Abstract
Microplitis bicoloratus parasitism induction of apoptotic DNA fragmentation of host Spodoptera litura hemocytes has been reported. However, how M. bicoloratus parasitism regulates the host signaling pathways to induce DNA fragmentation during apoptosis remains unclear. To address this question, we performed a new RNAseq-based comparative analysis of the hemocytes transcriptomes of non-parasitized and parasitized S. litura. We were able to assemble a total of more than 11.63 Gbp sequence, to yield 20,571 unigenes. At least six main protein families encoded by M. bicoloratus bracovirus are expressed in the parasitized host hemocytes: Ankyrin-repeat, Ben domain, C-type lectin, Egf-like and Mucin-like, protein tyrosine phosphatase. The analysis indicated that during DNA fragmentation and cell death, 299 genes were up-regulated and 2,441 genes were down-regulated. Data on five signaling pathways related with cell death, the gap junctions, Ca2+, PI3K/Akt, NF-κB, ATM/p53 revealed that CypD, which is involved in forming a Permeability Transition Pore Complex (PTPC) to alter mitochondrial membrane permeabilization (MMP), was dramatically up-regulated. The qRT-PCR also provided that the key genes for cell survival were down-regulated under M. bicoloratus parasitism, including those encoding Inx1, Inx2 and Inx3 of the gap junction signaling pathway, p110 subunit of the PI3K/Akt signaling pathway, and the p50 and p65 subunit of the NF-κB signaling pathway. These findings suggest that M. bicoloratus parasitism may regulate host mitochondria to trigger internucleosomal DNA fragmentation. This study will facilitate the identification of immunosuppression-related genes and also improves our understanding of molecular mechanisms underlying polydnavirus-parasitoid-host interaction.
Introduction
Polydnaviruses (PDVs) have a very special life cycle. Unlike many viruses, they are not always obligate intracellular parasites, replicating inside living host cells to produce virions that can transfer genes to other cells [1]–[4]. Rather, PDVs are obligate symbionts of many endoparasitic wasps in the families Braconidae (carrying bracovirus) and Ichneumonidae (carrying ichnovirus). Both viruses have similar life cycles, wherein viral DNAs are integrated into a wasp’s genome via Wasp Integration/Excision Motif (WIM) [5] and transmitted vertically to the wasp’s offspring in a proviral form. Viruses replicate in the nucleus of the calyx cell in wasp ovaries. Mature virions are stored in the lumen of the calyx and oviduct, and the suspension of virus and protein is called calyx fluid. When a female wasp finds a host, she injects calyx fluid, venom produced by the venom gland and one or more eggs into the hemocoel of the host caterpillar. Virions infect host cells and discharge their circular dsDNA into the host nuclei, which then rapidly integrates into the host genome via the Host Integration Motif (HIM) [6]. Virulence genes are then transcribed in host cell nuclei and the cytoplasm, resulting in expression of virulence proteins. During the development of the wasp’s offspring, the host hemocoel contains innate suppressive proteins from virus gene expression. In addition, specifically among the bracoviruses, the induction of host hemocyte apoptosis causes host immunosuppression [1], [3].
Apoptosis or programmed cell death (PCD), is common to all metazoan phyla, including insects. Braconidae-induced apoptosis, however, is specifically characterized by internucleosomal DNA fragmentation. Apoptotic DNA fragmentation involves a variety of elements, including AIF, EndoG and DFF40. Every element is regulated by different signaling pathways, defined as extrinsic and intrinsic pathways. Extrinsic apoptosis pathway is triggered by the ligand-induced oligomerization of specific cell surface receptors, and this process induces the intracellular assembly of the death-inducing signaling complex for the activation of a caspase cascade initiated from caspase 8 that results in activation of caspase 3 and further cascade activation of DFF (cleavage of DFF45 releases DFF40 into the nucleus). DFF, a heterodimeric protein comprising 45 kDa and 40 kDa subunits termed ICAD/DFF45 and CAD/DFF40 [7]. The DFF complex is localized in the cellular cytoplasm, resulting in the triggering of extrinsic apoptotic stress, and activated caspase 3 cleaves DFF45 and dissociates DFF40. Caspase 7 and Granzyme B also can cleave DFF45 but with a lower efficiency than caspase 3 [8]. Activated DFF40 translocates into the nucleus. In the nucleus, the activation of DFF40 is enhanced by interaction with the chromosomal protein Histone H1 and it cleaves chromosomal DNA at internucleosomal sites into fragments of ∼200 bp. [9]–[11]. In contrast, the intrinsic pathway is also controlled by mitochondria, which collects and integrates pro- and anti-apoptotic signal stimuli from other organelles as well as from the extracellular microenvironment, such as DNA damage produced by Ataxia-Telangiectasia Mutated (ATM), endoplasmic reticulum (ER) stress and calcium overload. The intrinsic pathway can mediate caspase-independent and caspase-dependent apoptosis. Following intrinsic apoptotic stress triggering, EndoG is released from the mitochondrial intermembrane space and moves to the nucleus to produce nucleosomal DNA fragmentation, giving rise to 200∼5,000 bp sized fragments in a caspase-independent manner. AIF is another endonuclease released from the mitochondrial intermembrane space. It is a flavoprotein that produces DNA fragments up to 5,000 bp in size, and it also does not require caspase activation [12]. Releasing cytochrome c can also mediate cell death via activation of caspase 8, which triggers a caspase-dependent apoptosis.
Numerous viruses are well known to modulate the mitochondrial apoptosis of infected host cells by altering Mitochondrial Membrane Permeabilization (MMP) in a direct and indirect manner with viral proteins. MMP regulation is performed via the Voltage-Dependent Anion Channel (VDAC) of the outer membrane (OM), the Adenine Nucleotide Translocase (ANT) of the inner membrane (IM), and cyclophilin D (CypD) of matrix proteins. Viral proapoptotic proteins are direct inducers of MMP. They include viral protein R (Vpr), which directly interacts with ANT and VDAC, thereby triggering MMP associated with mitochondrial membrane potential (ΔΨm) loss, mitochondrial intermembrane space (IMS) protein release, and caspase cascade activation. Viral proapoptotic proteins are also indirect MMP facilitators and promote apoptosis via both p53-dependent and -independent mechanisms [13]. The alteration of membrane permeability may release apoptotic-promoting factors from the mitochondria, such as AIF, EndoG, and Cyt c in the IMS, ultimately resulting in nuclear translocation. All of these signaling pathways involved in apoptotic DNA fragmentation are stimulated by intrinsic stress through the mitochondria via EndoG and AIF, in a process that is also called caspase-independent cell death, involving release of Cyt c, and extrinsic stress through caspase cascades via DFF40, which is also called caspase-dependent cell death [14].
After apoptotic stimulation, DFF40, EndoG and AIF migrate to the nucleus under the control of critical apoptosis-involved signaling pathways, including the gap junction signaling pathway, Ca2+ signaling pathway, PI3K/Akt signaling pathway, NF-κB signaling pathway, and ATM/p53 signaling pathway. The gap junction signaling pathway induces apoptosis via regulation of the permeability of the plasma membrane resulting in alteration of intracellular and extracellular communication via transmission of small molecules, such as apoptotic signaling ATP. Gap junction proteins are the target proteins of activated caspase 3 [15] and also Ca2+. The Ca2+ signaling pathway is involved in apoptosis via altering the permeability of the mitochondrial membrane to release apoptosis-inducing factors to trigger apoptotic caspase-dependent and -independent pathways [13]. Apoptotic caspase-dependent signaling pathways include the PI3K/Akt signaling pathway and NF-κB signaling pathway via regulation of caspase 3, and the apoptotic caspase-independent signaling pathways include regulation of the ATM/p53 signaling pathway by AIF expression [16]. The PI3K/Akt signaling pathway is crucial to many aspects of cell growth and survival, and its inhibition increases DNA fragmentation by the help of caspase 3 [17]. Baculoviruses inhibit cell apoptosis through activating the PI3K/Akt signaling pathway [18]. Nuclear Factor-κB (NF-κB) transcription factors regulate the expression of antimicrobial peptides (AMPs) and many genes involved in cell survival, such as c-IAP1/2, XIAP, and Bcl-XL. All NF-κBs are homo- or heterodimers of Rel proteins, such as p50/p65 subunits. p53 plays an important role in suppressing tumorigenesis through inducing genomic stability via DNA repair, cell cycle arrest and apoptosis. p53 promotes AIF activity and caspase-independent cell death by binding to a p53-responsive element (p53RE) in the AIF promoter, which ultimately results in efficient induction of large-scale DNA fragmentation (5 kb) [16].
In this paper, we aimed to clarify the mechanism of parasitism induction of host hemocyte apoptosis. To test the hypothesis that parasitism regulates host apoptotic signaling pathways to produce apoptotic DNA fragmentation involved in nuclear elements to the nucleus, resulting in internucleosomal DNA fragmentation from 5 kb to 200 bp, we sequenced the RNA from hemocytes of the Oriental Leafworm Moth Spodoptera litura parasitized by the wasp Microplitis bicoloratus and compared the transcriptome of hemocytes from non-parasitized controls. Using this transcription data, we obtained an overview on how M. bicoloratus parasitism regulates apoptosis signaling pathways during the immunosuppression and induced killing of host S. litura hemocytes. Furthermore, we proposed M. bicoloratus bracovirus products to regulate mitochondria permeability to trigger internucleosomal DNA fragmentation and block a set of key genes in the cell survival signaling pathway.
Results
Transcription sequencing and analysis
Gene expression profiling of S. litura hemocytes, both non-parasitized and parasitized, was achieved via sequencing with an Illumina Hiseq 2000 (Table S1). A million paired-end sequences (Table S2) from four samples, M1 and M2 from S. litura hemocytes parasitized by M. bicoloratus and samples S1 and S2 from non-parasitized S. litura hemocytes, were assembled into 3 different transcriptomes, M (M1+M2), S (S1+S2) and All (M1+M2+S1+S2), using Trinity. This gave a large number of EST cluster contigs: 15,208 (M), 15,206 (S) and 20,571 (All) (Table S3). A comparison of the transcriptome pattern of the average M and average S transcriptomes indicated that 299 consensus genes were up-regulated, and 2,441 genes were down-regulated, under M. bicoloratus parasitism in host hemocytes.
M. bicoloratus bracovirus genes transcribed in the hemocytes of parasitized host
It is well known that polydnaviruses manipulate host cell physiology [19]. Bracoviruses encode at least 20 gene families identified from 5 species of bracoviruses, Cotesia congregata bracovirus (CcBV) [20], Microplitis demolitor bracovirus (MdBV) [21], Glyptapanteles indiensis bracovirus (GiBV) [22], Glyptapanteles flavicoxis bracovirus (GfBV) [5], and Cotesia vestalis bracovirus (CvBV) [23]. In the present study, genes belonging to at least 6 conserved gene families were found to be expressed in the host hemocytes parasitized by M. bicoloratus including 1) Ankyrin-repeat, 2) BEN domain, 3) C-type lectin, 4) Epidermal growth factor-like (EGF-like), 5) Mucin-like, and 6) protein tyrosine phosphatases (PTPs) (Table 1). Some of the proteins encoded by these genes are likely to be involved in regulating host cell death.
