Abstract
Breath-holding techniques reduce the amount of radiation received by cardiac structures during tangential-field left breast radiotherapy. With these techniques, patients hold their breath while radiotherapy is delivered, pushing the heart down and away from the radiotherapy field. Despite clear dosimetric benefits, these techniques are not yet in widespread use. One reason for this is that commercially available solutions require specialist equipment, necessitating not only significant capital investment, but often also incurring ongoing costs such as a need for daily disposable mouthpieces. The voluntary breath-hold technique described here does not require any additional specialist equipment. All breath-holding techniques require a surrogate to monitor breath-hold consistency and whether breath-hold is maintained. Voluntary breath-hold uses the distance moved by the anterior and lateral reference marks (tattoos) away from the treatment room lasers in breath-hold to monitor consistency at CT-planning and treatment setup. Light fields are then used to monitor breath-hold consistency prior to and during radiotherapy delivery.
Keywords: Medicine, Issue 89, breast, radiotherapy, heart, cardiac dose, breath-hold
Introduction
Cancer is a leading cause of death worldwide, accounting for 7.6 million deaths in 20081. Of all cancers, breast cancer is the most common with an incidence of over 13.8 million worldwide, and this incidence is increasing1. However, improvements in the diagnosis and treatment of breast cancer mean that the number of women surviving their breast cancer is also increasing, and is estimated to treble to 1.7 million by 2040 in the UK alone2. Breast radiotherapy forms an important part of many women’s breast cancer treatment, halving their risk of breast cancer recurrence and reducing the risk of breast cancer death by 3.8%3. With improvements in breast cancer survivorship, any long term side effects caused by breast cancer treatments are increasingly important. An innocent bystander in breast radiotherapy is the heart, which is exposed to unwanted radiation as a result of its proximity to radiation fields, especially during left breast irradiation. It is this unwanted dose to the heart that accounts for the 1% increase in non-breast cancer deaths associated with breast radiotherapy4. Recent evidence suggests that there is no threshold dose below which the late cardiac effects of breast radiotherapy do not occur5, making it critical for the oncology community to establish techniques which minimize cardiac doses without compromising breast tissue coverage. However, since breast radiotherapy accounts for approximately 30% of all radiotherapy treatments6, any new technique must be simple and inexpensive in order to be sustainable and avoid an unacceptable burden on healthcare resources.
There are a number of techniques which may be employed to reduce heart doses during breast radiotherapy. Multileaf collimation (MLC) is widely used in the UK [Royal College of Radiologists’ (UK) audit 2012] and although effective at sparing heart tissue, it risks simultaneously shielding breast tissue. Inverse planned intensity modulated radiotherapy (IMRT) improves target tissue conformality7, but may also increase low-dose irradiation of the heart, lungs and contralateral breast7,8. An increase in low dose irradiation of the heart is undesirable, particularly in light of the data from Darby et al5. In addition, inverse-planned IMRT is more resource-intensive, requiring greater physics and quality assurance (QA) time and expertise. Treating women in the prone (face-down) position may reduce cardiac doses in larger-breasted women9, however, questions remain over the positional reproducibility of this technique10. Breath-holding techniques, in which patients hold their breath during radiotherapy delivery, result in the heart being pushed down and away from the radiotherapy fields and may minimize the need for a compromise between target tissue coverage and organ-at-risk (OAR) sparing (Figure 1)11.
There are currently two main breath-holding techniques in clinical use. The first consists of a digital spirometer attached to a balloon valve. Patients breathe through a mouthpiece and a clip is placed on their nose to avoid nasal respiration. The spirometry trace is visualized on a monitor, and inspiration interrupted and held at a predetermined lung volume. The second method was primarily designed for use as a respiratory gating system, although it also has an built-in breath-hold setting. This system uses a video camera to record the motion of an infrared-reflecting marker placed on the chest of the patient. The vertical movement of the marker is displayed in real-time on a monitor, and treatment delivery commences once the marker moves into a pre-specified threshold zone. Both systems markedly reduce cardiac doses in patients receiving left breast radiotherapy. The spirometry-based technique significantly reduces the volume of myocardium irradiated12-14, as well as demonstrating comparable intra- and inter-fraction reproducibility compared to standard supine free-breathing breast radiotherapy15. Similarly, treatment using the infrared-reflecting markers reduces the mean dose to the heart by over 50%11,16,17, whilst maintaining target tissue coverage11. Such dosimetric savings are projected to equate to a 10 fold reduction in cardiac deaths18.
