Abstract
Background
Toxoplasmosis, one of the most common zoonotic diseases worldwide, can induce various hormonal and behavioural alterations in infected hosts, and its most common form, latent toxoplasmosis, influences the course of pregnancy. Autoimmune thyroid diseases (AITD) belong to the well-defined risk factors for adverse pregnancy outcomes. The aim of this study was to investigate whether there is a link between latent toxoplasmosis and maternal AITD in pregnancy.
Methods
Cross-sectional study in 1248 consecutive pregnant women in the 9–12th gestational weeks. Serum thyroid-stimulating hormone (TSH), thyroperoxidase antibodies (TPOAb), and free thyroxine (FT4) were assessed by chemiluminescence; the Toxoplasma status was detected by the complement fixation test (CFT) and anti-Toxoplasma IgG enzyme-linked immunosorbent assay (ELISA).
Results
Overall, 22.5% of the women were positive for latent toxoplasmosis and 14.7% were screened positive for AITD. Women with latent toxoplasmosis had more often highly elevated TPOAb than the Toxoplasma-negative ones (p = 0.004), and latent toxoplasmosis was associated with decrease in serum TSH levels (p = 0.049). Moreover, we found a positive correlation between FT4 and the index of positivity for anti-Toxoplasma IgG antibodies (p = 0.033), which was even stronger in the TPOAb-positive Toxoplasma-positive women, (p = 0.014), as well as a positive correlation between FT4 and log2 CFT (p = 0.009).
Conclusions
Latent toxoplasmosis was associated with a mild increase in thyroid hormone production in pregnancy. The observed Toxoplasma-associated changes in the parameters of AITD are mild and do not seem to be clinically relevant; however, they could provide new clues to the complex pathogenesis of autoimmune thyroid diseases.
Introduction
The course of pregnancy is influenced by a number of environmental factors (e.g. infections), as well as endogenous factors in the mother. Often, it is difficult to identify the cause of an obstetric complication. Thyroid diseases are among the well defined risk factors for adverse pregnancy outcomes. Maternal autoimmune thyroid diseases (AITD) have been linked to infertility, spontaneous abortion, premature delivery, preeclampsia, caesarean section, and even foetal death [1].
In the general population, AITD are quite common. In our previous studies involving about 8500 pregnant women from the Czech Republic, we have found hypothyroidism (either manifest or subclinical) in more than 5% of these women and positivity for antibodies against thyroperoxidase (TPOAb) in 10% of the study cohort [2]–[4]. Such a high prevalence of AITD in pregnant women can be regarded as a serious public health issue.
The reasons for such a high prevalence of AITD in the population remain unclear, with the multifactorial aetiology being the most popular hypothesis. It is known that people with a genetic predisposition may develop AITD after an infectious disease [5], [6]. The molecular mimicry hypothesis suggests the resemblance between human thyroid autoantigens and molecular components of microorganisms is responsible for many autoimmune diseases including AITD [7], [8]. It has also been suggested that there is a link between AITD and the protozoan Toxoplasma gondii [9], [10]. In their study involving 1591 pregnant women, Wassermann et al. have found that T. gondii IgG seropositivity was the only significant infectious explanatory cofactor associated with the elevation of TPOAb in pregnancy. A smaller study on 414 pregnant women did not confirm this association, but its primary aim was to evaluate the association of toxoplasmosis and mood disturbances in pregnancy [11]. However, until now, no study has addressed the effect of toxoplasmosis on thyroid hormone levels in patients with AITD.
Toxoplasmosis is an endemic zoonosis caused by the parasitic protist Toxoplasma gondii, the most prevalent human parasite in developed countries. The prevalence of latent toxoplasmosis in the population ranges from 20% to 80% depending on various environmental and sociological factors, including the number of cats in the environment, latitude, moisture, hygienic standards, and kitchen habits [12]. Life-long latent toxoplasmosis is usually considered to pose no health threat to immunocompetent individuals; however, it is accompanied by specific changes in the psychomotor performance, behaviour, and personality profile [13]–[15]. A recent correlation study performed on a set of 88 countries has also shown that the prevalence of toxoplasmosis explains about 13% of the variation in the rates of congenital abnormalities between the countries [16].
Unlike acute toxoplasmosis in pregnancy, which has been described to damage significantly the foetal development [12], [17], latent toxoplasmosis seems to have no significant negative impact on the health of the offspring. However, latent toxoplasmosis leads to alterations in serum concentrations of testosterone both in men and mice [18], [19]. In addition to lower testosterone, the impairment of thyrotropin-releasing hormone (TRH) and thyroid-stimulating hormone (TSH) secretion as well as decreased serum thyroxine (T4) have been reported in T. gondii-infected mice [20], [21]. Latent toxoplasmosis is reportedly associated with higher concentration of cholesterol and LDL cholesterol in male schizophrenia patients [22]. A correlation study on a set of 88 countries has also shown a general positive association between prevalence of toxoplasmosis and incidence of endocrine disorders [16]. Moreover, it has been demonstrated that latent infection by T. gondii may lead to immune suppression both in mice and humans [23], [24].