Table 1. Transcription of M. bicoloratus bracovirus genes during development of parasitoid M. bicoloratus in host hemocytes.
| Protein Family | Protein | Consensus ID | Length | NCBI_E_value | NCBI_ID | Function | Species |
| Ankyrin-repeat | MbANK1 | comp576933_c0_seq1 | 207 | 1.00E-14 | ref|YP_239402.1| | viral ankyrin 1 | [Microplitis demolitor bracovirus] |
| MbANK1 | comp119151_c0_seq1 | 558 | 3.00E-58 | ref|YP_239402.1| | viral ankyrin 1 | [Microplitis demolitor bracovirus] | |
| MbANK1 | comp26305_c0_seq1 | 561 | 6.00E-40 | ref|YP_239402.1| | viral ankyrin 1 | [Microplitis demolitor bracovirus] | |
| MbANK2 | comp728608_c0_seq1 | 225 | 6.00E-35 | ref|YP_239372.1| | viral ankyrin 2 | [Microplitis demolitor bracovirus] | |
| MbANK3 | comp18868_c0_seq1 | 525 | 1.00E-83 | ref|YP_239406.1| | viral ankyrin; | [Microplitis demolitor bracovirus] | |
| Ben domain | MbBEN1 | comp20976_c0_seq1 | 1053 | 4.00E-54 | ref|YP_239364.1| | hypothetical protein | [Microplitis demolitor bracovirus] |
| MbBEN1 | comp20957_c0_seq1 | 1572 | 1.00E-115 | ref|YP_239364.1| | hypothetical protein | [Microplitis demolitor bracovirus] | |
| MbBEN2 | comp9824_c0_seq1 | 2046 | 1.00E-166 | ref|YP_184800.1| | CcBV_9.1 | [Microplitis demolitor bracovirus] | |
| MbBEN3 | comp177162_c0_seq1 | 618 | 2.00E-72 | ref|YP_184814.1| | CcBV_12.2 | [Cotesia congregata bracovirus] | |
| MbBEN4 | comp252441_c0_seq1 | 237 | 2.00E-34 | gb|AEE09539.1| | DUF-like 1 protein | [Cotesia congregata bracovirus] | |
| C-type lectin | MbCLECT1 | comp19781_c0_seq1 | 666 | 1.00E-34 | ref|YP_184818.1| | CcBV_2–13.1 | [Cotesia congregata bracovirus] |
| MbCLECT2 | comp37160_c0_seq1 | 474 | 1.00E-43 | gb|AEE09593.1| | lectin | [Cotesia vestalis bracovirus] | |
| MbCLECT3 | comp375850_c0_seq1 | 333 | 1.00E-31 | gb|AAS10157.1| | lectin | [Cotesia Plutellae Polydnavirus] | |
| EGF-like | MbCRP1 | comp22262_c0_seq1 | 561 | 8.00E-67 | gb|ABB922678.1| | CRP1, egf 1.5 | [Microplitis bicoloratus bracovirus] |
| Mucin-like | MbGlc1.8 | comp118173_c0_seq1 | 153 | 5.69E-14 | ref|YP_239419.1| | Glc1.8 | [Microplitis demolitor bracovirus] |
| MbGlc1.8 | comp85587_c0_seq1 | 126 | 1.46E-54 | ref|YP_239405.1| | Glc1.8 | [Microplitis demolitor bracovirus] | |
| PTP-like | MbPTP1 | comp360492_c0_seq1 | 444 | 7.00E-72 | ref|YP_239404.1| | PTP 1 | [Microplitis demolitor bracovirus] |
| MbPTP1 | comp330407_c0_seq1 | 417 | 7.00E-49 | ref|YP_239404.1| | PTP 1 | [Microplitis demolitor bracovirus] | |
| MbPTP2 | comp207973_c0_seq1 | 375 | 4.00E-64 | ref|YP_239382.1| | PTP 2 | [Microplitis demolitor bracovirus] | |
| MbPTP2 | comp130820_c0_seq1 | 618 | 1.00E-106 | ref|YP_239382.1| | PTP 2 | [Microplitis demolitor bracovirus] | |
| MbPTP3 | comp556935_c0_seq1 | 354 | 4.00E-59 | ref|YP_239383.1| | PTP 3 | [Microplitis demolitor bracovirus] | |
| MbPTP4 | comp188579_c0_seq1 | 177 | 3.00E-15 | ref|YP_239386.1| | PTP 4 | [Microplitis demolitor bracovirus] | |
| MbPTP4 | comp498102_c0_seq1 | 330 | 2.00E-33 | ref|YP_239386.1| | PTP 4 | [Microplitis demolitor bracovirus] | |
| MbPTP4 | comp584871_c0_seq1 | 201 | 9.00E-20 | ref|YP_239386.1| | PTP 4 | [Microplitis demolitor bracovirus] | |
| MbPTP5 | comp279111_c0_seq1 | 249 | 2.00E-11 | ref|YP_239381.1| | PTP | [Microplitis demolitor bracovirus] | |
| MbPTP5 | comp273967_c0_seq1 | 285 | 7.00E-35 | ref|YP_239381.1| | PTP | [Microplitis demolitor bracovirus] | |
| MbPTP5 | comp456541_c0_seq1 | 315 | 6.00E-38 | ref|YP_239381.1| | PTP | [Microplitis demolitor bracovirus] | |
| MbPTP5 | comp283025_c0_seq1 | 420 | 1.00E-41 | ref|YP_239381.1| | PTP | [Microplitis demolitor bracovirus] | |
| MbPTP6 | comp767898_c0_seq1 | 264 | 8.00E-42 | ref|YP_239390.1| | PTP | [Microplitis demolitor bracovirus] | |
| MbPTP | comp92610_c0_seq9 | 252 | 5.90E-37 | gb|ACE75309.1| | PTP | [Glyptapanteles indiensis bracovirus] |
Gap junction signaling pathway regulation by M. bicoloratus parasitism
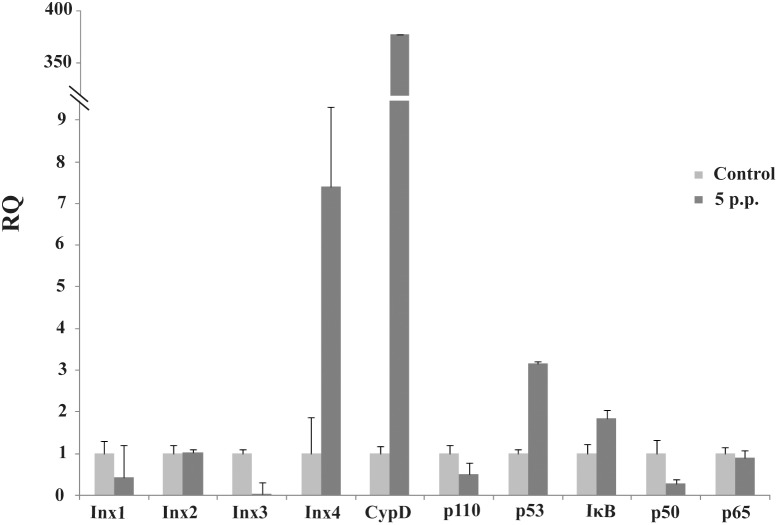
Gap junction proteins form gap junction channels connecting cells for cell-cell communication and form hemichannels facilitating extracellular and intracellular communication including between ER and mitochondria to exchange small molecular, such as ATP and Ca2+, to trigger apoptosis [24]. In the insect circulating hemocytes, gap junction proteins form hemichannels to allow communication between the cell and environment. Under lipopolysaccharide (LPS) immunochallenge, hemichannel dye uptake decreases [25]. Typically, the decrease of the transcription level of hemichannel components and the decrease in opening of hemichannels on the cell surface result in the decrease of dye uptake. Gap junction proteins, Spli-Inx2 and Inx3, have been characterized and functioned [26] and in this study, Spli-inx1 and inx4 also were detected from hemocytes (Fig. S1 and Table S4). Comparisons with S and M transcriptome data indicated that all 26 elements of the gap junction signaling pathway existed in the hemocytes. During immune challenge by M. bicoloratus parasitization, 2 genes (Spli-GNAS, ADCY5) were not expressed in the parasitized host hemocytes. To determine the differential expression of genes, all transcriptome were assembled into a combined pool, and S1, S2, M1, and M2 were mapped using this pool to obtain reads and the RPM values of S and M. Furthermore, the analysis obtained the fold change and p-value between parasitized and non-parasitized. These analyses indicated that 12 genes (ADCY8, CPKC, GNAS, INX1, INX2, INX3, INX4, ITPR1, PKA, PLCB, PRKG, and TUBA) were down-regulated (Table 2). The qRT-PCR results indicate that the parasitization down-regulated 3 key molecules, Inx1, Inx2, Inx3, on the cell membrane, not Inx4 (Fig. 1). These molecules are involved in forming hemichannels and gap junctions, suggesting that there might be disruptions of intracellular between ER, mitochondria and extracellular molecular exchanges.
Table 2. The differential expression of genes regulated by M. bicoloratus bracovirus in the host gap junction signaling pathway.
| M/S | ||||||||||
| A_ID | Function | read_M | RPKM_M | read_S | RPKM_S | log2(Fold_change)normalized | p-value | Result | S_ID | M-ID |
| comp95316_c0_seq3 | adenylate cyclase 8 | 414 | 1.538851011 | 5365 | 20.66052119 | −3.746951184 | 0 | down | comp59135_c1_seq10 | comp18779_c0_seq1 |
| comp96543_c0_seq4 | classical proteinkinase C | 1502 | 6.301173776 | 3740 | 16.25542916 | −1.367229143 | 4.6108E-232 | down | comp30329_c0_seq1 | comp20807_c0_seq1 |
| comp97909_c0_seq6 | guanine nucleotide-bindingprotein G(s) subunitalpha | 41 | 0.622358814 | 430 | 6.762398457 | −3.441716531 | 3.85048E-83 | down | comp59076_c0_seq5 | / |
| comp88846_c0_seq1 | gap junction | 628 | 3.260154915 | 1623 | 8.73E+00 | −1.420902221 | 2.19E-107 | down | comp57755_c2_seq1 | comp19421_c1_seq1 |
| comp65035_c0_seq1 | gap junction | 1808 | 16.23951848 | 11125 | 1.04E+02 | −2.672414439 | 0.00E+00 | down | comp45671_c0_seq1 | comp10397_c0_seq1 |
| comp99381_c0_seq1 | gap junction | 3994 | 22.25932132 | 36919 | 213.1714802 | −3.259532924 | 0 | down | comp59804_c0_seq1 | comp30941_c0_seq1 |
| comp121018_c0_seq1 | gap junction | 36 | 0.410377626 | 935 | 1.10E+01 | −4.749973239 | 7.01E-217 | down | comp59264_c0_seq1 | comp10397_c0_seq1 |
| comp96275_c0_seq13 | inositol1,4,5-triphosphatereceptor type 1 | 2068 | 3.72067313 | 5214 | 9.718904704 | −1.385230083 | 0 | down | comp59099_c0_seq4 | comp94669_c0_seq1 |
| comp106866_c0_seq1 | protein kinase A | 2326 | 9.047590091 | 7695 | 31.01038396 | −1.777145916 | 0 | down | comp65026_c0_seq1 | comp17984_c0_seq1 |
| comp97791_c0_seq2 | phosphatidylinositol phospholipase C,beta | 366 | 2.438775601 | 738 | 5.09474476 | −1.062852854 | 8.52689E-32 | down | comp55943_c2_seq1 | comp8084_c0_seq1 |
| comp95574_c0_seq5 | protein kinase,cGMP-dependent | 1795 | 5.701522537 | 4620 | 15.20350361 | −1.414984693 | 1.1324E-301 | down | comp58204_c1_seq7 | comp16873_c0_seq1 |
| comp63482_c0_seq3 | tubulin alpha | 20547 | 224.0520545 | 53712 | 606.8022006 | −1.437392362 | 0 | down | comp41562_c0_seq1 | comp14668_c0_seq1 |
| comp94424_c0_seq3 | adenylate cyclase 1 | 53 | 0.61819881 | 151 | 1.824754972 | −1.561559971 | 1.42371E-12 | comp29410_c0_seq1 | comp128727_c0_seq1 | |
| comp94556_c0_seq4 | adenylate cyclase 5 | 23 | 0.17008429 | 341 | 2.612558705 | −3.941141659 | 7.22672E-73 | comp55791_c2_seq2 | / | |
| comp69534_c0_seq1 | adenylate cyclase 9 | 17 | 0.46329195 | 29 | 0.81880247 | −0.82159384 | 0.069978649 | comp55534_c1_seq1 | comp4228_c0_seq1 | |
| comp76441_c0_seq1 | cyclin-dependentkinase 1 | 1207 | 7.410567531 | 2282 | 14.51560537 | −0.969948801 | 1.51194E-82 | comp27910_c0_seq1 | comp16516_c0_seq1 | |
| comp93202_c0_seq1 | epidermal growthfactor receptor | 6514 | 18.3968606 | 6149 | 17.99184785 | 0.032116227 | 0.238280263 | comp72419_c0_seq1 | comp33128_c0_seq1 | |
| comp83895_c0_seq2 | guanine nucleotide-binding protein G(q) subunit alpha | 4274 | 19.02930474 | 7980 | 36.81006701 | −0.951877524 | 5.7673E-277 | comp55254_c0_seq5 | comp17311_c0_seq3 | |
| comp103695_c0_seq1 | growth factorreceptor-bindingprotein 2 | 4553 | 31.55832529 | 7467 | 53.62135235 | −0.764786958 | 6.7036E-179 | comp59056_c4_seq1 | comp101386_c0_seq1 | |
| comp112119_c0_seq1 | GTPase KRas | 2904 | 11.39420805 | 3605 | 14.6544025 | −0.363033492 | 2.01055E-23 | comp46714_c0_seq1 | comp156998_c0_seq1 | |
| comp81191_c0_seq1 | mitogen-activatedprotein kinasekinase 1 | 1619 | 6.495897191 | 2927 | 12.16719112 | −0.905395446 | 3.63546E-94 | comp174562_c0_seq1 | comp20958_c0_seq1 | |
| comp23161_c0_seq1 | mitogen-activatedprotein kinase 1/3 | 5679 | 21.44919695 | 7831 | 30.64302995 | −0.514635322 | 3.1947E-93 | comp28328_c0_seq1 | comp9963_c0_seq1 | |
| comp96783_c0_seq11 | son of sevenless | 74 | 1.123281762 | 195 | 3.066669068 | −1.448952634 | 2.40343E-14 | comp47573_c1_seq1 | comp17032_c0_seq1 | |
| comp97420_c0_seq2 | tyrosine-protein kinase Src | 126 | 1.148163839 | 460 | 4.342766357 | −1.919285813 | 2.09308E-47 | comp56420_c1_seq1 | comp106968_c0_seq1 | |
| comp97925_c2_seq1 | tight junction protein 1 | 71 | 0.575127537 | 454 | 3.810105311 | −2.727877054 | 8.33036E-71 | comp58674_c2_seq3 | comp93783_c0_seq1 | |
| comp92127_c0_seq2 | tubulin beta | 63513 | 551.0471151 | 102657 | 922.7626088 | −0.74378387 | 0 | comp118372_c0_seq1 | comp18397_c1_seq1 | |
Figure 1. qRT-PCR detected key genes in five signaling pathways with hemocytes 5 days post-parasitization (p.p.).