A drawback of these systems, however, and a barrier to widespread implementation, is their cost. Both systems require investment in the devices themselves, however, in the case of the spirometry system there are also ongoing costs as the mouthpieces are disposable, requiring a new mouthpiece for planning-CT as well as for each fraction of treatment. Cost, coupled with a lack of staff training, explains why only 4% of UK breast treatments were performed using breath-holding techniques in 2012 [Royal College of Radiologists’ (UK) audit]. Breath-holding techniques are in more widespread use in the rest of Europe, with 20% of centers using these techniques in 201019. One explanation for this is the development and implementation of a simple, inexpensive and equipment-free breath-holding technique, voluntary breath-hold (VBH). Until recently, however, data was lacking on the reproducibility of the VBH technique. A randomized study conducted at the Royal Marsden Hospital (Sutton, UK), The UK HeartSpare Study, has demonstrated that interfraction reproducibility with the VBH technique is comparable to that with the spirometry-based device. In addition, the VBH technique offers a time advantage at planning-CT and treatment setup and is preferred by patients and radiographers alike20. The VBH technique is currently being rolled out to ten UK radiotherapy centers to confirm that the technique is feasible in a multicenter setting and that heart-sparing is maintained (HeartSpare II). It is expected that this will pave the way for the UK-wide uptake of heart-sparing breast radiotherapy, and is likely to lead to a significant reduction in heart disease among UK breast cancer survivors.
Protocol
The study through which this protocol was implemented was approved by the Royal Marsden Committee for Clinical Research (Sutton, UK) and the Research Ethics Committee (London – Riverside, UK) (ISRCTN 53485935).
1. Radiotherapy Clinic
Assess patient suitability for the voluntary breath-hold technique in the clinic: left breast or chest wall radiotherapy (without nodal irradiation) recommended by radiation oncologist.
Review the patient’s performance status and comorbidities (especially lung-related).
Ask the patient to practice holding their breath at home, while lying down, initially for 5 sec, and building up in 5 sec intervals to 20 sec.
2. Radiotherapy Planning-CT session
Position the patient on the CT couch in the standard treatment position.
Define the position of tattoos and place CT markers (crosses) on the patient midline in free breathing, approximately half way along the likely field edges. Add lateral markers to each side of the patient in free-breathing, in line with the midline marker.
Ask the patient to practice taking a deep breath in and holding it, initially for 5 sec, before building up in 5 sec intervals to 20 sec. Instruct the patient to breathe in and breathe out twice before asking them to hold their breath for up to 20 sec. This relaxes the patient, helps them prepare for the breath-hold and helps breath-hold consistency.
Record the maximum duration for which the patient can comfortably hold their breath.
Repeat the breath-hold and mark the position of the anterior and lateral tattoos in relation to the lasers in breath-hold to help establish reproducibility. Record the height of the lateral tattoo above the couch top during breath-hold before proceeding with the CT scan.
Give the standard breath-hold instructions to the patient and start the scan once satisfied the patient is in breath-hold.
Once the CT scan has been completed, check and record the height of the lateral tattoos on the CT scan to confirm that a consistent breath-hold was performed. If the lateral couch height differs by greater than 3 mm from the initial couch height, remeasure the anterior and lateral reference points.
3. Radiotherapy Treatment Planning
NOTE: The radiotherapy treatment planning process is the same as for a standard breast patient.
Apply tangential radiotherapy fields according to local protocol.
