Abstract
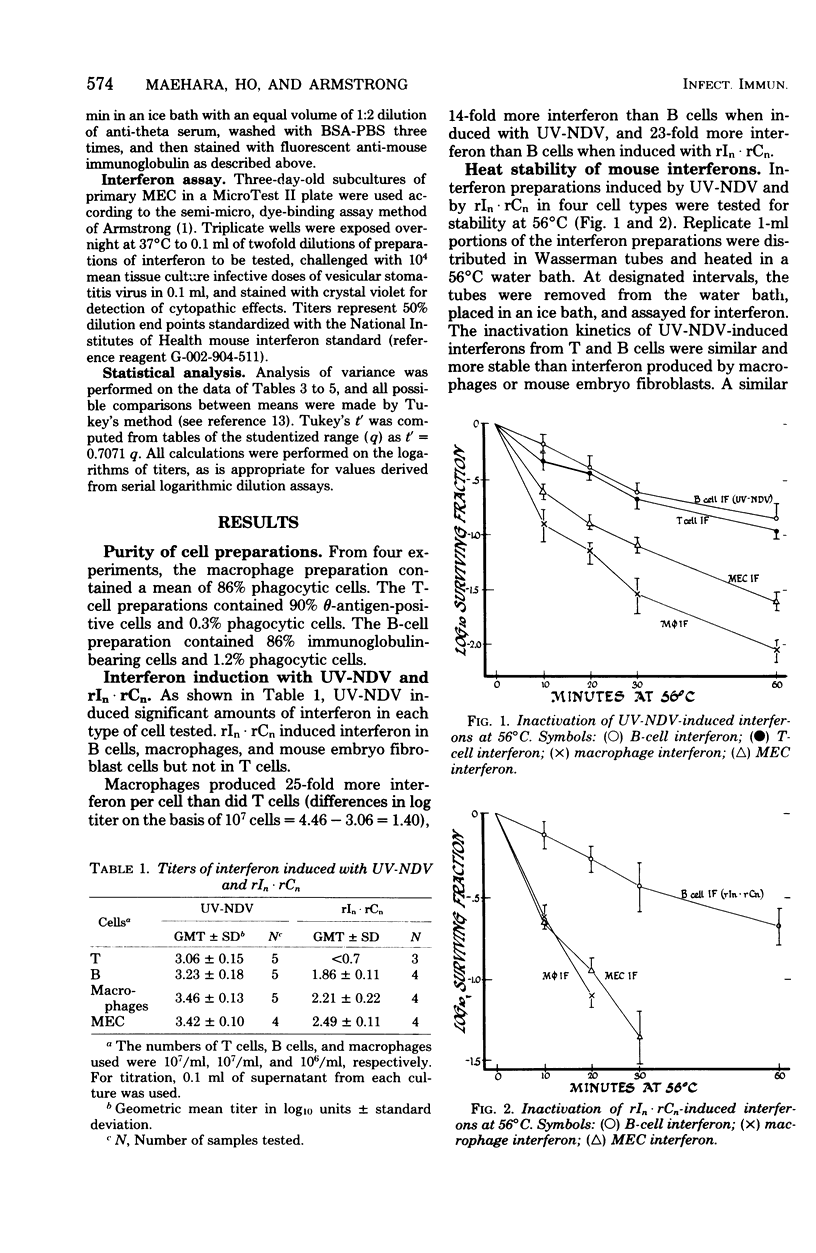
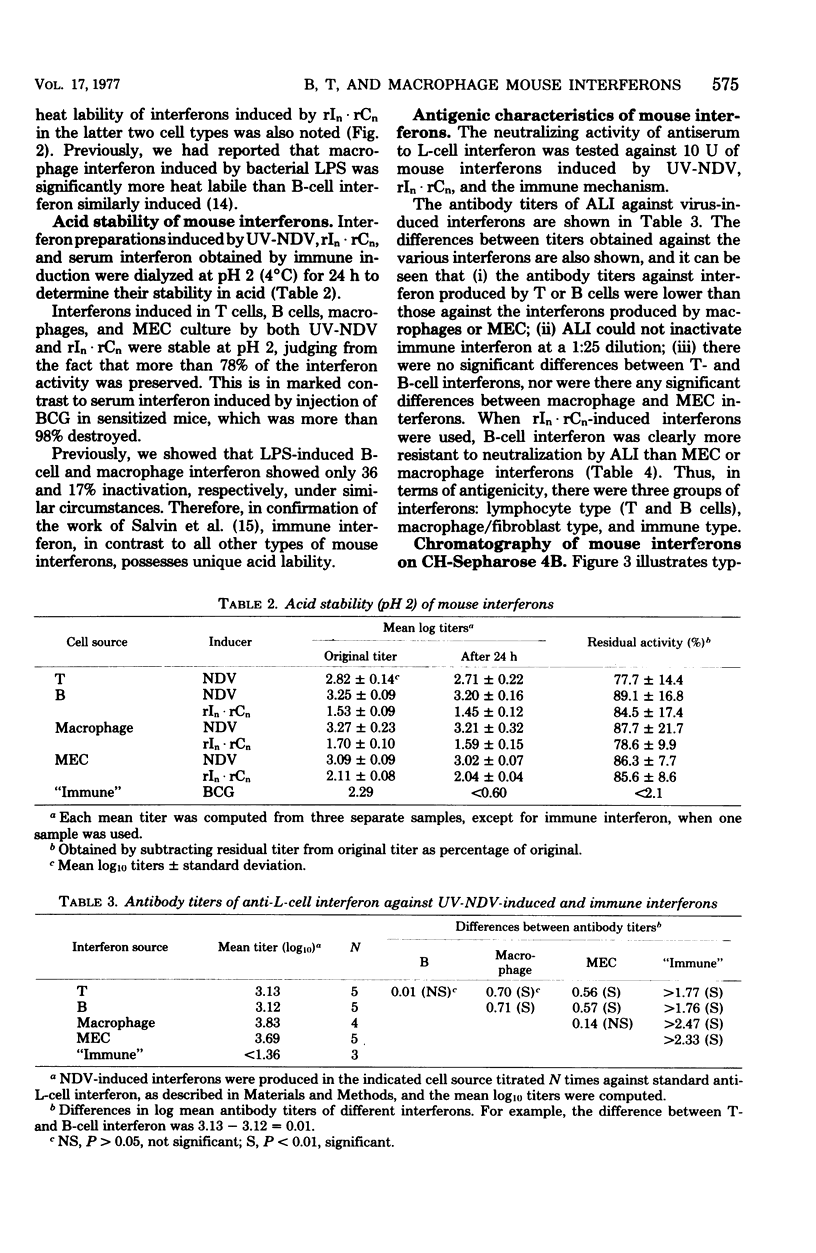
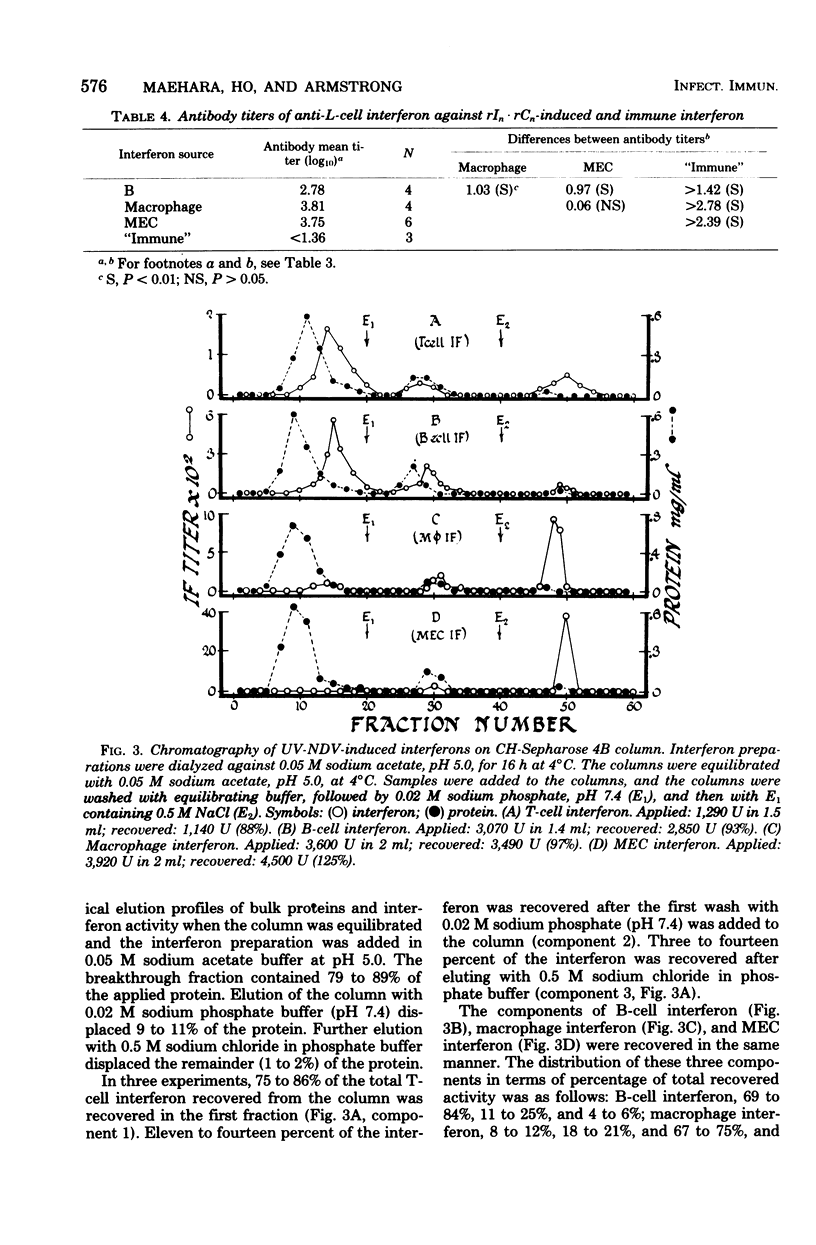
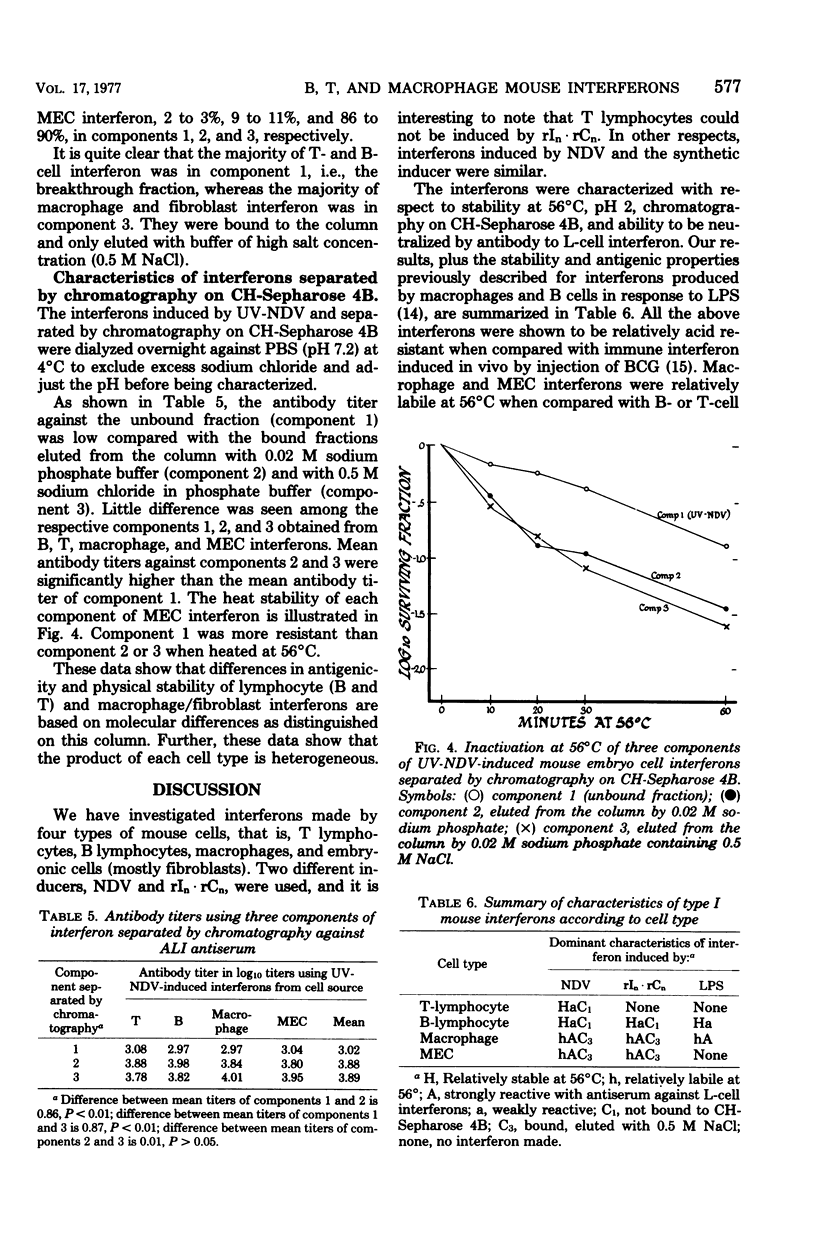
Mouse interferon induced by ultraviolet-irradiated Newcastle disease virus or polyriboinosinic-polyribocytidylic acid in T lymphocytes, B lymphocytes, macrophages, and primary mouse embryonic cell culture was studied. Irrespective of the inducer, interferons produced by T or B lymphocytes were relatively heat stable and of low antigenicity when reacted with antiserum against L-cell interferon (ALI), whereas interferons produced by macrophages and mouse embryo cells were heat labile and of high antigenicity against ALI. Mouse interferons induced by ultraviolet-irradiated Newcastle disease virus were separated into three components by chromatography on CH-Sepharose 4B. Interferons produced by T and B lymphocytes consisted primarily of component 1 (unbound fraction), whereas interferons produced by macrophages or mouse embryo cells consisted primarily of component 3 (eluted by 0.5 M NaCl). Component 1 was heat stable and of low antigenicity against ALI, properties characteristic of T- and B-cell