Ca2+ signaling pathway regulation by M. bicoloratus parasitism with respect to apoptosis
Calcium ions (Ca2+) control every aspect of cells as cellular messengers. Ca2+ ions also can become death signals when delivered at physiologically aberrant conditions. Mitochondria eventually decide whether Ca2+ signals are life or death signals via regulation of the mitochondrial membrane proteins Bcl-2 and Bax/Bak [27]. Comparisons of the transcription data from the S and M pools indicate that all 31 elements of the Ca2+ signaling pathway existed in the examined hemocytes. Under M. bicoloratus parasitism, 3 genes (Spli-ANT, CypD, PLCG2) increased in expression, and 1 genes (Spli-PDE1) was not expressed in the parasitized hemocytes. The other 13 genes (ADCY8, ATP2A, ATP2B, CPKC, GNAS, ITPR1, ORAI1, PHKA_B, PKA, PLCB, VDAC1, VDAC2 and VDAC3) had been down-regulated (Table 3). The qRT-PCR results indicate that the parasitism up-regulated a key molecule, CypD, in the mitochondria (Fig. 1). This molecule is involved in forming a permeability transition pore complex (PTPC), suggesting that the M. bicoloratus alters Ca2+ signaling pathway to promote apoptosis.
Table 3. The differential expression of genes regulated by M. bicoloratus bracovirus in the host Ca2+ signaling pathway.
| M/S | |||||||||||
| Gene family | A_ID | Function | read_M | RPKM_M | read_S | RPKM_S | log2(Fold_change) normalized | p-value | Result | S_ID | M-ID |
| ANT | comp95003_c0_seq1 | mitochondrialadenine nucleotidetranslocator | 482 | 5.819038231 | 0.5 | 0.006253879 | 9.86181365 | 7.35681E-80 | up | / | comp41118_c0_seq1 |
| CypD | comp93813_c0_seq1 | peptidyl-prolylisomerase F(cyclophilin D) | 448 | 6.696320619 | 0.5 | 0.007742898 | 9.756279236 | 2.02443E-75 | up | / | comp11549_c0_seq1 |
| ADCY8 | comp95316_c0_seq3 | adenylate cyclase 8 | 414 | 1.538851011 | 5365 | 20.66052119 | −3.746951184 | 0 | down | comp58820_c1_seq2 | comp18779_c1_seq1 |
| ATP2A | comp23165_c0_seq2 | Ca2+ transportingATPase | 4330 | 11.26751358 | 12042 | 32.46490454 | −1.526711778 | 0 | down | comp45209_c0_seq2 | comp20999_c0_seq2 |
| ATP2B | comp102625_c0_seq1 | Ca2+ transportingATPase | 6998 | 16.2324803 | 17020 | 40.90211972 | −1.333292153 | 0 | down | comp61676_c0_seq1 | comp19993_c0_seq2 |
| CPKC | comp96543_c0_seq4 | classical proteinkinase C | 1502 | 6.301173776 | 3740 | 16.25542916 | −1.367229143 | 4.6108E-232 | down | comp30329_c0_seq1 | comp20807_c0_seq1 |
| GNAS | comp97983_c1_seq2 | guanine nucleotide-bindingprotein G(s) subunit alpha | 149 | 2.139786276 | 363 | 5.400899151 | −1.335732903 | 7.99287E-23 | down | comp58416_c0_seq4 | comp20437_c1_seq1 |
| ITPR1 | comp96275_c0_seq13 | inositol 1,4,5-triphosphatereceptor type 1 | 2068 | 3.72067313 | 5214 | 9.718904704 | −1.385230083 | 0 | down | comp59099_c0_seq4 | comp94669_c0_seq1 |
| ORAI1 | comp97095_c0_seq1 | calcium release-activatedcalcium channel protein 1 | 238 | 1.972431074 | 588 | 5.048676205 | −1.355930267 | 4.85903E-37 | down | comp57934_c0_seq2 | comp71014_c0_seq1 |
| PHKA_B | comp92577_c0_seq1 | phosphorylase kinasealpha/beta subunit | 1191 | 3.399676742 | 3490 | 10.32111467 | −1.602129309 | 6.6379E-274 | down | comp58502_c0_seq5 | comp101238_c0_seq1 |
| PKA | comp106866_c0_seq1 | protein kinase A | 2326 | 9.047590091 | 7695 | 31.01038396 | −1.777145916 | 0 | down | comp65026_c0_seq1 | comp17984_c0_seq1 |
| PLCB | comp97791_c0_seq2 | Phosphatidylinositolphospholipase C, beta | 366 | 2.438775601 | 738 | 5.09474476 | −1.062852854 | 8.52689E-32 | down | comp55982_c0_seq1 | comp80726_c0_seq1 |
| VDAC1 | comp90986_c0_seq1 | voltage-dependentanion channel protein 1 | 4 | 0.179365731 | 42 | 1.95E+00 | −3.443393109 | 3.25E-09 | down | comp56820_c0_seq2 | comp79085_c0_seq1 |
| VDAC2 | comp99405_c0_seq1 | voltage-dependentanion channel protein 2 | 5522 | 67.03373029 | 10842 | 136.3583388 | −1.024443805 | 0 | down | comp60098_c0_seq1 | comp23968_c0_seq1 |
| VDAC3 | comp89185_c0_seq1 | voltage-dependentanion channel protein 3 | 2 | 0.093999153 | 26 | 1.27E+00 | −3.751515404 | 1.70E-06 | down | comp60098_c0_seq1 | comp23968_c0_seq1 |
| ADCY1 | comp94424_c0_seq3 | adenylate cyclase 1 | 53 | 0.61819881 | 151 | 1.824754972 | −1.561559971 | 1.42371E-12 | comp48930_c1_seq2 | comp128727_c0_seq1 | |
| ADCY9 | comp69534_c0_seq1 | adenylate cyclase 9 | 17 | 0.46329195 | 29 | 0.81880247 | −0.82159384 | 0.069978649 | comp55534_c1_seq1 | comp4228_c0_seq1 | |
| CALM | comp23241_c0_seq1 | calmodulin | 27610 | 270.0746841 | 42753 | 433.2707575 | −0.681910456 | 0 | comp45080_c1_seq2 | comp61330_c0_seq1 | |
| CAMK2 | comp97973_c0_seq1 | calcium/calmodulin-dependentprotein kinase II | 944 | 3.291051665 | 1737 | 6.273903548 | −0.930814675 | 5.27371E-59 | comp57321_c0_seq1 | comp19479_c0_seq1 | |
| EGFR | comp93202_c0_seq1 | epidermal growthfactor receptor | 6514 | 18.3968606 | 6149 | 17.99184785 | 0.032116227 | 0.238280263 | comp72419_c0_seq1 | comp33128_c0_seq1 | |
| GNAQ | comp83895_c0_seq2 | guanine nucleotide-binding protein G(q)subunit alpha | 4274 | 19.02930474 | 7980 | 36.81006701 | −0.951877524 | 5.7673E-277 | comp50512_c0_seq1 | comp166552_c0_seq1 | |
| ITPK | comp30903_c0_seq1 | 1D-myo-inositol-triphosphate 3-kinase | 962 | 11.05221153 | 1777 | 21.1512899 | −0.936410568 | 6.25084E-61 | comp55786_c0_seq2 | comp37996_c0_seq1 | |
| MYLK | comp95483_c0_seq1 | myosin-light-chainkinase | 65 | 1.078904349 | 198 | 3.404944919 | −1.658064493 | 1.86308E-17 | comp46122_c0_seq1 | comp119788_c0_seq1 | |
| PDE1 | comp96257_c0_seq5 | calcium/calmodulin-dependent 3′,5′-cyclicnucleotide phosphodiesterase | 8 | 0.058446689 | 531 | 4.019201305 | −6.103643737 | 1.0771E-127 | comp58443_c0_seq1 | / | |
| PHKG | comp97075_c0_seq1 | phosphorylase kinasegamma subunit | 350 | 2.198800775 | 633 | 4.119996628 | −0.905926263 | 2.4877E-21 | comp56788_c0_seq1 | comp57668_c0_seq1 | |
| PLCG1 | comp95371_c0_seq1 | phosphatidylinositolphospholipase C,gamma-1 | 155 | 1.337538268 | 289 | 2.583733252 | −0.949876963 | 3.71489E-11 | comp54883_c1_seq1 | comp81243_c0_seq1 | |
| PLCG2 | comp94580_c0_seq1 | phosphatidylinositolphospholipase C,gamma-2 | 110 | 1.770661702 | 159 | 2.651644792 | −0.582598928 | 0.00156832 | / | comp78811_c0_seq1 | |
| PPP3C | comp108295_c0_seq1 | serine/threonine-proteinphosphatase 2B catalyticsubunit | 2433 | 13.2733981 | 4265 | 24.1065111 | −0.860885107 | 1.4074E-125 | comp38261_c0_seq1 | comp20495_c0_seq2 | |
| PPP3R | comp109656_c0_seq1 | serine/threonine-proteinphosphatase 2B regulatorysubunit | 1743 | 13.13252377 | 2743 | 21.41173844 | −0.705257739 | 7.24698E-58 | comp42185_c0_seq1 | comp35682_c0_seq1 | |
| SPHK | comp92166_c0_seq3 | sphingosine kinase | 76 | 0.79803821 | 134 | 1.457774018 | −0.869237363 | 3.52398E-05 | comp52416_c0_seq1 | comp8718_c0_seq1 | |
| STIM1 | comp94633_c0_seq1 | stromal interactionmolecule 1 | 1970 | 8.552321068 | 2535 | 11.40173775 | −0.414865803 | 3.03169E-21 | comp55152_c1_seq2 | comp19536_c0_seq1 | |
PI3K/Akt signaling pathway regulation by M. bicoloratus parasitism
The PI3K/Akt signaling pathway is involved in multiple different pathways, including cell survival, apoptosis, cell cycle, and DNA repair, through different downstream molecules. A comparison of the transcription data from the S and M pools revealed that all 65 elements of the PI3K/Akt signaling pathway existed in the hemocytes. Under immune challenge, 4 genes (ATF4, RP-S6e, EIF4EBP1, and GNB1) were expressed in the parasitized hemocytes, and 7 genes (COL1AS, FGFR2, G6PC, p85, PPP2R3, THBS2S, and TSC1) were not expressed in the parasitized host hemocytes (Table 4). Another 19 genes (COL4A, CREB3, HSP90B, IRS1, ITGB1, LAMA3_5, LAMB1, LAMC1, PDPK1, PPP2C, PPP2R2, PPP2R5, PTEN, PTK2, RAC1, STK11, TSC2 and YWHAE) were down-regulated, (Table 4). The qRT-PCR results indicated that the parasitism down-regulated a key molecule, the p110 subunit, in the PI3K/Akt signaling pathway, suggesting that the disruption of cell survival signaling pathway by the parasitism may promote cell apoptosis (Fig. 1).