Produce clinical radiotherapy treatment plan which fulfills ICRU criteria.
Record the anterior beam source-to-skin distance (SSD) in addition to recording standard treatment planning data (according to local protocol). NOTE: The anterior beam SSD is used to check the anterior-posterior setup in the treatment room.
4. Radiotherapy Treatment Setup
Align the tattoos in free-breathing. Mark the posterior and inferior measurements from the left lateral tattoo and the anterior midline tattoo on the patient’s skin (from information recorded at planning-CT session).
Instruct the patient to breathe in and out twice before taking a deep breath in and holding. The reference mark on the patient’s skin should rise up to the level of the laser. Ask the patient to repeat the breath-hold procedure a couple of times to confirm reproducibility before proceeding with patient setup.
Ask the patient to perform a breath-hold and align the midline tattoo to the isocenter position superior/inferior and set the focus-to-surface distance (FSD) at the midline.
In free-breathing, move the bed laterally to the isocenter.
Measure and mark the medial and lateral field borders in free-breathing.
Set all other machine parameters (e.g., field size and gantry, collimator, and couch angles) for the first beam (anterior oblique). Ask the patient to perform a breath-hold and check the medial border aligns with mark made in Step 4.5.
Mark the field edge (as defined by the light field) with a pen at every fraction: this aids visualization of the patient’s breath-hold.
Repeat steps 4.6 and 4.7 for the posterior oblique beam, and treat using this beam first.
If patient setup is out of tolerance (according to local tolerance levels for a standard breast radiotherapy patient), refer to the troubleshooting algorithm (Figure 2).
If there are insufficient cameras to monitor the field edge as well as the position of the gantry relative to the couch from the control room, assess gantry rotation prior to leaving the treatment room in order to avoid collisions.
5. Radiotherapy Treatment Delivery
Once in the control room, zoom the treatment room cameras so that the field borders marked on the patient’s skin are visible on the control room monitors.
Once ready to commence treatment, ask the patient to perform a breath-hold (as detailed in 4.2) via the intercom system. Check the light field aligns satisfactorily with the marked field border and then commence treatment (Figure 3).
Monitor the patient’s breath-hold during treatment delivery. Treatment should be interrupted if there is concern that there has been a change in breath-hold depth.
6. Radiotherapy Treatment Verification
Perform imaging verification of patient position (such as with electronic portal imaging (EPI) or cone-beam CT), following local protocols for type/frequency of imaging and tolerance levels.
Correct for systematic errors with isocenter moves according to local protocols for standard breast radiotherapy patients. Adjustment of marks on the patient’s skin should not be necessary.
Representative Results
Real-time electronic portal images (EPI) were matched on-line to digitally reconstructed radiographs (DRRs) for 23 patients (172 treatment fractions). EPI displacements were analyzed for the right anterior and left posterior oblique beams in the (u,v)-plane (v-direction parallel to craniocaudal axis and u-direction perpendicular to this)21, and setup errors for the VBH technique estimated. EPI-based population systematic error range (for each beam and in each plane) was 1.5-1.8 mm and random error range 1.7-2.5 mm.
Tabular dose-volume histogram (DVH) data was used to derive the NTDmean (a biologically weighted mean of total dose to tissue normalized to 2 Gy fractions using a standard linear quadratic model22, α/β = 3Gy) for heart, left anterior descending coronary artery (LAD), ipsilateral, and whole lungs. In addition, the maximum dose received by the LAD (LADmax) was estimated. Normal tissue doses are shown in Table 1.
Times for planning-CT session, treatment setup, treatment delivery and total treatment session were recorded, and are shown in Table 2. The data demonstrate that planning-CTs can be completed within a standard 30 min session. Treatment times include CBCT imaging, which was performed for every third fraction. Treatment setup and total session times are, therefore, expected to be shorter than reported here for centers in which CBCT imaging is not part of standard treatment. However, even with CBCT imaging, treatments may be completed within a 20 min treatment session.
