Abstract
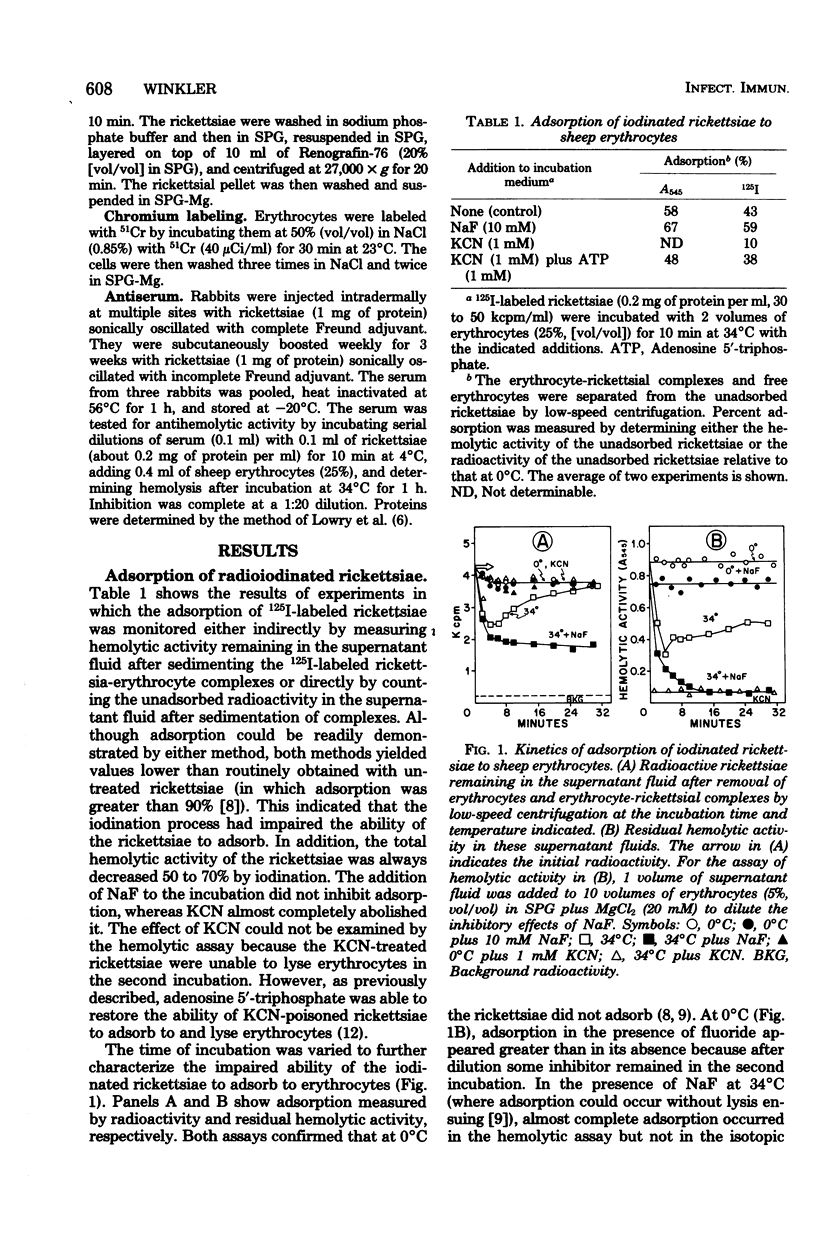
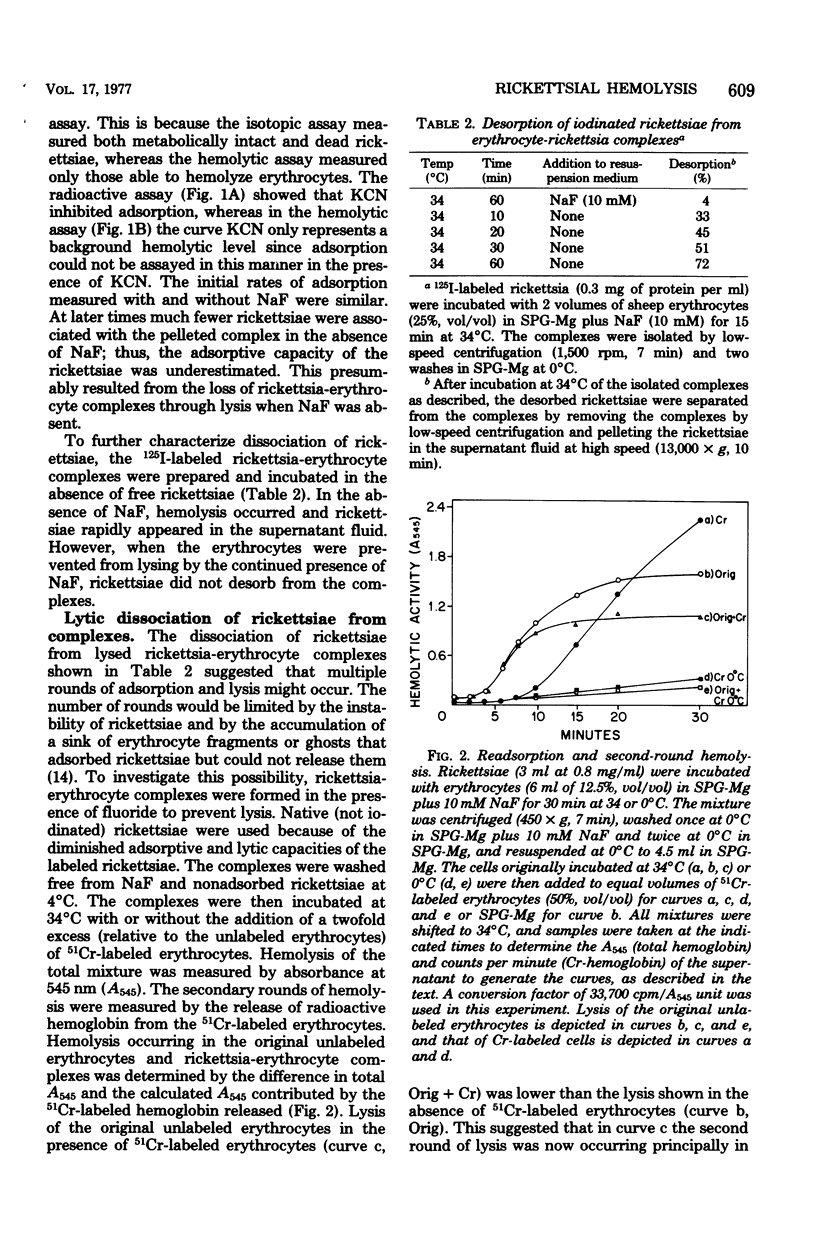
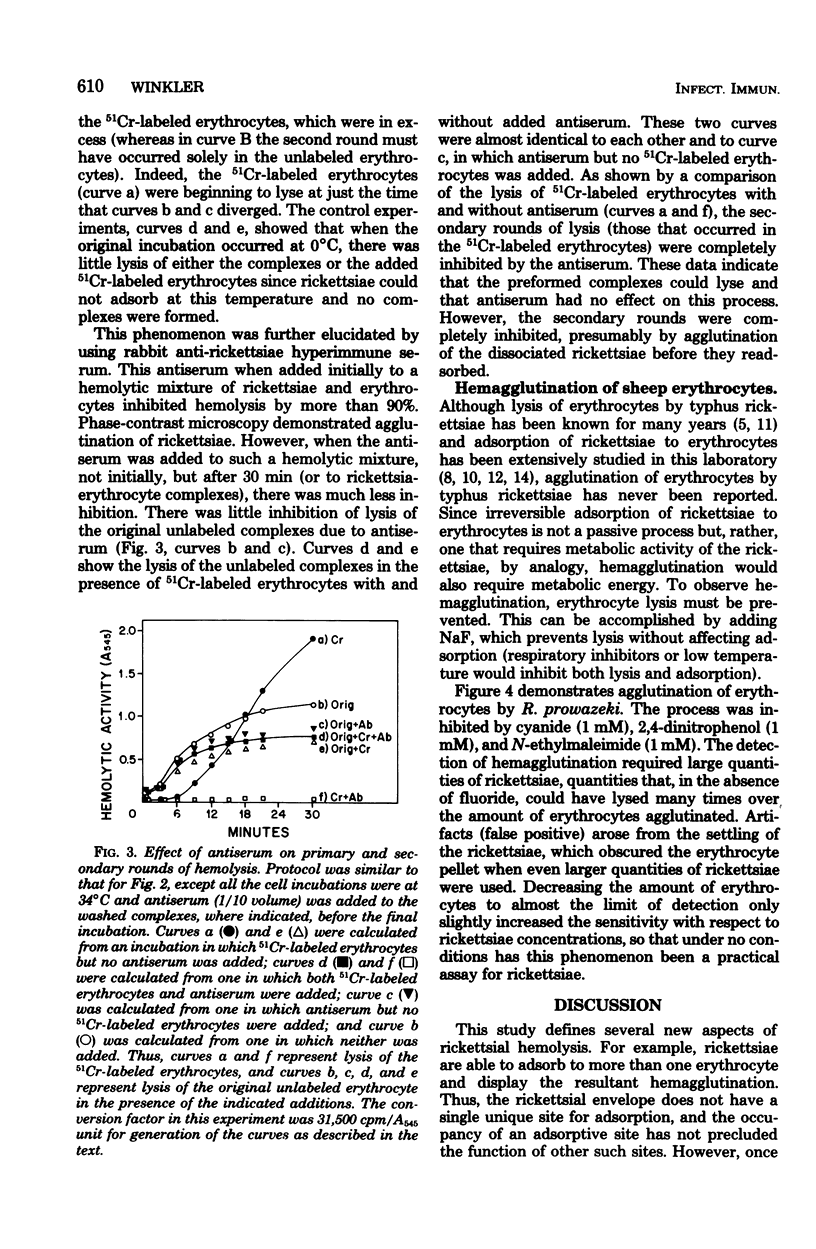
The energy-dependent adsorption of radioiodinated rickettsiae to sheep erythrocytes was demonstrated. The iodination procedure, however, decreased the hemolytic activity of the rickettsiae. No desorption of rickettsiae from isolated rickettsia-erythrocyte complexes (prevented from lysing by NaF) could be measured. On the other hand, rickettsiae desorbed from this complex during or after lysis and readsorbed and lysed other erythrocytes. Thus, the usual hemolytic assay measures multiple rounds of adsorption and lysis. Although lysis of the rickettsia-erythrocyte complex was insensitive to anti-rickettsial rabbit serum, adsorption and readsorption were completely inhibited by such antiserum. Hemagglutination of erythrocytes by rickettsiae was observed (in the presence of NaF to prevent lysis) and was sensitive to the same inhibitors as adsorption.