Abstract
Background
The incidence of diabetes is increasing. But the impact of diabetes and prediabetes on survival of patients with nasopharyngeal carcinoma (NPC) has received little evaluation.
Methods
In a cohort of 5,860 patients, we compared the disease specific survival (DSS), locoregional relapse-free survival (LRFS) and distant metastasis-free survival (DMFS) of patients with diabetes, prediabetes and normoglycemia defined by pretreatment fasting plasma glucose (FPG) using Kaplan–Meier method, log-rank test and Cox proportional hazards model.
Results
Comparing to normoglycemic patients, the diabetic and the prediabetic were generally older, fatter, had hypertension, heart diseases and hyperlipaemia and usually received radiotherapy alone. But both the diabetic and the prediabetic had similar DSS, LRFS and DMFS to normoglycemic patients, even adjusting for such important factors as age, gender, smoking, drinking, hypertension, heart diseases, body mass index, hyperlipaemia, titer of VCA-IgA and EA-IgA, pathology, T-stage, N-stage, chemotherapy and radiotherapy (P>0.05 for all). Additionally, the findings remained unchanged in sensitivity analysis by excluding patients with known diabetes history and in subgroups of the various factors.
Conclusions
The diabetic and prediabetic NPC patients had similar survival to normoglycemic NPC patients. These data, in the largest reported cohort, are the first to evaluate the association between diabetes, prediabetes and the survival in NPC. The findings are relevant to patient management and provided evidence of the effect on this disease exerted by comorbidities.
Introduction
The incidence of diabetes is increasing worldwide. Epidemiologic evidence suggests that people with diabetes are at an increased risk of cancers of liver, biliary tract, pancreatic, colorectal, as well as leukemia and melanoma [1]–[3]. Importantly, clinical studies observed a significantly poorer survival in several kinds of cancer patients with elevated blood glucose levels than those with normoglycemia, including extranodal natural killer (NK)/T-cell lymphoma (nasal type) [4], lung cancer [5], pancreatic cancer [6], breast cancer [7]–[9], acute lymphocytic leukemia [10] or colorectal cancer [11], [12].
However, no studies found significant association between diabetes and a higher risk of head and neck cancer [13], [14]. And Stott-Miller even observed weak inverse associations between type 2 diabetes and head and neck squamous cell cancer (HNSCC) [13], which was quite similar to the relation of diabetes with a lower risk of larynx cancer in the study by Atchison et al [3]. Additionally, nasopharyngeal carcinoma (NPC) is a non-lymphomatous, squamous-cell carcinoma that occurs in the epithelial lining of the nasopharynx. Of particular importance, it has distinct epidemiology, etiology [15], pathologic characteristics, clinical manifestation and treatment modes [16] compared to other cancers, including other types of head and neck cancer. Therefore, the finding that other types of cancer patients with diabetes had a lower survival than those without diabetes cannot be directly applied to the patients with NPC. To our best knowledge, only one study had reported the association between diabetes and the survival of NPC patients [17]. Unfortunately, only 37 patients with diabetes at diagnosis of NPC were enrolled into that study, and the influence of obesity, smoking, hypertension, heart diseases and hyperlipaemia were not taken into account.
In this largest study, with adjustment for various important covariates, we would provide convincing evidence of the association between diabetes, prediabetes defined by fasting plasma glucose (FPG) and the survival of NPC patients.
Materials and Methods
Patients
The study was reviewed and approved by the Human Ethics Approval Committee at Sun Yat-sen University Cancer Center. As a retrospective analysis of routine data, we therefore requested and were granted a waiver of individual informed consent from the ethics committee. Between January 2005 and December 2010, 6034 newly diagnosed, biopsy-proven, non-metastatic and hospitalized NPC patients who were at the age of 20 or>20 years were potentially eligible for this study. After excluding cases with missing data, we eventually enrolled 5860 patients who had complete pretreatment evaluation including history and physical examination, haematology and biochemistry profiles, fiberoptic nasopharyngoscopy with biopsy, magnetic resonance imaging (MRI) of the nasopharynx and neck, chest radiography, abdominal sonography and Technetium-99m-methylene diphosphonate (Tc-99-MDP) whole-body bone scan. The following pretreatment data were anonymously extracted and analyzed, including age, gender, smoking status, drinking status, hypertension history, heart diseases history, diabetes history, FPG, body mass index (BMI), total cholesterol (CHO), triglycerides (TG), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), titer of immunoglobulin A against viral capsid antigen (VCA-IgA) and early antigen (EA-IgA) and histological type.