Latent toxoplasmosis is thus a highly prevalent disease leading to alterations in the immune system and hormonal profile. A possible link between latent toxoplasmosis and AITD in pregnancy needs to be addressed. The goal of this study was to investigate whether or not there is an association between latent maternal toxoplasmosis, detected by immunological tests, and AITD in pregnant women diagnosed based on serum levels of TPOAb and thyroid hormones in the first trimester of pregnancy.
Subjects and Methods
The study was designed as a retrospective cross-sectional study and was performed in cooperation of three clinical settings of the General University Hospital in Prague (Dept. of Obstetrics and Gynecology, Institute of Clinical Biochemistry and Laboratory Diagnostics, and the Third Department of Medicine) and the Department of Philosophy and History of Science, Faculty of Science, Charles University in Prague.
Subjects
A total of 1250 consecutive women screened for AITD in 2008 and 2009 in the General University Hospital in Prague were included in the study. They were examined within an experimental universal screening programme for AITD conducted in 2006–2009 (gestational wks 9–12) [2]. The screening was focused on autoantibodies against thyroperoxidase (TPOAb), thyroid stimulating hormone (TSH), and free thyroxine (FT4). FT4 was assessed only in women with pathological TSH and/or positive for TPOAb. The laboratory assessment was performed in a single laboratory at the Institute of Clinical Biochemistry and Laboratory Diagnostics.
The AITD screening was carried out as part of the routine screening for chromosomal abnormalities (i.e. serum free beta-hCG and PAPP-A) in the 9–12th gestational weeks in all consecutive study subjects regardless of their medical history or symptoms. Serum samples collected at the time of screening, were frozen and stored at −70°C for later evaluation. They were used for retrospective measurement of antibodies against T. gondii.
Moreover, we assessed the history of the women and the clinical outcome of the pregnancy using data extraction from the hospital database.
During the whole time of the project, we worked with an anonymous data set; the key for the identification of the individual participants was only accessible to the main authors of the study. All participating women signed an informed consent form. The study was approved by the Ethical Committee of the General University Hospital and the First Medical Faculty of the Charles University in Prague.
Serological analysis and reference intervals
a) Screening for autoimmune thyroid disease
Serum samples were collected at inclusion after an overnight fast. The analysis of TSH, TPOAb, and FT4 was performed within eight hours. The rest of the serum sample was stored in aliquots frozen at −20°C until further use (screening for toxoplasmosis). TPOAb, TSH, and FT4 were assayed on the ADVIA Centaur Analyzer (Siemens Healthcare Diagnostics Inc., Tarrytown, NY) with chemiluminometric detection. TSH was determined using a direct sandwich chemiluminescence immunoassay and anti-TPO and FT4 were measured using a competitive chemiluminescence immunoassay.
The reference interval for TSH in the first trimester of pregnancy was set at 0.06–3.67 mIU/l [2], the upper limit for TPOAb at 143 kU/l, and the reference interval for FT4 at 9.8–23.1 pmol/l. A positive screening result was thus defined as TSH below 0.06 or above 3.67 mU/l and/or TPOAb above 143 kU/l. The upper limit of the detection range for TPOAb was 10000 kU/l when using automated sample dilution.
b) Immunological tests for toxoplasmosis
The complement-fixation test (CFT) determines the overall levels of IgM and IgG antibodies of particular specificity. This method has been used as a reference method in the Czech Republic in the last 50 years, which makes possible an analysis of the time trends in the seroprevalence of toxoplasmosis. Enzyme-Linked Immunosorbent Assays (ELISA) (IgG ELISA: SEVAC, Prague, IgM ELISA: TestLine, Brno) were used as a standard method. Using ELISA, individual classes of antibodies (IgG, IgM) were determined in order to discriminate between the acute and latent phase of the infection. Positivity for toxoplasmosis was defined as a CFT titre of 1∶8 and more, together with an index of positivity (IP) of >1.2 for IgG ELISA antibodies against Toxoplasma. The sera were retested when the results were ambiguous or when the results of two tests were in contradiction.
Statistics
The Statistica 9.0 software was used for all statistical analyses. The influence of the binary variable latent toxoplasmosis (positive/negative) on the probability of a positive screening result was evaluated using the method of contingency tables. The following parameters were tested: any positive screening results, TSH elevation, TPOAb positivity (TPOAb+), TPOAb >500 kU/l, and combination of TSH elevation and TPOAb+.
The log-linear analysis of frequency tables was used for the analysis of a model with three binary variables: toxoplasmosis (Toxo) (positive/negative), TPOAb (TPOAb+/TPOAb−), and TSH (suppressed/normal/elevated).