interferon. Components 2 and 3 were heat labile and of high antigenicity against ALI, properties characteristic of macrophage and mouse embryo cell interferon. In contrast, interferon induced in mice sensitized with BCG differed from these interferons induced in B cells, T cells, macrophages, and fibroblasts in being extremely acid labile and nonreactive against ALI.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A. Semi-micro, dye-binding assay for rabbit interferon. Appl Microbiol. 1971 Apr;21(4):723–725. doi: 10.1128/am.21.4.723-725.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey M. W., Sulkowski E., Carter W. A. Purification and characterization of mouse interferon with novel affinity sorbents. J Virol. 1976 Feb;17(2):439–445. doi: 10.1128/jvi.17.2.439-445.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcoff E., Falcoff R., Eyquem A., Sanceau J., Catinot L., de Vomecourt A. Induction d'interféron humain par le sérum antilymphocytaire. C R Acad Sci Hebd Seances Acad Sci D. 1970 Aug 3;271(5):545–548. [PubMed] [Google Scholar]
- Field A. K., Tytell A. A., Lampson G. P., Hilleman M. R. Inducers of interferon and host resistance. II. Multistranded synthetic polynucleotide complexes. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1004–1010. doi: 10.1073/pnas.58.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gery I., Krüger J., Spiesel S. Z. Stimulation of B-lymphocytes by endotoxin. Reactions of thymus-deprived mice and karyotypic analysis of dividing cells in mice bearing T 6 T 6 thymus grafts. J Immunol. 1972 Apr;108(4):1088–1091. [PubMed] [Google Scholar]
- HO M. INTERFERON-LIKE VIRAL INHIBITOR IN RABBITS AFTER INTRAVENOUS ADMINISTRATION OF ENDOTOXIN. Science. 1964 Dec 11;146(3650):1472–1474. doi: 10.1126/science.146.3650.1472. [DOI] [PubMed] [Google Scholar]
- Handwerger B. S., Schwartz R. H. Separation of murine lymphoid cells using nylon wool columns. Recovery of the B cell-enriched population. Transplantation. 1974 Dec;18(6):544–548. doi: 10.1097/00007890-197412000-00013. [DOI] [PubMed] [Google Scholar]
- Havell E. A., Berman B., Ogburn C. A., Berg K., Paucker K., Vilcek J. Two antigenically distinct species of human interferon. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2185–2187. doi: 10.1073/pnas.72.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M., Armstrong J. A. Interferon. Annu Rev Microbiol. 1975;29:131–161. doi: 10.1146/annurev.mi.29.100175.001023. [DOI] [PubMed] [Google Scholar]