Table 4. The differential expression of genes regulated by M. bicoloratus bracovirus in the host PI3K/Akt signaling pathway.
| M/S | |||||||||||
| Gene family | A_ID | Function | read_M | RPKM_M | read_S | RPKM_S | log2(Fold_change) normalized | p-value | Result | S_ID | M-ID |
| ATF4 | comp93717_c0_seq1 | CREB2; cyclic AMP-dependent transcription factor ATF-4 | 391 | 5.031431973 | 0.5 | 0.006665921 | 9.559949111 | 8.92893E-68 | up | / | comp42406_c0_seq1 |
| PEPCK | comp109757_c0_seq1 | phosphoenolpyruvate carboxykinase (GTP) | 3834 | 27.16382297 | 574 | 4.213334979 | 2.688652009 | 0 | up | comp58069_c0_seq1 | comp30301_c0_seq1 |
| RP-S6e | comp24289_c0_seq1 | small subunit ribosomal protein S6e | 1353 | 27.7189041 | 0.5 | 0.010612644 | 11.35087044 | 8.601E-176 | up | / | comp11154_c0_seq1 |
| COL4A | comp23243_c0_seq1 | type IV, alpha | 33659 | 235.0626028 | 160548 | 1161.615892 | −2.305016159 | 0 | down | comp45047_c0_seq1 | comp10045_c0_seq2 |
| CREB3 | comp101801_c0_seq1 | cyclic AMP-responsive element-binding protein 3 | 3995 | 24.04913606 | 11685 | 72.87636897 | −1.599466012 | 0 | down | comp28212_c0_seq1 | comp9969_c0_seq1 |
| GSK3B | comp23136_c0_seq1 | glycogen synthase kinase 3 beta | 1248 | 13.91068253 | 2965 | 34.2400086 | −1.299489856 | 1.9348E-170 | down | comp47318_c0_seq1 | comp20759_c0_seq2 |
| HSP90B | comp103187_c0_seq1 | heat shock protein 90kDa beta | 3701 | 23.9880501 | 9865 | 66.24426184 | −1.465479601 | 0 | down | comp28148_c0_seq1 | comp19950_c0_seq1 |
| IRS1 | comp97702_c0_seq2 | insulin receptor substrate 1 | 268 | 2.200308994 | 696 | 5.920159847 | −1.427929991 | 4.40292E-47 | down | comp58222_c1_seq1 | comp53773_c0_seq1 |
| ITGB1 | comp107868_c0_seq1 | integrin beta 1 | 2897 | 11.30115561 | 8616 | 34.82213249 | −1.623534251 | 0 | down | comp46010_c0_seq2 | comp20980_c0_seq2 |
| LAMA3_5 | comp99575_c0_seq1 | laminin, alpha 3/5 | 27851 | 42.99292765 | 112433 | 179.8147605 | −2.064340192 | 0 | down | comp59989_c0_seq1 | comp17635_c0_seq1 |
| LAMB1 | comp95243_c0_seq1 | laminin, beta 1 | 18108 | 49.24188678 | 51196 | 144.2366222 | −1.550479569 | 0 | down | comp60102_c0_seq1 | comp10145_c0_seq2 |
| LAMC1 | comp100060_c0_seq1 | laminin, gamma 1 | 15999 | 40.61052537 | 52368 | 137.716847 | −1.761779459 | 0 | down | comp60240_c0_seq1 | comp10076_c0_seq1 |
| PDPK1 | comp89371_c0_seq4 | 3-phosphoinositide dependent protein kinase-1 | 1184 | 5.484101314 | 2310 | 11.08513688 | −1.015299457 | 5.13123E-90 | down | comp57389_c0_seq3 | comp19669_c0_seq2 |
| PPP2C | comp99514_c0_seq1 | serine/threonine-protein phosphatase 2A catalytic subunit | 4007 | 52.05795373 | 8123 | 109.3350776 | −1.07056582 | 0 | down | comp134046_c0_seq1 | comp24560_c0_seq1 |
| PPP2R2 | comp95673_c0_seq2 | serine/threonine-protein phosphatase 2A regulatory subunit B | 362 | 3.276611055 | 761 | 7.136352468 | −1.122982442 | 1.08521E-35 | down | comp56391_c0_seq2 | comp21423_c0_seq2 |
| PPP2R5 | comp25110_c0_seq1 | serine/threonine-protein phosphatase 2A regulatory subunit B' | 414 | 4.316267161 | 1061 | 11.46037093 | −1.408797669 | 9.89141E-70 | down | comp226619_c0_seq1 | comp18379_c0_seq1 |
| PTEN | comp97411_c0_seq4 | PTEN | 233 | 1.759294286 | 911 | 7.126499544 | −2.018196785 | 5.8123E-99 | down | comp59211_c2_seq7 | comp62639_c0_seq1 |
| PTK2 | comp89387_c1_seq2 | focal adhesion kinase 1 | 1443 | 7.251236554 | 3747 | 19.50764073 | −1.427740364 | 3.4416E-248 | down | comp58747_c0_seq2 | comp20857_c0_seq2 |
| RAC1 | comp102261_c0_seq1 | Ras-related C3 botulinum toxin substrate 1 | 4703 | 22.53778331 | 13189 | 65.48222111 | −1.538757631 | 0 | down | comp61307_c0_seq1 | comp211969_c0_seq1 |
| STK11 | comp68494_c0_seq3 | serine/threonine-protein kinase 11 | 275 | 2.752794104 | 643 | 6.668487203 | −1.276462805 | 6.66465E-37 | down | comp28880_c0_seq2 | comp19353_c0_seq1 |
| TSC2 | comp93326_c0_seq1 | tuberous sclerosis 2 | 42 | 1.079341152 | 227 | 6.043807401 | −2.48530675 | 1.25293E-32 | down | comp31311_c0_seq1 | comp76972_c0_seq1 |
| YWHAE | comp96021_c0_seq1 | 14–3-3 protein epsilon | 55 | 1.03509774 | 357 | 6.960848804 | −2.749496236 | 2.16594E-56 | down | comp58841_c0_seq1 | comp18525_c1_seq1 |
| AKT | comp103304_c0_seq1 | RAC serine/threonine-protein kinase | 4590 | 25.54854119 | 8713 | 50.24541632 | −0.975751075 | 0 | comp62555_c0_seq1 | comp30027_c0_seq1 | |
| ATF2 | comp63925_c0_seq1 | CREBP1; cyclic AMP-dependent transcription factor ATF-2 | 33 | 1.162462472 | 51 | 1.861274773 | −0.679106908 | 0.041341344 | comp30829_c1_seq1 | comp100576_c0_seq1 | |
| BRCA1 | comp95658_c0_seq1 | breast cancer type 1 susceptibility protein | 355 | 2.744122154 | 366 | 2.931105739 | −0.09510031 | 0.409730291 | comp54774_c0_seq1 | comp10458_c0_seq1 | |
| CCND2 | comp92629_c0_seq2 | cyclin D2 | 1830 | 9.174971528 | 2968 | 15.41675056 | −0.748723129 | 2.87139E-69 | comp55005_c1_seq1 | comp20586_c0_seq2 | |
| CCNE | comp86772_c0_seq2 | cyclin E | 48 | 0.642601979 | 99 | 1.373128968 | −1.095469805 | 1.62283E-05 | comp29156_c0_seq1 | comp130315_c0_seq1 | |
| CDC37 | comp104439_c0_seq1 | cell division cycle protein 37 | 1645 | 17.75049078 | 2932 | 32.77809747 | −0.884873205 | 8.69703E-91 | comp28183_c0_seq1 | comp31800_c0_seq1 | |
| CDK4 | comp93505_c0_seq2 | cyclin-dependent kinase 4 | 91 | 1.026690365 | 248 | 2.898845626 | −1.497477356 | 9.30914E-19 | comp46659_c0_seq1 | comp104770_c0_seq1 | |
| COL1AS | comp140925_c0_seq1 | type I/II/III/V/XI/XXIV/XXVII, alpha | 0.5 | 0.035296871 | 59 | 4.315126435 | −6.933718735 | 8.23218E-15 | comp89703_c0_seq1 | / | |
| EGFR | comp93202_c0_seq1 | epidermal growth factor receptor | 6514 | 18.3968606 | 6149 | 17.99184785 | 0.032116227 | 0.238280263 | comp72419_c0_seq1 | comp33128_c0_seq1 | |
| EIF4B | comp103484_c0_seq1 | translation initiation factor 4B | 3735 | 23.01200087 | 6307 | 40.25890145 | −0.806921376 | 7.4266E-166 | comp45352_c0_seq1 | comp9966_c0_seq1 | |
| EIF4E | comp108936_c0_seq1 | translation initiation factor 4E | 671 | 7.905316174 | 1192 | 14.54950751 | −0.88007525 | 2.69219E-37 | comp38976_c0_seq1 | comp18053_c0_seq1 | |
| EIF4EBP1 | comp89898_c0_seq1 | eukaryotic translation initiation factor 4E binding protein 1 | 78 | 2.176304201 | 0.5 | 0.01445341 | 7.234326533 | 3.10204E-18 | / | comp65767_c0_seq1 | |
| FGFR2 | comp86525_c0_seq1 | fibroblast growth factor receptor 2 | 9 | 0.147300349 | 80 | 1.35652114 | −3.203078779 | 1.50516E-15 | comp134334_c0_seq1 | / | |
| FRAP | comp98229_c0_seq3 | FKBP12-rapamycin complex-associated protein | 501 | 1.205543964 | 1616 | 4.028672805 | −1.740620375 | 3.1793E-143 | comp57729_c1_seq1 | comp19045_c1_seq1 | |
| G6PC | comp92782_c0_seq1 | glucose-6-phosphatase | 2 | 0.039148868 | 87 | 1.764346079 | −5.494019182 | 5.9857E-22 | comp51802_c0_seq1 | / | |
| GBL | comp96734_c0_seq1 | G protein beta subunit-like | 166 | 2.709305209 | 307 | 5.191149459 | −0.9381311 | 1.47018E-11 | comp45689_c0_seq2 | comp52297_c0_seq1 | |
| GNB1 | comp81476_c0_seq1 | guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 | 7596 | 24.34298724 | 15821 | 52.52888221 | −1.109604667 | 0 | / | comp20574_c0_seq1 | |
| GNB5 | comp90181_c0_seq2 | guanine nucleotide-binding protein subunit beta-5 | 337 | 4.024274883 | 558 | 6.903466944 | −0.778592216 | 6.67432E-15 | comp45009_c0_seq1 | comp17886_c0_seq1 | |
| GNG13 | comp95223_c0_seq1 | guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-13 | 7200 | 46.4612554 | 8333 | 55.7102541 | −0.261914759 | 6.81673E-29 | comp61890_c0_seq1 | comp24329_c0_seq1 | |
| GRB2 | comp103695_c0_seq1 | growth factor receptor-binding protein 2 | 4553 | 31.55832529 | 7467 | 53.62135235 | −0.764786958 | 6.7036E-179 | comp59056_c4_seq1 | comp101386_c0_seq1 | |
| GYS | comp95471_c0_seq2 | glycogen(starch) synthase | 96 | 0.657627746 | 591 | 4.194417817 | −2.673127505 | 7.16393E-90 | comp55945_c1_seq1 | comp231427_c0_seq1 | |
| HSP90A | comp23248_c1_seq1 | molecular chaperone HtpG | 30676 | 217.7778149 | 38447 | 282.7825966 | −0.376836341 | 1.7963E-255 | comp55995_c0_seq2 | comp15914_c0_seq1 | |
| IKBKB | comp46046_c0_seq1 | inhibitor of nuclear factor kappa-B kinase subunit beta | 628 | 4.037631507 | 887 | 5.908346682 | −0.549245231 | 6.14389E-13 | comp46039_c0_seq1 | comp19169_c0_seq1 | |
| INSR | comp97941_c0_seq7 | insulin receptor | 381 | 2.063839032 | 405 | 2.27290447 | −0.139206596 | 0.201218987 | comp55149_c2_seq1 | comp9154_c1_seq1 | |
| JAK2 | comp98009_c1_seq1 | Janus kinase 2 | 407 | 1.908290622 | 691 | 3.356629968 | −0.814732602 | 1.51786E-19 | comp93258_c0_seq1 | comp16460_c1_seq1 | |