Nonparametric tests, e.g. the Mann-Whitney test, were used to search for the influence of toxoplasmosis on the TSH, TPOAb, and FT4 levels. The influence of the levels of anti-Toxoplasma-antibodies (IP for IgG antibodies and CFT antibodies in a nonparametric test or log2 CFT antibodies in parametric linear regression) on the TPOAb, TSH, and FT4 levels was evaluated using Spearman correlation and that on the FT4 level was analysed using linear regression in the group of the Toxoplasma-positive women (FT4 levels had normal distribution).
The general linear model (GLM) was used to analyse the influence of the binary variable toxoplasmosis on serum free beta-hCG and PAPP-A, with the gestational age on the day of the blood sampling as a continuous variable and log free beta-hCG or log PAPP-A as dependent variables.
Results
Overall, 1250 pregnant women, including 32 women who gave birth to twins, were enrolled in the study. Two women (one AITD negative and one AITD positive) were excluded due to suspected acute toxoplasmosis (tested positive for IgM antibodies against T. gondii). Our final set consisted of 1248 pregnant women; their mean age was 31 years (range 18–45 years). Of these, 183 (14.7%) were screened positive for AITD and 281 (22.5%) were diagnosed with latent toxoplasmosis. Basic characteristics of the women included in the study are shown in Table 1 and Table 2. Toxoplasma positivity frequencies in subgroups of pregnant women screened for thyroid disorders are shown in Table 3.
Table 1. Basic characteristics of women included in the study.
| Total | AITD+ | AITD− | Toxo+ | Toxo− | Toxo+ AITD+ | Toxo+ AITD– | Toxo– AITD+ | Toxo– AITD– | |
| n | 1248 | 183 | 1065 | 281 | 967 | 49 | 232 | 134 | 833 |
| Age (mean) | 31.0 | 31.3 | 30.9 | 31.7 | 30.8 | 32.5 | 31.5 | 30.8 | 30.8 |
| 1.25 (0.01–37.57) | 1.83 (0.01–37.57) | 1.21 (0.07–3.65) | 1.13 (0.01–21.75) | 1.27 (0.01–37.57) | 1.50 (0.01–21.75) | 1.08 (0.08–3.65) | 1.84 (0.01–37.57) | 1,24 (0.07–3.64) | |
| TPOAb (kU/l) | 32 (0–10000) | 279 (5–10000) | 30 (0–142) | 31.3 (0–10000) | 32 (0–10000) | 507 (11–10000) | 29.75 (0–134) | 247.5 (5–10000) | 30 (0–142) |
| TPOAb+ | 10.2% | 69.4% | - | 12.5% | 9.5% | 71.4% | - | 68.7% | - |
AITD+/−: women screened positive/negative for autoimmune thyroid disease in pregnancy (TPOAb positivity and/or pathological TSH); Toxo+/−: women with/without latent toxoplasmosis. The women were included in the 9th–12th gestational weeks. Except of age, values are expressed as medians and range.
Table 2. History of previous pregnancies and the outcome of the current pregnancy.
| Total (n) | Toxo+ AITD+ (n) | Toxo+ AITD– (n) | Toxo– AITD+ (n) | Toxo– AITD– (n) | OR | ||
| Total (n) | 1248 | 49 | 232 | 134 | 833 | 1.31 | |
| Number of previous pregnancies | 0 | 459 | 19 | 94 | 44 | 302 | 1.39 |
| 1 | 340 | 13 | 57 | 39 | 231 | 1.35 | |
| >1 | 125 | 4 | 27 | 13 | 81 | 0.92 | |
| Not available | 324 | 13 | 54 | 38 | 219 | 1.39 | |
| Current Pregnancy Outcome | Live birth | 678 | 31 | 127 | 71 | 449 | 1.54 |
| Abortion or fetus loss | 18 | 0 | 4 | 2 | 12 | - | |
| Not available | 552 | 18 | 101 | 61 | 372 | 1.09 |
AITD+/−: women screened positive/negative for autoimmune thyroid disease in pregnancy (TPOAb positivity and/or pathological TSH); Toxo+/−: women with/without latent toxoplasmosis. The last column shows OdsRatio reflecting increased (decreased) risk of autoimmune thyroid disease in Toxoplasma-infected subjects. No association was statistically significant.
Table 3. Frequencies of pregnant women screened for autoimmune thyroid disorders according to Toxoplasma positivity.
| Total | Negative in screening | Positive in screening | TPOAb | negative | TPOAb | positive | |||
| TSH | suppressed | elevated | normal | suppressed | elevated | all values | |||
| Toxo – | 967 | 833 | 134 | 20 | 22 | 71 | 3 | 18 | 92 |
| Toxo + | 281 | 232 | 49 | 10 | 4 | 27 | 1 | 7 | 35 |
| Total | 1248 | 1065 | 183 | 30 | 26 | 98 | 4 | 25 | 127 |
Toxo–: women without toxoplasmosis; Toxo+: women with latent toxoplasmosis. Positivity in screening for autoimmune thyroid disorders included either positivity of TPOAb and/or pathological TSH.