Patients and radiographers were asked to complete validated questionnaires23 at their planning-CT session as well as twice during their treatment. Sixty-five patient questionnaires and 64 radiographer questionnaires were analyzed. The questionnaires were summarized as patient comfort scores (PCS) and radiographer satisfaction scores (RSS) (out of 9, higher score = more comfortable/satisfactory). Median PCS was 8 (interquartile range 8-9) and median RSS was 7 (interquartile range 6-8).
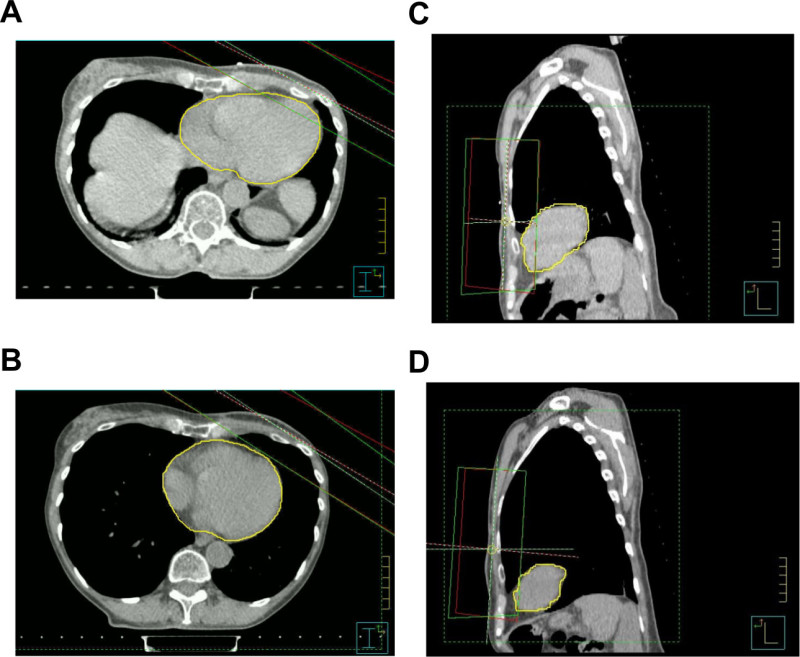 Figure 1. The heart-sparing effect of VBH. Axial and sagittal CT slices from the same patient at the same chest wall level in free-breathing (A and C) and using the VBH technique (B and D). Note that the heart (outlined in yellow) has been pushed down and away from the radiotherapy fields using the VBH technique. Please click here to view a larger version of this figure.
Figure 1. The heart-sparing effect of VBH. Axial and sagittal CT slices from the same patient at the same chest wall level in free-breathing (A and C) and using the VBH technique (B and D). Note that the heart (outlined in yellow) has been pushed down and away from the radiotherapy fields using the VBH technique. Please click here to view a larger version of this figure.
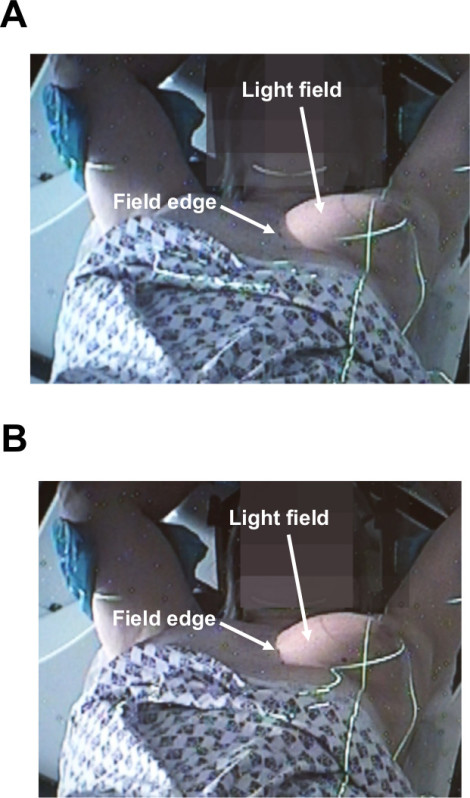 Figure 3. Checking breath-hold consistency from the control room. Control room CCTV stills demonstrating the position of the light field relative to the marked field border for a right anterior oblique beam in free-breathing (A) and the aligning of the light field and marked field border once the patient is in breath-hold (B).