| KRAS | comp112119_c0_seq1 | GTPase KRas | 2904 | 11.39420805 | 3605 | 14.6544025 | −0.363033492 | 2.01055E-23 | comp46714_c0_seq1 | comp37119_c0_seq1 | |
| MAP2K1 | comp81191_c0_seq1 | mitogen-activated protein kinase kinase 1 | 1619 | 6.495897191 | 2927 | 12.16719112 | −0.905395446 | 3.63546E-94 | comp174562_c0_seq1 | comp20958_c0_seq1 | |
| MAPK1_3 | comp23161_c0_seq1 | mitogen-activated protein kinase 1/3 | 5679 | 21.44919695 | 7831 | 30.64302995 | −0.514635322 | 3.1947E-93 | comp28328_c0_seq1 | comp9963_c0_seq1 | |
| MYB | comp93622_c1_seq4 | myb proto-oncogene protein | 1093 | 4.733328627 | 1565 | 7.021601066 | −0.568944942 | 2.91134E-23 | comp58816_c0_seq6 | comp9456_c0_seq2 | |
| MYC | comp63425_c0_seq2 | Myc proto-oncogene protein | 2255 | 7.977064372 | 3204 | 11.74260281 | −0.5578224 | 8.82957E-45 | comp47218_c0_seq2 | comp20721_c0_seq2 | |
| P110 | comp97931_c0_seq1 | phosphatidylinositol-4,5-bisphosphate 3-kinase, PIK3C | 165 | 1.192085437 | 623 | 4.663229594 | −1.967841825 | 6.96353E-66 | comp56297_c1_seq1 | comp85699_c0_seq1 | |
| P85 | comp27492_c0_seq1 | phosphoinositide-3-kinase, regulatory subunit, PIK3R | 0.5 | 0.03906187 | 15 | 1.214086421 | −4.957966281 | 0.000103715 | comp39459_c0_seq1 | / | |
| PKN; | comp67156_c0_seq1 | protein kinase N | 778 | 3.949050183 | 1366 | 7.183549165 | −0.863191109 | 3.18306E-41 | comp51044_c0_seq1 | comp19192_c0_seq1 | |
| PPP2R1 | comp101848_c0_seq1 | serine/threonine-protein phosphatase 2A regulatory subunit A | 4788 | 35.95160432 | 8113 | 63.11330498 | −0.811888022 | 2.797E-215 | comp28215_c0_seq1 | comp10104_c0_seq1 | |
| PPP2R3 | comp207060_c0_seq1 | serine/threonine-protein phosphatase 2A regulatory subunit B | 17 | 0.429343833 | 42 | 1.098957536 | −1.355930267 | 0.000936344 | comp141105_c0_seq1 | / | |
| PRKAA | comp88394_c0_seq1 | AMPK; 5′-AMP-activated protein kinase, catalytic alpha subunit | 935 | 5.517046633 | 1081 | 6.608395875 | −0.260403939 | 9.36228E-05 | comp46858_c0_seq1 | comp19779_c0_seq1 | |
| RAPTOR | comp96751_c0_seq3 | regulatory associated protein of mTOR | 186 | 1.325822604 | 550 | 4.061724643 | −1.615204683 | 1.12036E-44 | comp28927_c0_seq2 | comp85394_c0_seq1 | |
| RHEB | comp94584_c0_seq1 | Ras homolog enriched in brain | 150 | 2.527973388 | 197 | 3.43971752 | −0.444308815 | 0.006072962 | comp83256_c0_seq1 | comp65541_c0_seq1 | |
| RPS6KB | comp110869_c0_seq1 | p70 ribosomal S6 kinase | 1391 | 8.265982996 | 2672 | 16.4505016 | −0.992873274 | 3.286E-100 | comp70041_c0_seq1 | comp10389_c0_seq1 | |
| SOS | comp96783_c0_seq11 | son of sevenless | 74 | 1.123281762 | 195 | 3.066669068 | −1.448952634 | 2.40343E-14 | comp47573_c1_seq1 | comp17032_c1_seq1 | |
| THBS2S | comp28902_c0_seq1 | thrombospondin 2/3/4/5 | 31 | 0.720784511 | 15 | 0.361335244 | 0.996230029 | 0.029739095 | comp378223_c0_seq1 | / | |
| TSC1 | comp87058_c0_seq1 | tuberous sclerosis 1 | 15 | 0.611757829 | 46 | 1.943665035 | −1.667747046 | 5.20854E-05 | comp30877_c0_seq1 | / | |
| YWHAB_Q_Z | comp63845_c0_seq2 | 14-3-3 protein beta/theta/zeta | 25941 | 59.75452622 | 34171 | 81.54871003 | -0.448614059 | 0 | comp45161_c0_seq1 | comp17512_c0_seq2 | |
NF-κB signaling pathway regulation by M. bicoloratus parasitism
The NF-κB signaling pathway regulates gene expression via regulation of nuclear transcription factor. Comparison of the transcription data from the S and M pools indicates that all 18 elements of NF-κB signaling pathway existed in the hemocytes. Under M. bicoloratus parasitism, 1 gene (Spli-PLCG2) was expressed in the parasitized host hemocytes, and 5 genes (Spli-CSNK2A, MYD88, P50, P65 and XIAP) were down-regulated (Table 5). The qRT-PCR results indicate that the parasitism down-regulated two key molecules, the p50 (Relish) and p65 (Dorsal) subunits in the NF-κB signaling pathway, suggesting the disruption of the cell survival signaling pathway (Fig. 1).
Table 5. The differential expression of genes regulated by M. bicoloratus bracovirus in the host NF-κB signaling pathway.
| M/S | |||||||||||
| Gene family | A_ID | Function | read_M | RPKM_M | read_S | RPKM_S | log2(Fold_change)normalized | p-value | Result | S_ID | M-ID |
| CSNK2A | comp67611_c0_seq1 | casein kinase II subunitalpha | 1490 | 10.76489273 | 2927 | 21.90894546 | −1.025186101 | 1.5073E-115 | down | comp56988_c0_seq2 | comp10015_c0_seq2 |
| MYD88 | comp68137_c0_seq1 | myeloid differentiationprimary response proteinMyD88 | 577 | 6.026390147 | 1241 | 13.42853162 | −1.155935577 | 4.14962E-60 | down | comp76362_c0_seq1 | comp49638_c0_seq1 |
| P50 | comp97501_c11_seq1 | nuclear factor kappa-Bp105/100, Relish 1A | 756 | 8.18783011 | 1588 | 1.78E+01 | −1.121828459 | 4.83E-73 | down | comp57569_c0_seq1 | comp46759_c0_seq1 |
| P65 | comp89974_c0_seq4 | nuclear factorkappa-B/Rel, Dorsal 1A | 725 | 6.340266258 | 2092 | 1.90E+01 | −1.579905637 | 2.35E-161 | down | comp58671_c0_seq4 | comp19997_c0_seq5 |
| XIAP | comp66362_c0_seq1 | E3 ubiquitin-proteinligase XIAP, Bcl-2 | 3120 | 19.18253441 | 7814 | 49.77372136 | −1.375590912 | 0 | down | comp62965_c0_seq1 | comp17943_c0_seq1 |
| ATM | comp98156_c1_seq4 | ataxia telangiectasiamutated family protein | 659 | 2.224231496 | 928 | 3.245023613 | −0.544922026 | 2.64245E-13 | comp56187_c0_seq2 | comp18276_c0_seq1 | |
| BIRC2_3 | comp98152_c0_seq6 | baculoviral IAPrepeat-containing protein2/3 | 1252 | 4.419168216 | 1858 | 6.794495692 | −0.620591625 | 8.2147E-32 | comp57322_c0_seq1 | comp52055_c0_seq1 | |
| CSNK2B | comp102869_c0_seq1 | casein kinase II subunitbeta | 2230 | 21.59701753 | 3660 | 36.72360578 | −0.765875624 | 1.66408E-88 | comp55287_c0_seq1 | comp20218_c0_seq1 | |
| IKB | comp108698_c0_seq1 | inhibitor of nuclearfactor kappa-B, Cactus | 3403 | 15.84566756 | 3613 | 1.74E+01 | −0.137465789 | 0.000117519 | comp46039_c0_seq1 | comp19169_c0_seq1 | |
| IKKB | comp46046_c0_seq1 | inhibitor of nuclearfactor kappa-B kinasesubunit beta | 628 | 4.037631507 | 887 | 5.908346682 | −0.549245231 | 6.14389E-13 | comp46039_c0_seq1 | comp19169_c0_seq1 | |
| IRAK4 | comp50303_c0_seq1 | interleukin-1receptor-associatedkinase 4 | 486 | 4.085524166 | 723 | 6.296875769 | −0.624115019 | 3.09261E-13 | comp55403_c1_seq1 | comp50008_c0_seq1 | |
| MALT1 | comp86328_c0_seq1 | MALT1 | 110 | 1.662564556 | 120 | 1.879067547 | −0.176606568 | 0.386011433 | comp29501_c0_seq1 | comp69294_c0_seq1 | |
| MAP3K7 | comp97891_c0_seq2 | mitogen-activatedprotein kinase kinasekinase 7 | 541 | 3.421954775 | 876 | 5.740588546 | −0.746377962 | 3.47289E-21 | comp47143_c1_seq1 | comp18892_c0_seq2 | |
| MAP3K7IP1 | comp96428_c0_seq2 | TAK1-binding protein 1 | 226 | 2.447684663 | 387 | 4.342434795 | −0.82708648 | 9.53466E-12 | comp27058_c0_seq1 | comp60240_c0_seq1 | |
| PLCG1 | comp95371_c0_seq1 | phosphatidylinositolphospholipase C,gamma-1 | 155 | 1.337538268 | 289 | 2.583733252 | −0.949876963 | 3.71489E-11 | comp54883_c1_seq1 | comp81243_c0_seq1 | |
| PLCG2 | comp94580_c0_seq1 | phosphatidylinositol phospholipase C, gamma-2 | 110 | 1.770661702 | 159 | 2.651644792 | −0.582598928 | 0.00156832 | / | comp78811_c0_seq1 | |
| TRAF6 | comp91031_c0_seq1 | TNF receptor-associatedfactor 6 | 38 | 0.903867364 | 54 | 1.330730718 | −0.558035675 | 0.079258626 | comp52687_c2_seq1 | comp130761_c0_seq1 | |
| UBE2I | comp106025_c0_seq1 | ubiquitin-conjugatingenzyme E2 I | 1630 | 22.02296837 | 1997 | 27.95384991 | −0.344038054 | 2.46942E-12 | comp57828_c0_seq1 | comp16034_c0_seq1 | |
ATM/p53 signaling pathway regulation by M. bicoloratus parasitism
The ATM/p53 signaling pathway plays an important role in cell cycle control and apoptosis. In normal cells, the p53 protein level is low. DNA damage and stress signaling may trigger an increase of p53 protein levels, which has three major functions: cell cycle arrest, DNA repair and apoptosis. The cell cycle arrest prevents replication of proteins involved in DNA repair. Apoptosis avoids proliferation of cells containing abnormal DNA. p53 is a transcriptional activator that regulates the expression of MDM2. A comparison of the transcription data from the S and M pools indicate that all 21 elements of the ATM/p53 signaling pathway existed in the hemocytes. Under M. bicoloratus parasitism, 1 gene (Spli-SESN), was expressed in the parasitized host hemocytes, and 1 gene (CYC) was not expressed in the parasitized host hemocytes. Another 3 genes (Spli-PPM1D, PTEN, and TSC2) were down-regulated (Table 6). The qRT-PCR results indicate that the parasitism increased expression of a key molecule, p53, in the ATM/p53 signaling pathway (Fig. 1).