Latent toxoplasmosis and thyroid autoimmunity
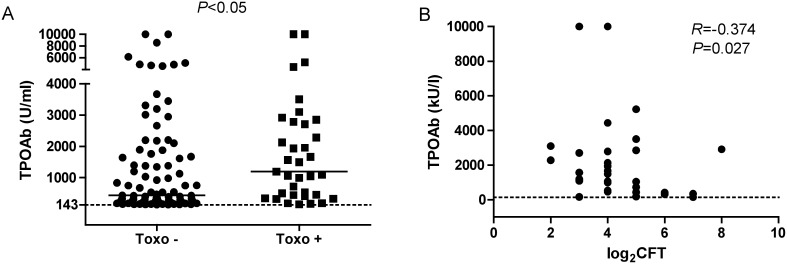
Although the overall comparison did not show an increased prevalence of TPOAb positivity in the Toxoplasma-positive women, we found an association of latent toxoplasmosis with high TPOAb levels. Among the 967 Toxoplasma-negative women, 134 (13.9%) were screened positive for AITD as compared to 49 (17.4%) of the 281 Toxoplasma-positive women (p = 0.135). Overall, TPOAb (>143 k/l) were present in 92 Toxoplasma-negative women (9.5%) and in 35 Toxoplasma-positive ones (12.5%) (p = 0.151). However, a subgroup analysis showed that the Toxoplasma-positive women were more frequently highly positive for TPOAb (>500 kU/l) than the Toxoplasma-negative ones: 25/281 (8.9%) vs. 43/967 (4.4%) (χ2 = 8.37, p = 0.004). Moreover, latent toxoplasmosis in the TPOAb–positive pregnant women was associated with elevated TPOAb levels (n = 127, Z = 2.45, p = 0.014) (Fig. 1A). Although there was no correlation between serum TPOAb levels and the index of positivity for IgG antibodies against T. gondii (IP for IgG), we found a negative association between CFT antibody titres and TPOAb levels (n = 35, Spearman R = −0.37, p = 0.026) in the TPOAb–positive pregnant women with latent toxoplasmosis (Fig. 1B).
Figure 1. Association of latent toxoplasmosis and serum TPOAb levels in pregnant women (9–12th gest. wks).
A: Serum TPOAb levels in Toxoplasma-negative vs. Toxoplasma-positive pregnant TPOAb-positive women. Black lines represent median values. (Mann-Whitney test). B: Negative correlation between log2 CFT (complement fixation test) T. gondii antibodies and serum TPOAb levels in the TPOAb-positive pregnant women with latent toxoplasmosis (Spearman correlation).
Latent toxoplasmosis and thyroid hormones
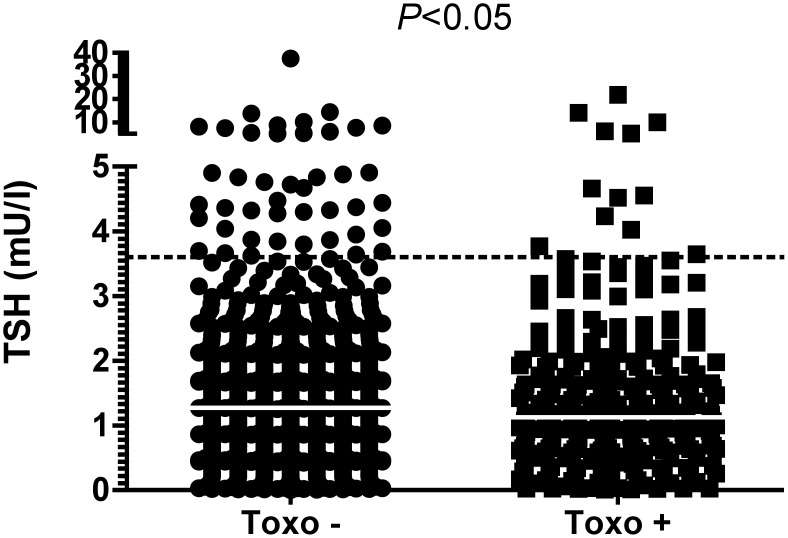
Latent toxoplasmosis was associated with a decrease in TSH levels. Women with latent toxoplasmosis had lower TSH levels than the Toxoplasma-negative ones (Z = 1.97, p = 0.049; median 1.13 vs. 1.27 in the Toxo+ vs. Toxo– women; SD = 1.82) (Fig. 2). Decreased levels of TSH (<0.06 mIU/l) were detected in 23 Toxoplasma-negative women (2.5%) and in 11 Toxoplasma-positive ones (4.1%) (p = 0.166, chi-square test).
Figure 2. Comparison of serum TSH in pregnant women (9–12th gest. wks) with and without latent toxoplasmosis.
The dotted lines represent the upper cut-off for normal values. White lines represent median values. Mann-Whitney test.