- Jankowski W. J., Davey M. W., O'Malley J. A., Sulkowski E., Carter W. A. Molecular structure of human fibroblast and leukocyte interferons: probe by lectin and hydrophobic chromatography. J Virol. 1975 Nov;16(5):1124–1130. doi: 10.1128/jvi.16.5.1124-1130.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kvarstein B. The effect of temperature, mabolic inhibitors, andEDTA on pgocytosis of polystyrene latex particles by human leucocytes. Scand J Clin Lab Invest. 1969 Oct;24(3):271–277. doi: 10.3109/00365516909080162. [DOI] [PubMed] [Google Scholar]
- Maehara N., Ho M. Cellular origin of interferon induced by bacterial lipopolysaccharide. Infect Immun. 1977 Jan;15(1):78–83. doi: 10.1128/iai.15.1.78-83.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvin S. B., Youngner J. S., Lederer W. H. Migration inhibitory factor and interferon in the circulation of mice with delayed hypersensitivity. Infect Immun. 1973 Jan;7(1):68–75. doi: 10.1128/iai.7.1.68-75.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H., Jackson L., Paul W. E. T lymphocyte-enriched murine peritoneal exudate cells. I. A reliable assay for antigen-induced T lymphocyte proliferation. J Immunol. 1975 Nov;115(5):1330–1338. [PubMed] [Google Scholar]
- Stewart W. E., 2nd Distinct molecular species of interferons. Virology. 1974 Sep;61(1):80–86. doi: 10.1016/0042-6822(74)90243-8. [DOI] [PubMed] [Google Scholar]
- Tan Y. H., Armstrong J. A., Ke Y. H., Ho M. Regulation of cellular interferon production: enhancement by antimetabolites. Proc Natl Acad Sci U S A. 1970 Sep;67(1):464–471. doi: 10.1073/pnas.67.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trizio D., Cudkowicz G. Separation of T and B lymphocytes by nylon wool columns: evaluation of efficacy by functional assays in vivo. J Immunol. 1974 Oct;113(4):1093–1097. [PubMed] [Google Scholar]
- Valle M. J., Jordan G. W., Haahr S., Merigan T. C. Characteristics of immune interferon produced by human lymphocyte cultures compared to other human interferons. J Immunol. 1975 Jul;115(1):230–233. [PubMed] [Google Scholar]
- Youngner J. S., Salvin S. B. Production and properties of migration inhibitory factor and interferon in the circulation of mice with delayed hypersensitivity. J Immunol. 1973 Dec;111(6):1914–1922. [PubMed] [Google Scholar]