Table 6. The differential expression of genes regulated by M. bicoloratus bracovirus in the host ATM/p53 signaling pathway.
| M/S | |||||||||||
| Gene family | A_ID | Function | read_M | RPKM_M | read_S | RPKM_S | log2(Fold_change)normalized | p-value | Result | S_ID | M-ID |
| PPM1D | comp23378_c0_seq1 | protein phosphatase 1D | 436 | 5.195890813 | 968 | 11.95154904 | −1.201754598 | 4.38033E-50 | down | comp45971_c0_seq1 | comp9608_c0_seq1 |
| PTEN | comp97411_c0_seq4 | PTEN | 233 | 1.759294286 | 911 | 7.126499544 | −2.018196785 | 5.8123E-99 | down | comp59211_c2_seq7 | comp62639_c0_seq1 |
| TSC2 | comp93326_c0_seq1 | tuberous sclerosis 2 | 42 | 1.079341152 | 227 | 6.043807401 | −2.48530675 | 1.25293E-32 | down | comp31311_c0_seq1 | comp12675_c0_seq1 |
| ATM | comp98156_c1_seq4 | ataxia telangiectasia mutated family protein | 659 | 2.224231496 | 928 | 3.245023613 | −0.544922026 | 2.64245E-13 | comp56187_c0_seq2 | comp18276_c0_seq1 | |
| ATR | comp85208_c0_seq1 | serine/threonine-protein kinase ATR | 62 | 1.180743424 | 98 | 1.933593758 | −0.71158922 | 0.00305328 | comp53816_c1_seq1 | comp15900_c0_seq1 | |
| CCNB | comp86097_c0_seq2 | cyclin B | 984 | 4.996128296 | 1852 | 9.742149268 | −0.963429564 | 1.7509E-66 | comp57367_c0_seq1 | comp19132_c0_seq2 | |
| CCND2 | comp92629_c0_seq2 | cyclin D2 | 1830 | 9.174971528 | 2968 | 15.41675056 | −0.748723129 | 2.87139E-69 | comp55005_c1_seq1 | comp20586_c0_seq2 | |
| CCNE | comp86772_c0_seq2 | cyclin E | 48 | 0.642601979 | 99 | 1.373128968 | −1.095469805 | 1.62283E-05 | comp29156_c0_seq1 | comp130315_c0_seq1 | |
| CCNG2 | comp81700_c0_seq1 | cyclin G2 | 2216 | 12.41316031 | 3124 | 18.1300477 | −0.546512258 | 3.78105E-42 | comp58929_c0_seq2 | comp21305_c1_seq2 | |
| CDK1 | comp76441_c0_seq1 | cyclin-dependent kinase 1 | 1207 | 7.410567531 | 2282 | 14.51560537 | −0.969948801 | 1.51194E-82 | comp27910_c0_seq1 | comp16516_c0_seq1 | |
| CDK4 | comp93505_c0_seq2 | cyclin-dependent kinase 4 | 91 | 1.026690365 | 248 | 2.898845626 | −1.497477356 | 9.30914E-19 | comp46659_c0_seq1 | comp104770_c0_seq1 | |
| CHK2 | comp93714_c0_seq1 | serine/threonine-protein kinase Chk2 | 116 | 1.042448685 | 212 | 1.973821482 | −0.921015145 | 3.88281E-08 | comp29305_c0_seq1 | comp185379_c0_seq1 | |
| CYC | comp93023_c0_seq1 | cytochrome c | 1 | 0.030570159 | 77 | 2.438730115 | −6.317862227 | 1.87344E-19 | comp96131_c0_seq1 | / | |
| EI24 | comp94889_c0_seq2 | etoposide-induced 2.4 mRNA | 143 | 1.901385289 | 220 | 3.030624197 | −0.672564063 | 2.18683E-05 | comp87761_c0_seq1 | comp67505_c0_seq1 | |
| GADD45 | comp87685_c0_seq2 | growth arrest and DNA-damage-inducible protein | 933 | 10.69806017 | 999 | 11.86763536 | −0.149683283 | 0.029048024 | comp58271_c0_seq2 | comp19840_c0_seq1 | |
| P53 | comp63894_c0_seq1 | p53 | 1659 | 10.61581334 | 2957 | 1.96E+01 | −0.884896043 | 1.49E-91 | comp27951_c0_seq1 | comp20024_c0_seq1 | |
| RCHY1 | comp94630_c0_seq1 | RING finger and CHY zinc finger domain-containing protein 1 | 80 | 1.340540829 | 140 | 2.430487591 | −0.858430608 | 2.87028E-05 | comp30489_c0_seq1 | comp128683_c0_seq1 | |
| RFWD2 | comp93760_c0_seq1 | E3 ubiquitin-protein ligase RFWD2 | 122 | 1.853497561 | 146 | 2.298054677 | −0.310162907 | 0.094260381 | comp99768_c0_seq1 | comp10686_c1_seq1 | |
| RRM2 | comp100970_c0_seq1 | ribonucleoside-diphosphate reductase subunit M2 | 4699 | 44.24117718 | 6338 | 61.82281522 | −0.482749578 | 1.81544E-67 | comp61643_c0_seq1 | comp10097_c0_seq1 | |
| SESN | comp313998_c0_seq1 | sestrin | 12 | 0.967587612 | 0.5 | 0.041769028 | 4.533886815 | 0.000805877 | / | comp150130_c0_seq1 | |
| SIAH1 | comp38385_c0_seq1 | E3 ubiquitin-protein ligase SIAH1 | 886 | 4.980482149 | 1456 | 8.479580246 | −0.767707437 | 4.40084E-36 | comp189698_c0_seq1 | comp20596_c1_seq1 | |
Discussion
M. bicoloratus parasitism regulated host hemocyte apoptosis, resulting in DNA fragmentation. In this study, we examined the impacts of both the apoptotic caspase-dependent and -independent signaling pathways on the host hemocytes based on transcriptome data. Our results demonstrated that bracovirus proteins are expressed in the host hemocytes, suggesting their roles in DNA fragmentation by regulating key signaling pathways, resulting in the triggering of caspase-dependent and -independent pathways.
First, we found that M. bicoloratus parasitism regulated genes involved in forming the PTPC, which control mitochondrial apoptosis. Following M. bicoloratus parasitization, Spli-CypD was dramatically up-regulated (Table 3, Fig. 1). PTPC, which is a large multiprotein structure assembled at the contact sites between outer membrane (OM) and inner membrane (IM) of mitochondria, regulates MMP. PTPC activation provokes a sudden increase in the IM permeability to solutes of low molecular weight, causing the unregulated entry of water and osmotic swelling of the mitochondrial matrix. Numerous studies suggest that the PTPC is assembled by ANT (in the IM), VDAC (in the OM) and mitochondrial matrix protein cyclophilin D (Cyp D) [13]. According to our data, under M. bicoloratus parasitism, PTPC can form in the mitochondria of host hemocytes. Some DNA viral proteins may be direct inducers of MMP, and some may be indirect MMP facilitators, resulting in the activation of the mitochondrial apoptosis pathway [13]. This suggests an inducing condition of PTPC. M. bicoloratus parasitism may promote cell death via regulation of PTPC formation to release factors involved in DNA fragmentation from mitochondria into nuclei to cleave DNA.
PTPC formed suddenly during immunochallenge, AIF, EndoG, and Cyt c in the mitochondria were released from the inter-mitochondrial space into the cytoplasm. EndoG and AIF directly move into the nucleus to digest DNA [28], [29]. In mammals, the endonuclease DFF40 initiates DNA fragmentation. A recent report found that in Caenorhabditis elegans, there is an unexpected connection between Dicer and DNA degradation during cell death [30]. The Dicer-family RNase III enzymes include helicase, PAZ, RNaseIII, and dsRNA-binding domains [30]. CED-3 cleaves DCR-1, the C. elegans Dicer orthologue, as a candidate, at a specific position to yield a short isoform termed tDCR-1, which lacks the helicase and PAZ domain, and gains the capacity to cleave DNA into fragments [31]. Once DNA suffers double-strand breaks, the ATM signaling pathway activates and interacts with many different proteins to induce cell cycle arrest, increase DNA repair, and inhibit apoptosis, which involves the p53 signaling pathway, NF-κB signaling pathway and PI3K/Akt signaling pathway via the activation of IKKβ and p53 [32]. Typically, the activated ATM signaling pathway should inhibit host cell apoptosis for cell survival [33], [34].
At this point, we wish to examine how the parasitism inhibited the ATM-triggered DNA repair and cell survival signaling pathways. During DNA damage in the host hemocytes, ATM is expressed (Table 6). The ATM signaling pathway is responsible for DNA repair via activation of the related cell survival signaling pathway [35]. DNA damage may activate protein kinases, such as ATM, to phosphorylate p53 at one of these three residues, which thereby increases the p53 level. After the DNA damage is repaired, the ATM kinase is no longer active. p53 will be quickly dephosphorylated and destroyed by the accumulated MDM2 [36]. p53 is conserved across eukaryotic organisms, and the decrease of transcriptional levels of genes regulated by p53 leads to a subdued resistance to pathogens infections. In C. elegans, p53/CEP-1 are inhibited by the nucleolar protein NOL-6, a nucleolar RNA-associated protein, causing innate immune suppression [37].