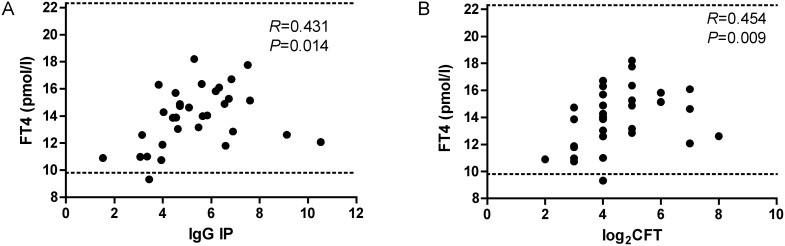
The IP for IgG in the Toxoplasma-positive women positively correlated with the FT4 level (n = 76, F = 4.71, R = 0.24, p = 0.033). This correlation was even stronger in the TPOAb–positive pregnant women with latent toxoplasmosis (n = 32, Spearman R = 0.43 p = 0.014; Fig. 3A). In this subgroup, also a positive correlation existed between CFT antibody titres and FT4 levels (n = 32, Spearman R = 0.45, p = 0.009; Fig. 3B).
Figure 3. Relationship between free thyroxine (FT4) and positivity for Toxoplasma antibodies in TPOAb-positive pregnant women with latent toxoplasmosis.
A: Correlation of free thyroxine (FT4) and index of positivity for anti-Toxoplasma IgG antibodies (IP for IgG). B: Correlation of FT4 and logarithmic values of the complement fixation test (CFT) antibodies against T. gondii (Spearman correlation). Dotted lines represent the reference range for FT4.
The statistical analysis did not show any correlation either between the IP for IgG or between the CFT antibody titres and TSH or TPOAb in the Toxoplasma-positive women.
In our data set, no association was observed between latent toxoplasmosis and hypothyroidism in pregnancy. Elevated TSH levels (>3.67 mIU/l) were found in 40 (4.2%) Toxoplasma-negative women and in 11 (4.1%) Toxoplasma-positive women (Table 1). A model with three variables: latent toxoplasmosis (positive vs. negative), TPOAb (TPOAb+ v.s. TPOAb−), and TSH (suppressed/normal/elevated) did not show any significant association between the binary variables analysed (p = 0.398).
Latent toxoplasmosis and serum free beta-hCG and PAPP-A
The statistical analysis did not show any significant association of latent toxoplasmosis either with the serum free beta-hCG (p = 0.808) or the serum PAPP-A (p = 0.465) in the pregnant women in the 9–12th gestational weeks.
Discussion
In this study, we assess the prevalence of latent toxoplasmosis in pregnancy and analyse its possible association with autoimmune thyroid diseases. The study cohort were consecutive women in the first trimester of pregnancy (9–12th gestational weeks) screened for AITD by measurement of TSH, TPOAb, and FT4. The presence of latent toxoplasmosis was defined as a positive complement-fixation test and anti-Toxoplasma IgG positivity in ELISA (women with IgM positivity were excluded).
Of the 1248 pregnant women, 22.5% were screened positive for latent toxoplasmosis and 14.7% for AITD. Although we could not detect any strong effect of latent toxoplasmosis on the presence of AITD, we found that women with latent toxoplasmosis had more often highly elevated TPOAb than the Toxoplasma-negative ones. Furthermore, latent toxoplasmosis had a week stimulatory effect on serum levels of thyroid hormones.
The prevalence of latent toxoplasmosis in our cohort is in line with the data of the Centers for Disease Control and Prevention (CDC) reporting that 22.5% of the North American population older than 12 years of age are positive for Toxoplasma [25], but it is slightly lower than the rate, i.e. 26.7%, found by Wasserman et al. in a large mixed group of pregnant women residing both in rural and urban areas [10]. The pregnant women included in our study were mostly residents of the capital city and only a few of them lived in rural areas. It is highly probable that the prevalence of latent toxoplasmosis would be higher if more rural residents were included. Toxoplasmosis has been reported to be more prevalent in rural areas in many countries, including the Czech Republic [26], probably due to the more frequent contact with Toxoplasma oocytes excreted by cats.
In contrast to Tozzoli et al., who found a 65.5% prevalence of latent toxoplasmosis among patients with AITD [27], only 27.1% of the 127 TPOAb-positive pregnant women were positive for latent toxoplasmosis in our study. Tozzoli analysed a group of 120 AITD patients from different areas of north-western Italy. Although the numbers of AITD patients analysed are similar, there are some potentially important differences between these two groups. Our cohort consisted of pregnant women with mostly mild forms of Hashimoto’s thyroiditis (HT) including subclinical cases, with no identified Graves’ disease (GD) cases; and it was relatively homogeneous, consisting mostly of capital city residents. Tozzoli et al. have not characterised their AITD group in detail, but it appeared to be more heterogeneous with a large proportion of Graves’ disease cases (there was no difference in the prevalence of toxoplasmosis in HT and GD, however).
