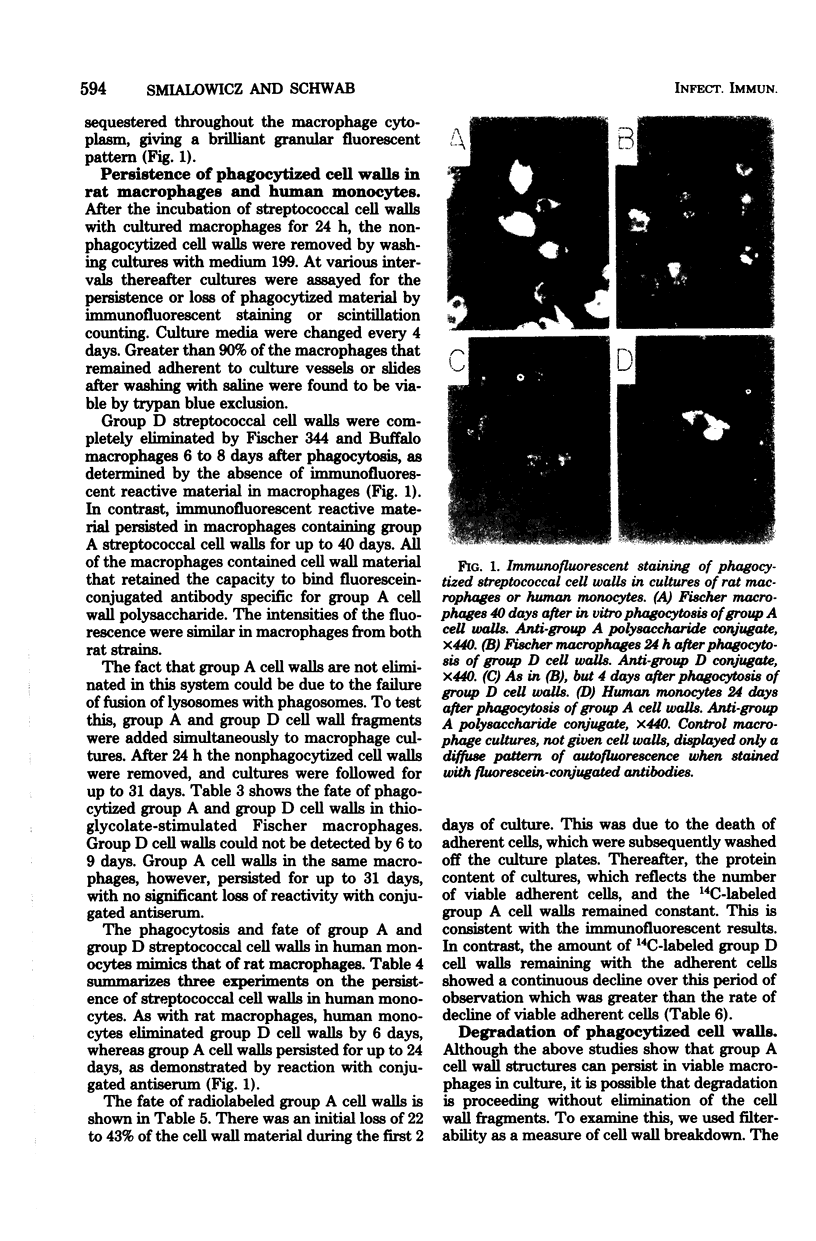
Abstract
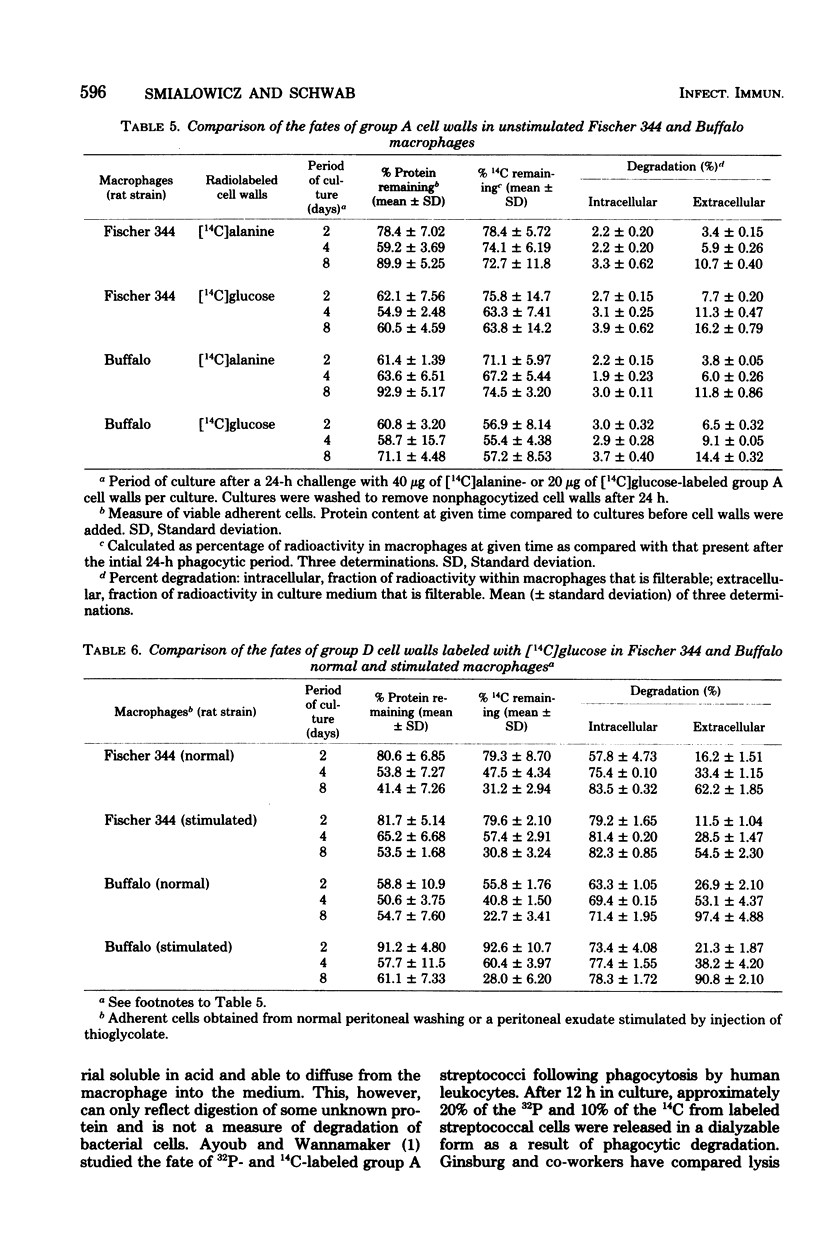
Phagocytosis and degradation of cell walls by peritoneal macrophages obtained from Fischer 344 or Buffalo rats was measured in tissue culture. Group A cell wall antigen, detected by immunofluorescence, persisted in cultured rat macrophages for at least 40 days, whereas group D cell wall material was eliminated by 6 to 8 days. This same pattern of persistence of group A cell walls and elimination of group D cell walls was observed in cultures of human monocytes followed for 24 days in culture. Group A streptococcal cell walls labeled with either [14C]alanine or [14C]glucose were handled in a similar manner by macrophages from either Fischer 344 or Buffalo rats. In contrast, [14C]glucose-labeled group D cell walls were degraded at a much faster rate. Buffalo macrophages were more efficient than Fischer 344 macrophages in degrading group D cell walls. The inability of macrophages to degrade group A cell walls was not due to a failure of lysosomes to fuse with phagosomes. Neither serum lysozyme in the culture medium nor cell wall-associated autolysin contributed to the degradation of group D cell walls by macrophages. Neither immune serum nor macrophages obtained from specifically immunized rats influenced phagocytosis or persistence of group A cell walls.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayoub E. M., Wannamaker L. W. The fate of group A streptococci following phagocytosis. In vitro phagocytic studies of isotope-labeled streptococci. J Immunol. 1967 Dec;99(6):1099–1105. [PubMed] [Google Scholar]