All the included patients were restaged according to the seventh edition of the UICC/AJCC Staging System for NPC [18]. And all were treated by definitive intensity-modulated radiotherapy (IMRT) or conventional radiotherapy (CRT) with or without chemotherapy; further details of the radiation techniques had been described previously [19]. Institutional guidelines recommended no chemotherapy for patients in early stage, and induction, concurrent and adjuvant chemotherapy or combined treatment for those in locoregionally advanced stage. Induction or adjuvant chemotherapy consisted of cisplatin with 5-fluorouracil, cisplatin with taxane or triplet of cisplatin and 5-fluorouracil plus taxane every 3 weeks for two to three cycles. Concurrent chemotherapy consisted of cisplatin given on weeks 1, 4 and 7 of radiotherapy or cisplatin given weekly. Deviation from the institutional guidelines was result from organ dysfunction, treatment intolerance and/or patient refusal.
Patients were examined every 3–6 months during the first 3 years, with follow-up examinations every 6–12 months thereafter or until death. The assessment included history and physical examination and a series of conventional examination equipment at each follow-up visit, to detect the possible relapse or distant metastasis. Local relapses were confirmed by biopsy, MRI scan, or both. Regional relapses were diagnosed by clinical examination and an MRI scan of the neck and, in doubtful cases, by fine needle aspiration of the lymph nodes. Distant metastases were diagnosed by clinical symptoms, physical examinations, and imaging methods including chest radiography, bones scan, MRI, and abdominal sonography. Patients with relapse, distant metastasis or in persistent disease were delivered with salvage treatment including reirradiation, chemotherapy and surgery. Those patients without recent examination tests in the medical records were followed up by telephone call.
Diabetes and prediabetes assessment
According to the 2014 diagnosis and classification of diabetes mellitus by American Diabetes Association (ADA) [20], patients were classified into the normoglycemic (FPG <5.6 mmol/L), the prediabetic (FPG 5.6–6.9 mmol/L) and the diabetic (FPG ≥7.0 mmol/L) group based on FPG only. Patients with known diabetes at diagnosis were classified into the diabetic group and were excluded in sensitivity analysis.
End points
The primary end point was disease specific survival (DSS), defined as the time from treatment to death resulting from NPC or treatment complications [21]. Secondary end points were locoregional relapse-free survival (LRFS) and distant metastasis-free survival (DMFS), defined as the time from treatment to the first locoregional relapse and distant metastasis, respectively.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics version 20.0. Clinical parameters, including CHO, TG, HDL-C and LDL-C, were stratified into normal and abnormal group. Age and titer of VCA-IgA and EA-IgA were classified according to the criteria adopted in the previous studies [22], [23]. Comparisons of categorical characteristics were performed using χ2 statistic. Univariate stratified survival analyses were performed using Kaplan–Meier methods and log-rank test [24]. Multivariate analyses for hazard ratios (HRs) and 95% confidence intervals (CIs) were performed using the Cox proportional hazards model [25] with forward selection method for important covariates such as gender, smoking and BMI, and enter method for FPG. Two-sided P-values <0.05 were considered to be significant.
Results
Patients
The median follow-up duration (from the first day of therapy) was 55.6 months (range, 3.1–119.2 months), with 612 (10.4%) cases of lost-to-follow up. There were 569 (9.7%) cases of locoregional relapse, 762 (13.0%) cases of distant metastasis and 889 (15.2%) cases of disease-cause death, respectively. The 5-year survival rates were as follows: DSS 84.9%, LRFS 89.2% and DMFS 86.0%.
The clinicopathologic characteristics of the 5860 patients were shown in Table 1. Of the 121 patients who had known diabetes at diagnosis, 17 patients had a FPG level <5.6 mmol/L and 44 patients <7.0 mmol/L. Drinking, HDL-C level, titer of VCA-IgA and EA-IgA, histological type, T-stage, N-stage, clinical stage and radiotherapy did not significantly differ for group of the diabetic versus the normoglycemic or the prediabetic versus the normoglycemic. Comparing to normoglycemic patients, the diabetic and the prediabetic were generally older, fatter, had hypertension, heart diseases and higher levels of CHO, TG and LDL-C and usually received radiotherapy alone. In the diabetic group, we observed a significantly higher proportion of smoker.