Figure 3. Checking breath-hold consistency from the control room. Control room CCTV stills demonstrating the position of the light field relative to the marked field border for a right anterior oblique beam in free-breathing (A) and the aligning of the light field and marked field border once the patient is in breath-hold (B).
| Median dose (Gy) | Minimum dose (Gy) | Maximum dose (Gy) | ||||
| VBH | FB | VBH | FB | VBH | FB | |
| Heart NTDmean | 0.6 | 0.8 | 0.4 | 0.4 | 0.9 | 2.1 |
| LAD NTDmean | 2.5 | 6.0 | 1.3 | 1.2 | 10.0 | 22.6 |
| LADmax | 28.6 | 43.7 | 9.7 | 4.6 | 41.8 | 51.3 |
| Ipsilateral lung NTDmean | 4.1 | - | 2.8 | - | 5.6 | - |
| Whole lungs NTDmean | 2.0 | - | 1.3 | - | 2.7 | - |
Table 1. Normal tissue doses for the voluntary breath-hold (VBH) technique. Median, minimum and maximum NTDmean (Gy) for heart, LAD, ipsilateral and whole lungs are shown, as are median, minimum, and maximum LADmax (Gy). In addition, median, minimum, and maximum cardiac doses for standard free-breathing (FB) left breast radiotherapy at our center are shown for comparison.
| Time (min) | |||
| Event | Median | Minimum | Maximum |
| Planning-CT session | 22 | 14 | 44 |
| Treatment setup | 9 | 6 | 15 |
| Treatment delivery | 7 | 5 | 12 |
| Total treatment session | 18 | 14 | 27 |
Table 2. Planning-CT and treatment session times for the voluntary breath-hold technique. Median, minimum, and maximum planning-CT, treatment setup, treatment delivery, and total treatment session times are shown (min).
Discussion
Critical steps in the protocol include: 1) checking for breath-hold consistency at planning-CT and treatment setup; 2) checking the lateral couch height measured on CT is consistent with that measured pre-CT; 3) aligning tattoos in free-breathing but setting FSD in breath-hold; 4) ensuring light field aligns with marked field borders prior to commencing treatment.
The number of breath-holds required during treatment delivery varies from patient to patient, and is primarily dependent on the number of segments being delivered. Suitable interruption points during treatment delivery (to enable the patient to relax before repeating a breath-hold) should be determined on an individual basis depending on the method of delivery. We would strongly suggest that for initial implementation of VBH that a consistent team is used. This enables those involved to become competent more quickly and helps maintain treatment quality. Where problems are encountered during treatment setup, the patient may be asked to modify their breath-hold (deeper or shallower as required). If this fails to improve setup, the patient should be set up again. Vector couch moves should be employed as a last resort. A troubleshooting algorithm is shown in Figure 2.