- Bültmann B., Heymer B., Schachenmayr W., Haferkamp O., Schmidt W. C. Inhibition of macrophage migration by nucleotide-containing streptococcal preparations. Infect Immun. 1973 Nov;8(5):700–707. doi: 10.1128/iai.8.5.700-707.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A. The fate of bacteria within phagocytic cells. I. The degradation of isotopically labeled bacteria by polymorphonuclear leucocytes and macrophages. J Exp Med. 1963 Jan 1;117:27–42. doi: 10.1084/jem.117.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromartie W. J., Craddock J. G. Rheumatic-like cardiac lesions in mice. Science. 1966 Oct 14;154(3746):285–287. doi: 10.1126/science.154.3746.285. [DOI] [PubMed] [Google Scholar]
- Gallis H. A., Miller S. E., Wheat R. W. Degradation of 14C-labeled streptococcal cell walls by egg white lysozyme and lysosomal enzymes. Infect Immun. 1976 May;13(5):1459–1466. doi: 10.1128/iai.13.5.1459-1466.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahav M., Ne'eman N., James J., Ginsburg I. The effect of leukocyte hydrolases on bacteria. III. Bacteriolysis induced by extracts of different leukocyte populations and the inhibition of lysis by macromolecular substances. J Infect Dis. 1975 Feb;131(2):149–157. doi: 10.1093/infdis/131.2.149. [DOI] [PubMed] [Google Scholar]
- MICHEL M. F., GOODER H. Amino acids, amino sugars and sugars present in the cell wall of some strains of Streptococcus pyogenes. J Gen Microbiol. 1962 Oct;29:199–205. doi: 10.1099/00221287-29-2-199. [DOI] [PubMed] [Google Scholar]
- OYAMA V. I., EAGLE H. Measurement of cell growth in tissue culture with a phenol reagent (folin-ciocalteau). Proc Soc Exp Biol Med. 1956 Feb;91(2):305–307. doi: 10.3181/00379727-91-22245. [DOI] [PubMed] [Google Scholar]