It is well known that PI3K/Akt signaling pathway regulates cell survival and apoptosis. PI3K is composed of heterodimers of inhibitory adaptor/regulatory (p85) and a catalytic (p110) subunits. p85 binds and integrates signals from various cellular proteins, including transmembrane tyrosine kinase-linked receptors and intracellular proteins, providing an integration point for activation of p110. Akt, which contains a PH domain in the N-terminal region, is the primary downstream mediator of the effects of PI3K. The PH domain of Akt interacts with 3′-phosphoinositides, contributing to recruitment of Akt to the plasma membrane. Recruitment to the membrane results in a conformation change, contributing to exposure of two crucial phosphorylation sites, serine 473 and threonine 308, for activation. An unexpected finding is that p85 was not expressed under M. bicoloratus parasitism (Table 4). HSV-1, herpes simplex virus, induces the phosphorylation of Akt during infection of oral epithelial cells, leading to anti-apoptosis, and inhibition of HSV-1-induced PI3K activity increases DNA fragmentation [17]. Insect baculovirus AcMNPV activates PI3K/Akt signaling pathway antiapoptosis to replicate itself in the host cell via enhancing phosphorylation of Ser 473 of Akt [18]. In our laboratory, overexpression of the gap junction proteins Inx2 and Inx3 caused dramatic apoptosis in Sf9 and Spli221 cells but no phosphorylation of Akt in Hi5 cell lines, which reveals an anti-apoptosis function [26].
NF-κB signaling pathway regulates cell survival and apoptosis. In innate immunosuppression in invertebrates, it is well known that PDV protein vankyrins, which lack the phosphorylation and ubiquitination domains, function as IκB mimics via completion for the NF-κB site with IκB [38]. This results in retention of NF-κB in the cytoplasm, which inhibits immune gene expression for products such as antimicrobial peptides (AMPs) [39]. Three vankyrin genes were expressed in the host hemocytes (Table 1). NF-κB is constituted of p50 and p65 subunits. Normally, the p50/p65 complex is released from IκB and translocated to the nucleus to activate the transcription of genes involved in cell survival. During the immunochallenge, p50 and p65 were down-regulated by M. bicoloratus parasitism (Table 5, Fig. 1) suggesting that M. bicoloratus blocked the critical signaling pathway to promote cell apoptosis.
Ca2+ overload from the ER to mitochondria is required for initiation of programmed cell death. An unexpected result concerns Ca2+ loading between the endoplasmic reticulum and mitochondria. Previously, we proposed that innexin hemichannels on the ER can be Ca2+ channels, providing a pannexin 3-like function in the mammal to deliver Ca2+ [24], [31]. In such a case, inx genes should be up-regulated to produce more hemichannels, but 3 inx genes were been down-regulated, only inx4 was up-regulated (Table 2, Fig. 1). This suggests a disruption in hemichannel activation under M. bicoloratus parasitism.
In Table 1 and Fig. 1, we show six types of gene transcriptions in the parasitized host hemocytes related to the Ankyrin-repeat, PTP, C-type lectin, Ben domain, Mucin-like and EGF-like families. Recent research indicates that C-type lectin (SIGN-R1) enhances uptake and the processing of circulating apoptotic cells in the spleen [40]. CpBV-lectin encoded by C. plutellae bracovirus is secreted into plasma and binds to the surface of parasitoid eggs to induce host immunosuppression via inhibition of host hemocyte non-self recognition [41]. In our research system, considering the interaction between M. bicoloratus bracovirus proteins and apoptosis, whether MbBV-lectin provides a relative contribution to apoptotic cell clearance, similar to SIGN-R1, requires further examination. However, it is reasonable to indicate that most important genes displayed less transcription in the host hemocytes during apoptosis. The Ben domain-containing proteins are well known to be involved in the transcriptional repression through its interaction with histone deacetylase, and overexpression causes cell cycle arrest [42]. The ankyrin-repeat protein family acts as inhibitors of nuclear transcription factors via binding of NF-κB homodimers [39]. Protein tyrosine phosphatases are the largest family encoded by bracovirus, and PTPs are well known as a regulator of apoptosis in human [43], such as PTP-1B regulation of the PI3K/Akt cascade to influence the nuclear localization of FOXO1, a transcription factor that regulates the expression of several pro-apoptotic genes [44], and SHP-1 that disrupts anti-apoptotic pathways through the regulation of the p85 subunit of PI3K [45], and TC-PTP also regulates p53 expression during apoptosis [46]. PTP-H2 from MdBV is a functional tyrosine phosphatase [47] and induces apoptosis of Sf21 cells [48]. MbCrp (egf-like) disrupts the cytoskeleton of host hemocytes [49].
In conclusion, our findings demonstrated that M. bicoloratus parasitism could regulate critical signaling pathways of host hemocytes to promote apoptosis to suppress host cellular immunity. Bracovirus may regulate proteins to form a PTPC structure that altered mitochondrial permeability, resulting in the release of DNA fragmentation elements, causing DNA damage and keeping ATM expression. This might have implications for better understanding of the mechanism of innate immunosuppression via the apoptosis pathway. However, analysis of the bracovirus proteins regulation of the critical signaling pathway may involve three levels in the cell, as a ligand binding to receptor on the cell surface, as a mini-protein to compete with scaffold proteins, as a nuclear factor to promote gene expression, as a host translation inhibitory factor to inhibit host protein translation or utilization of an RNAi mechanism [50] to inhibit gene expression on the mRNA level. The proteins responsible for specific signaling molecules in host hemocytes remain to elucidated.
Materials and Methods
Insect rearing and experimental animals
The S. litura colony was reared on an artificial diet (formulated according to [51]) at 27±1°C, RH 60–80%, and under a 12∶12 h photoperiod regimen. The parasitoid M. bicoloratus colony was maintained on S. litura larvae reared in the laboratory according to established methods [52]. Adults were also provided with honey as a dietary supplement.
Isolation of hemocytes from larvae of S. litura
Hemocytes were collected 5 days post-parasitization from parasitized S. litura larvae (more than 1,000) (when immature parasitoids in the host developed to the second larvae [52], approximately 21% hemocytes underwent apoptosis [1]) and named ‘M’ (parasitized by M. bicoloratus) in this paper. The fourth instar S. litura larvae were used to collect hemocytes to serve as the control group, named ‘S’ (non-parasitized S. litura hemocytes) in this paper.
Total RNA extraction
Total RNA was isolated from hemocytes using an RNeasy Plus Universal Mini Kit (QIAGEN, Maryland, USA), which is specific for genome DNA elimination, according to the manufacturer’s instructions. The concentration of each RNA sample was determined by measuring OD at A260/A280 using the NanoDrop 2000 and running 1 x TBE agarose gel. High quality samples (with an A260/A280 ratio >2.0, A260/A230>2.0, concentration>500 ng/ul) showing 28S and 18S RNA bands clearly were stored at −80°C until use. RNA was prepared from at least two biological replicates and used for independent library preparations.
Transcription mRNA sequencing, assemble, gene predicted
Sequencing libraries were prepared using a RNA-Seq sample preparation kit from Illumina following the manufacturer’s instructions. The transcription sequences were sequenced using an Illumina Hiseq2000, and the total base number was more than 26.3 Gb per sample. There were two replications for the M1, M2, S1, and S2 pools. RNA-seq de novo assembly was performed using Trinity [53]. GetORF in EBOSS were used to find protein from contigs [54].
Gene Ontology (GO) and KEGG data
GO Slim test were assigned to the NR-annotated transcripts using a local Blast2GO pipeline b2g4pipe [55] with access to a local GO MySQL database (version of April 2013). The Kyoto Encyclopedia of Genes and Genomes (KEGG) was used for analysis of molecular networks [56].
Definition of up- or down-regulated genes based on fold change
Clean reads were mapping to assembled contigs, to get RPM value based on reads number [57]. Statistical analysis of data was performed using DESeq [58]. Transcript abundances for each gene were expressed as a weighted mean of counts from each replicate normalized to the overall library size (known as ‘base mean’). p-values (adjusted for false discovery rate) were generated for each gene in pair-wise comparisons between different conditions. In our analyses, we used an adjusted p-value of 0.001 as a criteria for identifying significant differences in gene expression.
Total RNA isolation, cDNA synthesis and qRT-PCR
Total RNA was isolated from hemocytes of parasitized S. litura larvae 5 days post-parasitization using RNAiso Plus (TaKaRa, Dalian, China), according to manufacturer’s instructions, including DNase treatment. The concentration and purity of each RNA sample was determined by measuring OD at A260/A280 using NanoDrop 2000. Samples with an A260/A280 ratio >2.0) were used to synthesize cDNA using Oligo d (T) 18 primers following manufacturer’s instruction (TaKaRa, Dalian, China). All cDNA samples were stored at −80°C for preservation. qRT-PCR was performed using SYBR PCR Kit (Takara, Dalin, China) with the ABI 7500 system following the cycling parameters: 50°C, 2 min; 95°C, 10 mim; 95°C, 5 sec, 60°C, 34 sec, 40 cycles; 95°C, 15 sec; 60°C, 1 min; 95°C, 30 sec; 60°C, 15 sec. The 2-ΔΔCT method was used to get the relative mRNA levels [59]. 18S rDNA gene was used as the housekeeping genes for normalization. Three replications have been carried out for per sample.
GenBank accession numbers
The whole RNA-Seq project was deposited into DDBJ/DRA/GenBank under the accession DRA001149.
Supporting Information
Completed ORF and short qRT-PCR products.
(TIF)
Sample information.
(XLS)
Sequencing output and quality.
(XLSX)
EST cluster contigs.
(XLSX)
Primers of completed ORF and short qRT-PCR.
(XLSX)
Acknowledgments
The authors are grateful to Pierre Golstein and Dr. Jonathan Ewbank (Aix-Marseille University, CIML, France) for valuable comments and suggestions on the manuscript.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All RNA-Seq projects are available from the DDBJ/DRA/GenBank database accession numbers DRA001149. 110967 data avaliablity.