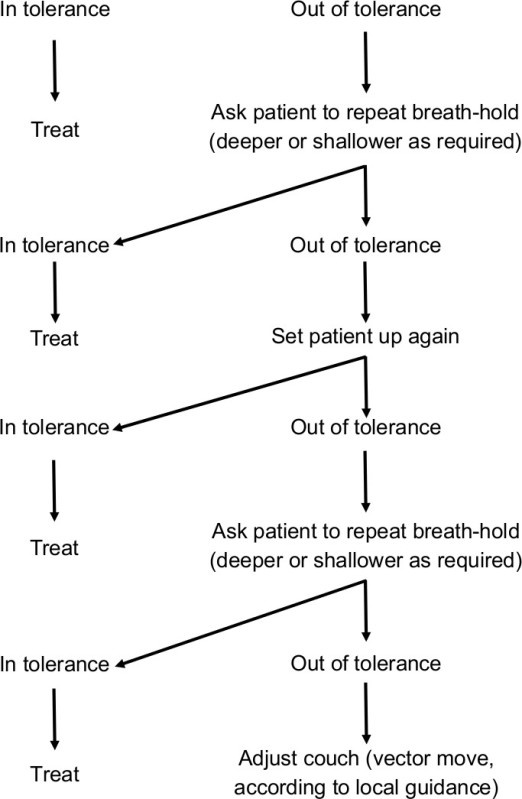 Figure 2. Troubleshooting algorithm for voluntary breath-hold technique treatment setup. This algorithm may be used to assist treatment setup where patients are not setting up within tolerance (according to local tolerance levels). As an example, our center uses a 5 mm tolerance level. The algorithm should be followed from top to bottom. In the majority of cases, setup can be brought within tolerance by asking the patient to modify their breath-hold depth (deeper or shallower as required).
Figure 2. Troubleshooting algorithm for voluntary breath-hold technique treatment setup. This algorithm may be used to assist treatment setup where patients are not setting up within tolerance (according to local tolerance levels). As an example, our center uses a 5 mm tolerance level. The algorithm should be followed from top to bottom. In the majority of cases, setup can be brought within tolerance by asking the patient to modify their breath-hold depth (deeper or shallower as required).
Population systematic and random treatment setup errors are less than those seen in free-breathing tangential field breast radiotherapy24, and consistent with other published data on breath-holding techniques25,26. VBH reduces median normal tissue doses by 25-58% compared with standard free-breathing breast radiotherapy at our center (Table 1). Cardiac doses with VBH are lower than those seen in other published work on breath-holding techniques11,16,17,26,27, although methods of recording dose data vary between these studies.
As described in the introduction, all breath-hold techniques use a surrogate to measure inter- and intrafraction reproducibility. The VBH technique uses alignment of the light field with marked field borders to check for consistency before commencing treatment and during treatment delivery. Although not yet formally reported, we have found intrafraction reproducibility (measured using multiple intrafraction EPIs) to be extremely good, with little, if any, intrafraction movement. This is consistent with previously published work26. Given the consistent intrafraction reproducibility observed, radiotherapy systems in which light fields do not remain on during treatment delivery need not be a barrier to implementation of VBH. In-room lasers may be used as an alternative to light fields for checking that the breath-hold is maintained during treatment delivery. In addition, the reference point from which breath-hold reproducibility is monitored may be adapted; for example, centers using an asymmetric field technique may wish to use the superior tangential field border.
VBH offers significant advantages over other heart-sparing techniques, some of which have already been alluded to. It minimizes the trade-off between target and OAR compromise often required when using MLC, it reduces the low-dose irradiation of the heart and is much less resource-intensive than IMRT, and it is more reproducible than prone irradiation whilst benefiting women of all breast sizes. With respect to other breath-holding techniques, VBH gives comparable reproducibility and heart-sparing, while being less expensive to implement as no specialist equipment is required. The low cost of the technique means that there is a very real opportunity for it to benefit other healthcare systems, especially those with limited resources.
There is already published work demonstrating the feasibility of delivering nodal irradiation in addition to whole breast/chest wall irradiation using the infrared-reflecting markers17 and spirometry-based14 systems. Our center is now performing further work to confirm the feasibility of using VBH for nodal irradiation in breast cancer patients. Inverse-planned IMRT is likely to be of benefit in selected patients, especially when delivering a simultaneous integrated boost, and the feasibility of using VBH in these patients needs to be assessed. Finally, breath-holding techniques may be of benefit when treating other tumors, including lung28, liver29, and gastric30 cancers. Further work is needed to assess the suitability of using the VBH technique for treating sites other than breast.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors are grateful to Dr Liesbeth Boersma and colleagues at the Maastro Clinic for their advice on the voluntary breath-hold technique. The authors are also very grateful to the Pink Ribbon Foundation for funding the dissemination of the VBH technique. This article presents independent research funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number PB-PG-1010-23003). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. The work was undertaken in The Royal Marsden NHS Foundation Trust which receives a proportion of its funding from the NHS Executive. We acknowledge NHS funding to the NIHR Biomedical Research Centre and the support of the NIHR, through the South London Cancer Research Network.
