;3:1077–1084. doi: 10.1038/nprot.2008.67. [DOI] [PubMed] [Google Scholar]
- 39.Liu CG, Calin GA, Volinia S, Croce CM. MicroRNA expression profiling using microarrays. Nat Protoc. 2008;3:563–578. doi: 10.1038/nprot.2008.14. [DOI] [PubMed] [Google Scholar]
- 40.Carrette O, Burkhard PR, Sanchez JC, Hochstrasser DF. State-of-the-art two-dimensional gel electrophoresis: a key tool of proteomics research. Nat Protoc. 2006;1:812–823. doi: 10.1038/nprot.2006.104. [DOI] [PubMed] [Google Scholar]
- 41.Shiio Y, Aebersold R. Quantitative proteome analysis using isotope-coded affinity tags and mass spectrometry. Nat Protoc. 2006;1:139–145. doi: 10.1038/nprot.2006.22. [DOI] [PubMed] [Google Scholar]
- 42.de Planell-Saguer M, Rodicio MC, Mourelatos Z. Rapid in situ codetection of noncoding RNAs and proteins in cells and formalin-fixed paraffin-embedded tissue sections without protease treatment. Nat Protoc. 2010;5:1061–1073. doi: 10.1038/nprot.2010.62. [DOI] [PubMed] [Google Scholar]