Funding Statement
This study was supported in part by a grant (31260448;31060251) from National Natural Science Foundation of China, a grant (2013CB127600) from Major State Basic Research Development Program and a grant (2013FA003) from Yunnan Department of Science and Technology to KL, and a grant (31360454) from National Natural Science Foundation of China to ML. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Luo KJ, Pang Y (2006) Spodoptera litura multicapsid nucleopolyhedrovirus inhibits Microplitis bicoloratus polydnavirus-induced host granulocytes apoptosis. J Insect Physiol 52: 795–806. [DOI] [PubMed] [Google Scholar]
- 2. Amaya KE, Asgari S, Jung R, Hongskula M, Beckage NE (2005) Parasitization of Manduca sexta larvae by the parasitoid wasp Cotesia congregata induces an impaired host immune response. J Insect Physiol 51: 505–512. [DOI] [PubMed] [Google Scholar]
- 3. Strand MR, Pech LL (1995) Microplitis demolitor polydnavirus induces apoptosis of a specific haemocyte morphotype in Pseudoplusia includens. J Gen Virol 76: 283–291. [DOI] [PubMed] [Google Scholar]
- 4.Strand MR, Burke GR (2012) Polydnaviruses as symbionts and gene delivery systems. PLoS Pathogens 8: doi:10.1372/journal.ppat.1002757. [DOI] [PMC free article] [PubMed]
- 5. Desjardins CA, Gundersen-Rindal DE, Hostetler JB, Tallon LJ, Fadrosh DW, et al. (2008) Comparative genomics of mutualistic viruses of Glyptapanteles parasitic wasps. Genome Biol 9: R183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beck MH, Zhang S, Bitra K, Burke GR, Strand MR (2011) The encapsidated genome of Microplitis demolitor bracovirus integrates into the host Pseudoplusia includens. J Virol 85: 11685–11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu X, Zou H, Slaughter C, Wang X (1997) DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell 89: 175–184. [DOI] [PubMed] [Google Scholar]
- 8. Widlak P, Li P, Wang X, Garrard WT (2000) Cleavage preferences of the apoptotic endonuclease DFF40 (caspase-activated DNase or nuclease) on naked DNA and chromatin substrates. J Biol Chem 275: 8226–8232. [DOI] [PubMed] [Google Scholar]
- 9. Ninios YP, Sekeri-Pataryas KE, Sourlingas TG (2010) Histone H1 subtype preferences of DFF40 and possible nuclear localization of DFF40/45 in normal and trichostatin A-treated NB4 leukemic cells. Apoptosis 15: 128–138. [DOI] [PubMed] [Google Scholar]
- 10. Zhou P, Lugovskoy AA, McCarty JS, Li P, Wagner G (2001) Solution structure of DFF40 and DFF45 N-terminal domain complex and mutual chaperone activity of DFF40 and DFF45. Proc Natl Acad Sci U S A 98: 6051–6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gu J, Dong RP, Zhang C, McLaughlin DF, Wu MX, et al. (1999) Functional interaction of DFF35 and DFF45 with caspase-activated DNA fragmentation nuclease DFF40. J Biol Chem 274: 20759–20762. [DOI] [PubMed] [Google Scholar]
- 12. Cregan SP, Dawson VL, Slack RS (2004) Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene 23: 2785–2796. [DOI] [PubMed] [Google Scholar]
- 13. Galluzzi L, Brenner C, Morselli E, Touat Z, Kroemer G (2008) Viral control of mitochondrial apoptosis. PLoS Pathog 4: e1000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang J, Xu M (2002) Apoptotic DNA fragmentation and tissue homeostasis. Trends Cell Biol 12: 84–89. [DOI] [PubMed] [Google Scholar]
- 15. Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, et al. (2010) Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 467: 863–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stambolsky P, Weisz L, Shats I, Klein Y, Goldfinger N, et al. (2006) Regulation of AIF expression by p53. Cell Death Differ 13: 2140–2149. [DOI] [PubMed] [Google Scholar]
- 17. Hsu MJ, Wu CY, Chiang HH, Lai YL, Hung SL (2010) PI3K/Akt signaling mediated apoptosis blockage and viral gene expression in oral epithelial cells during herpes simplex virus infection. Virus Res 153: 36–43. [DOI] [PubMed] [Google Scholar]
- 18. Xiao W, Yang Y, Weng QB, Lin TH, Yuan MJ, et al. (2009) The role of the PI3K–Akt signal transduction pathway in Autographa californica multiple nucleopolyhedrovirus infection of Spodoptera frugiperda cells. Virology 391: 83–89. [DOI] [PubMed] [Google Scholar]
- 19.Luo KJ, Turnbull M (2008) Manipulations of Host Cell Physiology by Polyndaviruses. In Recent advances in insect physiology, toxicology and molecular biology (ed Nannan Liu): 93–115.
- 20. Espagne E, Dupuy C, Huguet E, Cattolico L, Provost B, et al. (2004) Genome sequence of a polydnavirus: insights into symbiotic virus evolution. Science 306: 286–289. [DOI] [PubMed] [Google Scholar]
- 21. Webb BA, Strand MR, Dickey SE, Beck MH, Hilgarth RS, et al. (2006) Polydnavirus genomes reflect their dual roles as mutualists and pathogens. Virology 347: 160–174. [DOI] [PubMed] [Google Scholar]
- 22. Desjardins CA, Gundersen-Rindal DE, Hostetler JB, Tallon LJ, Fuester RW, et al. (2007) Structure and evolution of a proviral locus of Glyptapanteles indiensis bracovirus. BMC Microbiol 7: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen YF, Gao F, Ye XQ, Wei SJ, Shi M, et al. (2011) Deep sequencing of Cotesia vestalis bracovirus reveals the complexity of a polydnavirus genome. Virology 414: 42–50. [DOI] [PubMed] [Google Scholar]
- 24. Ishikawa M, Iwamoto T, Nakamura T, Doyle A, Fukumoto S, et al. (2011) Pannexin 3 functions as an ER Ca(2+) channel, hemichannel, and gap junction to promote osteoblast differentiation. J Cell Biol 193: 1257–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luo K, Turnbull MW (2011) Characterization of nonjunctional hemichannels in caterpillar cells. J Insect Sci 11: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu T, Li M, Zhang Y, Pang Z, Xiao W, et al. (2013) A role for Innexin2 and Innexin3 proteins from Spodoptera litura in apoptosis. PLoS One 8: e70456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Demaurex N, Distelhorst C (2003) Cell biology. Apoptosis–the calcium connection. Science 300: 65–67. [DOI] [PubMed] [Google Scholar]
- 28. Loeffler M, Kroemer G (2000) The mitochondrion in cell death control: certainties and incognita. Exp Cell Res 256: 19–26. [DOI] [PubMed] [Google Scholar]
- 29. Bouaziz C, Sharaf El Dein O, El Golli E, Abid-Essefi S, Brenner C, et al. (2008) Different apoptotic pathways induced by zearalenone, T-2 toxin and ochratoxin A in human hepatoma cells. Toxicology 254: 19–28. [DOI] [PubMed] [Google Scholar]
- 30. Nakamura M, Ando R, Nakazawa T, Yudazono T, Tsutsumi N, et al. (2007) Dicer-related drh-3 gene functions in germ-line development by maintenance of chromosomal integrity in Caenorhabditis elegans. Genes Cells 12: 997–1010. [DOI] [PubMed] [Google Scholar]
- 31. Okamura K, Lai EC (2010) A deathly DNase activity for dicer. Dev Cell 18: 692–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bensimon A, Aebersold R, Shiloh Y (2011) Beyond ATM: The protein kinase landscape of the DNA damage response. FEBS Letters 585: 1625–1639. [DOI] [PubMed] [Google Scholar]
- 33. Hirao A, Kong YY, Matsuoka S, Wakeham A, Ruland J, et al. (2000) DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science 287: 1824–1827. [DOI] [PubMed] [Google Scholar]
- 34. Borner C (2003) The Bcl-2 protein family: sensors and checkpoints for life-or-death decisions. Mol Immunol 39: 615–647. [DOI] [PubMed] [Google Scholar]
- 35. Brodsky MH, Weinert BT, Tsang G, Rong YS, McGinnis NM, et al. (2004) Drosophila melanogaster MNK/Chk2 and p53 Regulate Multiple DNA Repair and Apoptotic Pathways following DNA Damage. Molecular and Cellular Biology 24: 1219–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schumacher B, Hanazawa M, Lee MH, Nayak S, Volkmann K, et al. (2005) Translational repression of C. elegans p53 by GLD-1 regulates DNA damage-induced apoptosis. Cell 120: 357–368. [DOI] [PubMed] [Google Scholar]
- 37. Fuhrman LE, Goel AK, Smith J, Shianna KV, Aballay A (2009) Nucleolar proteins suppress Caenorhabditis elegans innate immunity by inhibiting p53/CEP-1. PLoS Genet 5: e1000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thoetkiattikul H, Beck MH, Strand MR (2005) Inhibitor kappaB-like proteins from a polydnavirus inhibit NF-kappaB activation and suppress the insect immune response. Proc Natl Acad Sci U S A 102: 11426–11431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bitra K, Suderman RJ, Strand MR (2012) Polydnavirus Ank proteins bind NF-kappaB homodimers and inhibit processing of Relish. PLoS Pathog 8: e1002722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Prabagar MG, Do Y, Ryu S, Park JY, Choi HJ, et al. (2013) SIGN-R1, a C-type lectin, enhances apoptotic cell clearance through the complement deposition pathway by interacting with C1q in the spleen. Cell Death Differ 20: 535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee S, Nalini M, Kim Y (2008) A vial lectin encoded in Cotesia plutellae bracovirus and its immunosuppressive effect on host hemcytes. Comparative Biochemistry and Physiology, Part A: Molecular & Integrative Physiology 149: 351–261. [DOI] [PubMed] [Google Scholar]
- 42. Sathyan KM, Shen Z, Tripathi V, Prasanth KV, Prasanth SG (2011) A BEN-domain-containing protein associates with heterochromatin and represses transcription. J Cell Sci 124: 3149–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Halle M, Tremblay ML, Meng TC (2007) Protein tyrosine phosphatases: emerging regulators of apoptosis. Cell Cycle 6: 2773–2781. [DOI] [PubMed] [Google Scholar]
- 44. Gonzalez-Rodriguez A, Escribano O, Alba J, Rondinone CM, Benito M, et al. (2007) Levels of protein tyrosine phosphatase 1B determine susceptibility to apoptosis in serum-deprived hepatocytes. J Cell Physiol 212: 76–88. [DOI] [PubMed] [Google Scholar]
- 45. Yousefi S, Simon HU (2003) SHP-1: a regulator of neutrophil apoptosis. Semin Immunol 15: 195–199. [DOI] [PubMed] [Google Scholar]
- 46. Gupta S, Radha V, Sudhakar C, Swarup G (2002) A nuclear protein tyrosine phosphatase activates p53 and induces caspase-1-dependent apoptosis. FEBS Lett 532: 61–66. [DOI] [PubMed] [Google Scholar]
- 47. Eum JH, Bottjen RC, Pruijssers AJ, Clark KD, Strand MR (2010) Characterization and kinetic analysis of protein tyrosine phosphatase-H2 from Microplitis demolitor bracovirus. Insect Biochem Mol Biol 40: 690–698. [DOI] [PubMed] [Google Scholar]
- 48. Suderman RJ, Pruijssers AJ, Strand MR (2008) Protein tyrosine phosphatase-H2 from a polydnavirus induces apoptosis of insect cells. J Gen Virol 89: 1411–1420. [DOI] [PubMed] [Google Scholar]
- 49. Luo KJ, Pang Y (2006) Disruption effect of Microplitis bicoloratus polydnavirus EGF-like protein, MbCRP, on actin cytoskeleton in lepidopteran insect hemocytes. Acta Biochim Biophys Sin (Shanghai) 38: 577–585. [DOI] [PubMed] [Google Scholar]
- 50. Sharma S, Fitzgerald KA (2011) Innate immune sensing of DNA. PLoS Pathog 7: e1001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li G, Chen Q, Pang Y (1998) Studies of artificial diets for the beet armyworm, Spodoptera exigua. Acta Scientiarum Naturalium Unviersitatis Sunyatseni 4: 1–5. [Google Scholar]
- 52. Luo KJ, Trumble JT, Pang Y (2007) Development of Microplitis bicoloratus on Spodoptera litura and implications for biological control. Biocontrol 52: 309–321. [Google Scholar]
- 53. Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, et al. (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29: 644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rice P, Longden I, Bleasby A (2000) EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet 16: 276–277. [DOI] [PubMed] [Google Scholar]
- 55. Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, et al. (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21: 3674–3676. [DOI] [PubMed] [Google Scholar]
- 56. Kanehisa M, Goto S, Furumichi M, Tanabe M, Hirakawa M (2010) KEGG for representation and analysis of molecular networks involving diseases and drugs. Nucleic Acids Res 38: D355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5: 621–628. [DOI] [PubMed] [Google Scholar]
- 58. Anders S, Huber W (2010) Differential expression analysis for sequence count data. Genome Biol 11: R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Livak KJ, Schmittgen TD (2001) Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Completed ORF and short qRT-PCR products.
(TIF)
Sample information.
(XLS)
Sequencing output and quality.
(XLSX)
EST cluster contigs.
(XLSX)
Primers of completed ORF and short qRT-PCR.
(XLSX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All RNA-Seq projects are available from the DDBJ/DRA/GenBank database accession numbers DRA001149. 110967 data avaliablity.