References
- Ferlay J, et al. International Agency for Research on Cancer. Lyon: 2010. GLOBOCAN 2008 v1.2. [Google Scholar]
- Maddams J, Utley M, Moller H. Projections of cancer prevalence in the United Kingdom, 2010-2040. Br. J. Cancer. 2012;107:1195–1202. doi: 10.1038/bjc.2012.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby S, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- Darby SC, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- Team DoHCP. London, UK: 2012. Radiotherapy Services in England 2012. [Google Scholar]
- Zhang F, Zheng M. Dosimetric evaluation of conventional radiotherapy, 3-D conformal radiotherapy and direct machine parameter optimisation intensity-modulated radiotherapy for breast cancer after conservative surgery. J Med Imaging Radiat Oncol. 2011;55:595–602. doi: 10.1111/j.1754-9485.2011.02313.x. [DOI] [PubMed] [Google Scholar]
- Schubert LK, et al. Dosimetric comparison of left-sided whole breast irradiation with 3DCRT forward-planned IMRT, inverse-planned IMRT, helical tomotherapy, and topotherapy. Radiother. Oncol. 2011;100:241–246. doi: 10.1016/j.radonc.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Kirby AM, et al. Prone versus supine positioning for whole and partial-breast radiotherapy: A comparison of non-target tissue dosimetry. Radiother. Oncol. 2010;96:178–184. doi: 10.1016/j.radonc.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Kirby AM, et al. A randomised trial of Supine versus Prone breast radiotherapy (SuPr study): Comparing set-up errors and respiratory motion. Radiother. Oncol. 2011;100:221–226. doi: 10.1016/j.radonc.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Vikstrom J, Hjelstuen MH, Mjaaland I, Dybvik KI. Cardiac and pulmonary dose reduction for tangentially irradiated breast cancer, utilizing deep inspiration breath-hold with audio-visual guidance, without compromising target coverage. Acta Oncol. 2011;50:42–50. doi: 10.3109/0284186X.2010.512923. [DOI] [PubMed] [Google Scholar]
- Sixel KE, Aznar MC, Ung YC. Deep inspiration breath hold to reduce irradiated heart volume in breast cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2001;49:199–204. doi: 10.1016/s0360-3016(00)01455-3. [DOI] [PubMed] [Google Scholar]
- Krauss DJ, et al. MRI-based volumetric assessment of cardiac anatomy and dose reduction via active breathing control during irradiation for left-sided breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2005;61:1243–1250. doi: 10.1016/j.ijrobp.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Remouchamps VM, et al. Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2003;55:392–406. doi: 10.1016/s0360-3016(02)04143-3. [DOI] [PubMed] [Google Scholar]
- Remouchamps VM, et al. Three-dimensional evaluation of intra- and interfraction immobilization of lung and chest wall using active breathing control: A reproducibility study with breast cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2003;57:968–978. doi: 10.1016/s0360-3016(03)00710-7. [DOI] [PubMed] [Google Scholar]
- Johansen S, et al. Dose evaluation and risk estimation for secondary cancer in contralateral breast and a study of correlation between thorax shape and dose to organs at risk following tangentially breast irradiation during deep inspiration breath-hold and free breathing. Acta Oncol. 2011;50:563–568. doi: 10.3109/0284186X.2010.541933. [DOI] [PubMed] [Google Scholar]
- Hjelstuen MH, Mjaaland I, Vikstrom J, Dybvik KI. Radiation during deep inspiration allows loco-regional treatment of left breast and axillary-, supraclavicular- and internal mammary lymph nodes without compromising target coverage or dose restrictions to organs at risk. Acta Oncol. 2012;51:333–344. doi: 10.3109/0284186X.2011.618510. [DOI] [PubMed] [Google Scholar]