The results of our study differ also from the data reported by Wassermann et al. They have found a strong association between TPOAb and antibodies against T. gondii in a comparably large group of pregnant women analysed in the second half of pregnancy [10]. In our study, there was a significant association between latent toxoplasmosis and TPOAb levels only in the TPOAb-positive women. Interestingly, we found a negative correlation between the CFT titres of anti-Toxoplasma antibodies and TPOAb levels in these women. It is known that the CFT titres decrease with the duration of the latent infection by the protozoan [28], therefore, we can speculate that the production of the TPOAb increases with the time from the infection by T. gondii. This suggests that the observed phenomenon represents a cumulative effect of latent toxoplasmosis, rather than a fade away effect of past acute toxoplasmosis.
Different results obtained in our study and works by others [10], [27] concerning the percentages of women with positive autoantibody tests could be explain by geographical differences in autoantibodies generation patterns [29].
Our study is the first to address the effect of latent toxoplasmosis on thyroid hormones production. We demonstrate that the infection is not associated with hypothyroidism, as could be expected, as a result of the promotion of thyroid autoimmunity, but that it is associated with a slight decrease in TSH and an increase in FT4 (as observed only in TPOAb-positive/Toxoplasma-positive women). Moreover, the correlation analysis showed that women with a higher index of positivity for T. gondii IgG antibodies, as well as with a higher CFT antibody titre, had higher concentrations of FT4. These concentrations remain, however, at subclinical levels. Furthermore, we could exclude an effect of latent toxoplasmosis on the serum concentration of HCG, which is known to have a TSH-like effect in pregnancy leading to transitory gestational hyperthyroidism [30]. Our data thus indicate that latent toxoplasmosis per se might have a moderate stimulatory effect on thyroid hormone production in pregnancy. We can just speculate about the mechanisms responsible for the observed phenomena. During early pregnancy, an initial shift from Th1 to Th2 immune reactions occurs as part of the mechanisms of maternal immune tolerance of the foetus, as a semi-allograft [31]. This may also lead to a reactivation of Toxoplasma infection, because under normal conditions, this intracellular parasite is controlled mostly by Th1 immune reactions [32], [33]. It is possible that this Th1 to Th2 shift could also be behind a partial escape of T. gondii from immunological control with subsequent activation of autoimmune mechanisms that advance the progression of pre-existing thyroiditis with a transient release of thyroid hormones. Thus, it would be interesting to analyse the values of maternal thyroid parameters in relationship to the Toxoplasma positivity in the second half of pregnancy, when the Th1 response shifts back to Th2, and also after delivery.
A possible role of the NK cells in the interaction between Toxoplasma and thyroid autoimmunity should be mentioned. As Solerte et al. have shown, important alterations in the NK cell function are associated with the autoimmune thyroid disorders and these changes are probably related to the onset and progression of the autoimmune mechanism [34]. NK cells are important mediators of the immune response against Toxoplasma via a robust IFN-γ-mediated effect that limits parasite replication and allows for parasite clearance [35]. A defect of the NK cell activity in autoimmune thyroiditis could thus eventually result in a higher risk of reinfection by T. gondii during pregnancy [36].
Also, fetal microchimeric cells have been more frequently found in the thyroid gland of women with Hashimoto’s thyroiditis and Graves’ disease compared to those women without thyroid autoimmunity [37], [38]. It was suggested that Toxoplasma-containing cells of fetal origin could disseminate the parasite in the maternal organism [39]. It was reported recently that Toxoplasma increases migration activity of infected leukocytes, which could help spread infection in various tissues of the host body [40].
Our study has several limitations. Most importantly, the numbers of pregnant women with thyroid dysfunction (hypo−/hyperthyroidism) are too low to perform a reliable statistical analysis of the thyroid hormonal secretion with regard to the Toxoplasma status. Moreover, we are lacking the follow-up data on the thyroid hormone levels, which would improve the understanding of the mechanism by which Toxoplasma influences thyroid autoimmunity/function.
In conclusion, our study provides evidence for the existence of an association between latent toxoplasmosis and thyroid autoimmunity and thyroid function in pregnancy. This association does not seem to be clinically relevant; however, they could provide new clues to the understanding of the complex pathogenesis of autoimmune thyroid diseases.