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton L. P., Burgdorfer W. Fine structure of Rickettsia canada in tissues of Dermacentor andersoni Stiles. J Bacteriol. 1971 Mar;105(3):1149–1159. doi: 10.1128/jb.105.3.1149-1159.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton P. R., Stueckemann J., Paretsky D. Electron microscopy studies of the limiting layers of the rickettsia Coxiella burneti. J Bacteriol. 1975 Apr;122(1):316–324. doi: 10.1128/jb.122.1.316-324.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ramm L. E., Winkler H. H. Identification of cholesterol in the receptor site for rickettsiae on sheep erythrocyte membranes. Infect Immun. 1976 Jan;13(1):120–126. doi: 10.1128/iai.13.1.120-126.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramm L. E., Winkler H. H. Rickettsial hemolysis: adsorption of rickettsiae to erythrocytes. Infect Immun. 1973 Jan;7(1):93–99. doi: 10.1128/iai.7.1.93-99.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramm L. E., Winkler H. H. Rickettsial hemolysis: effect of metabolic inhibitors upon hemolysis and adsorption. Infect Immun. 1973 Apr;7(4):550–555. doi: 10.1128/iai.7.4.550-555.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNYDER J. C., BOVARNICK M. R., MILLER J. C., CHANG R. S. M. Observations on the hemolytic properties of typhus rickettsiae. J Bacteriol. 1954 Jun;67(6):724–730. doi: 10.1128/jb.67.6.724-730.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISSEMAN C. L., Jr, JACKSON E. B., HAHN F. E., LEY A. C., SMADEL J. E. Metabolic studies of rickettsiae. I. The effects of antimicrobial substances and enzyme inhibitors on the oxidation of glutamate by purified rickettsiae. J Immunol. 1951 Aug;67(2):123–136. [PubMed] [Google Scholar]
- Winkler H. H. Inhibitory and restorative effects of adenine nucleotides on rickettsial adsorption and hemolysis. Infect Immun. 1974 Jan;9(1):119–126. doi: 10.1128/iai.9.1.119-126.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Ramm L. E. Adsorption of typhus rickettsiae to ghosts of sheep erythrocytes. Infect Immun. 1975 Jun;11(6):1244–1251. doi: 10.1128/iai.11.6.1244-1251.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