- Korreman SS, et al. Cardiac and pulmonary complication probabilities for breast cancer patients after routine end-inspiration gated radiotherapy. Radiother. Oncol. 2006;80:257–262. doi: 10.1016/j.radonc.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Laan HP, Hurkmans CW, Kuten A, Westenberg HA. Current technological clinical practice in breast radiotherapy; results of a survey in EORTC-Radiation Oncology Group affiliated institutions. Radiother. Oncol. 2010;94:280–285. doi: 10.1016/j.radonc.2009.12.032. [DOI] [PubMed] [Google Scholar]
- Bartlett FR, et al. The UK HeartSpare Study: Randomised evaluation of voluntary deep-inspiratory breath-hold in women undergoing breast radiotherapy. Radiother. Oncol. 2013;108:242–247. doi: 10.1016/j.radonc.2013.04.021. [DOI] [PubMed] [Google Scholar]
- Penninkhof J, Quint S, Baaijens M, Heijmen B, Dirkx M. Practical use of the extended no action level (eNAL) correction protocol for breast cancer patients with implanted surgical clips. Int. J. Radiat. Oncol. Biol. Phys. 2012;82:1031–1037. doi: 10.1016/j.ijrobp.2010.12.059. [DOI] [PubMed] [Google Scholar]
- Scrimger RA, et al. Reduction in radiation dose to lung and other normal tissues using helical tomotherapy to treat lung cancer, in comparison to conventional field arrangements. Am. J. Clin. Oncol. 2003;26:70–78. doi: 10.1097/00000421-200302000-00014. [DOI] [PubMed] [Google Scholar]
- Nutting CM, et al. A randomised study of the use of a customised immobilisation system in the treatment of prostate cancer with conformal radiotherapy. Radiother. Oncol. 2000;54:1–9. doi: 10.1016/s0167-8140(99)00181-4. [DOI] [PubMed] [Google Scholar]
- Hurkmans CW, Remeijer P, Lebesque JV, Mijnheer BJ. Set-up verification using portal imaging; review of current clinical practice. Radiother. Oncol. 2001;58:105–120. doi: 10.1016/s0167-8140(00)00260-7. [DOI] [PubMed] [Google Scholar]
- Remouchamps VM, et al. Initial clinical experience with moderate deep-inspiration breath hold using an active breathing control device in the treatment of patients with left-sided breast cancer using external beam radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2003;56:704–715. doi: 10.1016/s0360-3016(03)00010-5. [DOI] [PubMed] [Google Scholar]
- Borst GR, et al. Clinical results of image-guided deep inspiration breath hold breast irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2010;78:1345–1351. doi: 10.1016/j.ijrobp.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Hayden AJ, Rains M, Tiver K. Deep inspiration breath hold technique reduces heart dose from radiotherapy for left-sided breast cancer. J Med Imaging Radiat Oncol. 2012;56:464–472. doi: 10.1111/j.1754-9485.2012.02405.x. [DOI] [PubMed] [Google Scholar]
- Marchand V, et al. Dosimetric comparison of free-breathing and deep inspiration breath-hold radiotherapy for lung cancer. Strahlenther. Onkol. 2012;188:582–589. doi: 10.1007/s00066-012-0129-9. [DOI] [PubMed] [Google Scholar]
- Bloemen-van Gurp E, et al. Active breathing control in combination with ultrasound imaging: a feasibility study of image guidance in stereotactic body radiation therapy of liver lesions. Int. J. Radiat. Oncol. Biol. Phys. 2013;85:1096–1102. doi: 10.1016/j.ijrobp.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Hu W, Ye J, Wang J, Xu Q, Zhang Z. Incorporating breath holding and image guidance in the adjuvant gastric cancer radiotherapy: a dosimetric study. Radiat Oncol. 2012;7 doi: 10.1186/1748-717X-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]