Acknowledgments
The author would like to sincerely thank Dr. Petr Kodym and Blanka Širocká from the National Reference Laboratory for Toxoplasmosis of the National Institute of Public Health, Czech Republic for their assistance with the laboratory investigation of Toxoplasma infection and Assoc. Prof. Zdeňka Límanová for her helpful comments.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
The research was supported by project UNCE 204004 of the Charles University, the grant PRVOUK 25 of the Charles University, and research grant No. RVO-VFN64165 from the Ministry of Health of the Czech Republic. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, et al. (2011) Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 21: 1081–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Springer D, Zima T, Limanova Z (2009) Reference intervals in evaluation of maternal thyroid function during the first trimester of pregnancy. Eur J Endocrinol 160: 791–797. [DOI] [PubMed] [Google Scholar]
- 3. Potlukova E, Potluka O, Jiskra J, Limanova Z, Telicka Z, et al. (2012) Is age a risk factor for autoimmune thyroid disorders in pregnancy? An analysis of 5223 pregnant women. J Clin Endocrinol Metab 97(6): 1945–1952. [DOI] [PubMed] [Google Scholar]
- 4. Limanova Z, Springer D (2011) Thyreopathy examination during pregnancy–results of pilot project. Časop Lék Česk 150: 389–393. [PubMed] [Google Scholar]
- 5. Morohoshi K, Takahashi Y, Mori K (2011) Viral infection and innate pattern recognition receptors in induction of Hashimoto's thyroiditis. Discov Medic 12: 505–511. [PubMed] [Google Scholar]
- 6. Bach JF (2005) Infections and autoimmune diseases. J Autoimmun 25: 74–80. [DOI] [PubMed] [Google Scholar]
- 7. Benvenga S, Guarneri F, Vaccaro M, Santarpia L, Trimarchi F (2004) Homologies between proteins of Borrelia burgdorferi and thyroid autoantigens. Thyroid 14: 964–966. [DOI] [PubMed] [Google Scholar]
- 8. Benvenga S, Santarpia L, Trimarchi F, Guarneri F (2006) Human thyroid autoantigens and proteins of Yersinia and Borrelia share amino acid sequence homology that includes binding motifs to HLA-DR molecules and T-cell receptor. Thyroid 16: 225–236. [DOI] [PubMed] [Google Scholar]
- 9. Shapira Y, Agmon-Levin N, Selmi C, Petrikova J, Barzilai O, et al. (2012) Prevalence of anti-toxoplasma antibodies in patients with autoimmune diseases. J Autoimmun 39: 112–116. [DOI] [PubMed] [Google Scholar]
- 10. Wasserman EE, Nelson K, Rose NR, Rhode C, Pillion JP, et al. (2009) Infection and thyroid autoimmunity: A seroepidemiologic study of TPOaAb. Autoimmunity 42: 439–446. [DOI] [PubMed] [Google Scholar]
- 11. Groer MW, Yolken RH, Xiao JC, Beckstead JW, Fuchs D, et al. (2011) Prenatal depression and anxiety in Toxoplasma gondii-positive women. Am J Obstet Gynecol 204 433: e1–433e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tenter AM, Heckeroth AR, Weiss LM (2000) Toxoplasma gondii: from animals to humans. Int J Parasitol 30: 1217–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Flegr J, Zítková S, Kodym P, Frynta D (1996) Induction of changes in human behaviour by the parasitic protozoan Toxoplasma gondii . Parasitology 133: 49–54. [DOI] [PubMed] [Google Scholar]
- 14. Lindová J, Novotná M, Havlíček J, Jozífková E, Skallová A, et al. (2006) Gender differences in behavioural changes induced by latent toxoplasmosis. Int J Parasitol 36: 1485–1492. [DOI] [PubMed] [Google Scholar]
- 15. Flegr J (2013) Influence of latent Toxoplasma infection on the human personality, physiology, and morphology: The Toxoplasma-human model in studying the manipulation hypothesis - pros and cons. J Exp Biol 216: 127–133. [DOI] [PubMed] [Google Scholar]
- 16. Flegr J, Prandota J, Sovičková M, Israili ZH (2014) Toxoplasmosis – A global threat. Correlation between latent toxoplasmosis and specific disease burden – a WHO data-based study of 29 European and 59 non-European countries. PLoS ONE 9(3): e90203 10.1371/journal.pone.0090203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wolf A, Cowen D, Paige BH (1939) Toxoplasmic encephalomyelititis. III. A new case of granulomatoces encephalomyelititis due to a protozoon. Am J Pathol 15: 657. [PMC free article] [PubMed] [Google Scholar]
- 18. Flegr J, Lindová J, Kodym P (2008) Sex-dependent toxoplasmosis-associated differences in testosterone concentration in humans. Parasitology 135: 427–431. [DOI] [PubMed] [Google Scholar]
- 19. Kaňková Š, Kodym P, Flegr J (2011) Direct evidence of Toxoplasma-induced changes in serum testosterone in mice. Exp Parasitol 128: 181–183. [DOI] [PubMed] [Google Scholar]
- 20. Stahl W, Kaneda Y (1998) Impaired thyroid function in murine toxoplasmosis. Parasitology 117(Pt 3): 217–222. [DOI] [PubMed] [Google Scholar]
- 21. Stahl W, Kaneda Y (1998) Aetiology of thyroidal dysfunction in murine toxoplasmosis. Parasitology 117(Pt 3): 223–227. [DOI] [PubMed] [Google Scholar]
- 22. Flegr J, Hampl R, Bičíková M, Ripova D, Mohr P (2014) Difference of neuro- and immunomodulatory steroids and selected hormone and lipid concentrations between Toxoplasma-free and Toxoplasma-infected schizophrenia patients. Neuroendocrinol Lett 35: 20–27. [PubMed] [Google Scholar]
- 23. Kankova S, Holan V, Zajicova A, Kodym P, Flegr J (2010) Modulation of immunity in mice with latent toxoplasmosis–the experimental support for the immunosuppression hypothesis of Toxoplasma-induced changes in reproduction of mice and humans. Parasitol res 107: 1421–1427. [DOI] [PubMed] [Google Scholar]
- 24. Flegr J, Stříž I (2011) Potential immunomodulatory effects of latent toxoplasmosis in humans. BMC Infect Dis 11: 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CDC. Toxoplasmosis: epidemiology & risk factors. 2013. Available: http://www.cdc.gov/toxoplasmosis/epi.html. Accessed 2014 October 2.