- Ohanian S. H., Schwab J. H., Cromartie W. J. Relation of rheumatic-like cardiac lesions of the mouse to localization of group A streptococcal cell walls. J Exp Med. 1969 Jan 1;129(1):37–49. doi: 10.1084/jem.129.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohanian S. H., Schwab J. H. Persistence of group a streptococcal cell walls related to chronic inflammation of rabbit dermal connective tissue. J Exp Med. 1967 Jun 1;125(6):1137–1148. doi: 10.1084/jem.125.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWAB J. H., CROMARTIE W. J. Studies on a toxic cellular component of group A streptococci. J Bacteriol. 1957 Nov;74(5):673–679. doi: 10.1128/jb.74.5.673-679.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab J. H., Ohanian S. H. Degradation of streptococcal cell wall antigens in vivo. J Bacteriol. 1967 Nov;94(5):1346–1352. doi: 10.1128/jb.94.5.1346-1352.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Conover M. J., Kolb J. J., Riley L. S., Toennies G. NUTRITIONAL REQUIREMENTS FOR BACTERIAL CELL WALL SYNTHESIS. J Bacteriol. 1961 Jan;81(1):44–50. doi: 10.1128/jb.81.1.44-50.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Thompson J. S., Conover M. J. The autolytic enzyme system of Streptococcus faecalis. II. Partial characterization of the autolysin and its substrate. Biochemistry. 1967 Apr;6(4):1054–1065. doi: 10.1021/bi00856a014. [DOI] [PubMed] [Google Scholar]
- Smialowicz R. J., Schwab J. H. Cytotoxicity of rat macrophages activated by persistent or biodegradable bacterial cell walls. Infect Immun. 1977 Sep;17(3):599–606. doi: 10.1128/iai.17.3.599-606.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector W. G., Reichhold N., Ryan G. B. Degradation of granuloma-inducing micro-organisms by macrophages. J Pathol. 1970 Aug;101(4):339–354. doi: 10.1002/path.1711010407. [DOI] [PubMed] [Google Scholar]