- 26. Kolbeková P, Kourbatova E, Novotná M, Kodym P, Flegr J (2007) New and old risk-factors for Toxoplasma gondii infection: prospective cross-sectional study among military personnel in the Czech Republic. Clin Microb Inf 13: 1012–1017. [DOI] [PubMed] [Google Scholar]
- 27. Tozzoli R, Barzilai O, Ram M, Villalta D, Bizzaro N, et al. (2008) Infections and autoimmune thyroid diseases: Parallel detection of antibodies against pathogens with proteomic technology. Autoimmun Rev 8: 112–115. [DOI] [PubMed] [Google Scholar]
- 28. Kodym P, Machala L, Roháčová H, Širocká B, Malý M (2007) Evaluation of a commercial IgE ELISA in comparison with IgA and IgM ELISAs, IgG avidity assay and complement fixation for the diagnosis of acute toxoplasmosis. Clin Microb Inf 13: 40–47. [DOI] [PubMed] [Google Scholar]
- 29. Shapira Y, Poratkatz B, Gilburd B, Barzilai O, Ram M, et al. (2012) Geographical differences in autoantibodies and antiinfectious agents antibodies among healthy adults. Clin Rev Allergy Immunol 42: 154–63. [DOI] [PubMed] [Google Scholar]
- 30. Glinoer D, de Nayer P, Bourdoux P, Lemone M, Robyn C, et al. (1990) Regulation of maternal thyroid during pregnancy. J Clin Endocrinol Metab 71: 276–287. [DOI] [PubMed] [Google Scholar]
- 31. Challis JR, Lockwood CJ, Myat L, Norman JE, Strauss JF 3rd, et al. (2009) Inflammation and pregnancy. Reprod Sci 16(2): 206–215 10.1177/1933719108329095 [DOI] [PubMed] [Google Scholar]
- 32. Denkers EY, Gazzinelli RT (1998) Regulation and fuction of T-cell-mediated imunity during Toxoplasma gondii infection. Clin Microbiol Rev 11(4): 569–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gazzinelli RT, Denkers EY, Sher A (1993) Host resistance to Toxoplasma gondii: model for studying the selective induction of cell-mediated immunity by intracellular parasites. Infect Agents Dis 2: 139–149. [PubMed] [Google Scholar]
- 34. Solerte SB, Precerutti S, Gazzaruso C, Locatelli E, Zamboni M, et al. (2005) Defect of a subpopulation of natural killer immune cells in Graves’ disease and Hashimoto’s thyroiditis: normalizing effect of dehydroepiandrosterone sulfate. Eur J Endocrinol 152: 703–712. [DOI] [PubMed] [Google Scholar]
- 35. Ronet C, Darche S, Leite de Moraes M, Miyake S, Yamamura T, et al. (2005) NKT cells are critical for the initiation of an inflammatory bowel response against Toxoplasma gondii . J Immunol 175(2): 899–908. [DOI] [PubMed] [Google Scholar]
- 36. Elbez-Rubinstein A, Ajzenberg D, Dardé ML, Cohen R, Dumètre A, at al (2009) Congenital toxoplasmosis and reinfection during pregnancy: case report, strain characterization, experimental model of reinfection, and review. J Infect Dis 199(2): 280–285 10.1086/595793 [DOI] [PubMed] [Google Scholar]
- 37. Nelson JL (2012) The otherness of self: microchimerism in health and disease. Trends Immunol 33: 421–427 10.1016/j.it.2012.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fugazzola L, Cirello V, Beck-Peccoz P (2012) Microchimerism and endocrine disorders. J Clin Endocrinol Metab 97: 1452–1461. [DOI] [PubMed] [Google Scholar]
- 39.Prandota J (2012). Increased generation of antibodies and autoantibodies directed against brain proteins in patients with autism and their families may be caused by T. gondii infection. Maternal and fetal microchimerisms probably play an important role in these processes acting as a “Trojan horse” in dissemination of the parasite. In: Gemma C, editor. Neuroinflammation Pathogenesis, Mechanisms and Management. New York: Nova Science Publishers. 447–638.
- 40. Fuks JM, Arrighi RB, Weidner JM, Kumar Mendu S, Jin Z, et al. (2012) GABAergic signaling is linked to a hypermigratory phenotype in dendritic cells infected by Toxoplasma gondii . PLoS Pathog 8: e1003051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.