Table 1. Clinicopathologic characteristics of 5860 patients with nasopharyngeal carcinoma.
| Characteristics | Normoglycemia | Diabetes | Prediabetes | P1 | P2 |
| No. (%) | No. (%) | No. (%) | |||
| Total | 3949 | 345 | 1566 | ||
| Age | <0.001 | <0.001 | |||
| 20–30 | 254 (6.4) | 3 (0.9) | 26 (1.7) | ||
| 30–40 | 1138 (28.8) | 26 (7.5) | 290 (18.5) | ||
| 40–50 | 1286 (32.6) | 104 (30.1) | 553 (35.3) | ||
| 50–60 | 875 (22.2) | 104 (30.1) | 445 (28.4) | ||
| ≥60 | 396 (10.0) | 108 (31.3) | 252 (16.1) | ||
| Gender | 0.072 | 0.133 | |||
| Male | 2916 (73.8) | 270 (78.3) | 1187 (75.8) | ||
| Female | 1033 (26.2) | 75 (21.7) | 379 (24.2) | ||
| Smoking | 0.004 | 0.862 | |||
| Yes | 1677 (42.5) | 174 (50.4) | 661 (42.2) | ||
| No | 2272 (57.5) | 171 (49.6) | 905 (57.8) | ||
| Drinking | 0.495 | 0.107 | |||
| Yes | 477 (12.1) | 46 (13.3) | 165 (10.5) | ||
| No | 3472 (87.9) | 299 (86.7) | 1401 (89.5) | ||
| Hypertension | <0.001 | 0.012 | |||
| Yes | 169 (4.3) | 67 (19.4) | 92 (5.9) | ||
| No | 3780 (95.7) | 278 (80.6) | 1474 (94.1) | ||
| Heart disease | <0.001 | <0.001 | |||
| Yes | 25 (0.6) | 46 (13.3) | 28 (1.8) | ||
| No | 3924 (99.4) | 299 (86.7) | 1538 (98.2) | ||
| BMI (kg/m2) § | <0.001 | <0.001 | |||
| <18.5 | 346 (8.8) | 11 (3.2) | 85 (5.4) | ||
| 18.5–22.9 | 1819 (46.1) | 85 (24.6) | 562 (35.9) | ||
| 22.9–27.5 | 1505 (38.1) | 194 (56.2) | 744 (47.5) | ||
| ≥ 27.5 | 279 (7.1) | 55 (15.9) | 175 (11.2) | ||
| CHO (mmol/L) ¶ | <0.001 | <0.001 | |||
| ≤6.47 | 3629 (91.9) | 296 (85.8) | 1367 (87.3) | ||
| >6.47 | 320 (8.1) | 49 (14.2) | 199 (12.7) | ||
| TG (mmol/L) ¶ | <0.001 | 0.003 | |||
| ≤1.7 | 2826 (71.6) | 190 (55.1) | 1058 (67.6) | ||
| >1.7 | 1123 (28.4) | 155 (44.9) | 508 (32.4) | ||
| HDL-C (mmol/L) ¶ | 0.954 | 0.402 | |||
| ≥0.78 | 3848 (97.4) | 336 (97.4) | 1532 (97.8) | ||
| <0.78 | 101 (2.6) | 9 (2.6) | 34 (2.2) | ||
| LDL-C (mmol/L) ¶ | 0.001 | <0.001 | |||
| ≤3.4 | 2416 (61.2) | 179 (51.9) | 839 (53.6) | ||
| >3.4 | 1533 (38.8) | 166 (48.1) | 727 (46.4) | ||
| VCA-IgA # | 0.085 | 0.334 | |||
| ≤80 | 988 (25.0) | 73 (21.2) | 418 (26.7) | ||
| 80–320 | 2024 (51.3) | 198 (57.4) | 771 (49.2) | ||
| >320 | 937 (23.7) | 74 (21.4) | 377 (24.1) | ||
| EA-IgA # | 0.076 | 0.293 | |||
| ≤10 | 1755 (44.4) | 136 (39.4) | 673 (43.0) | ||
| 10–40 | 1260 (31.9) | 130 (37.7) | 534 (34.1) | ||
| >40 | 934 (23.7) | 79 (22.9) | 359 (22.9) | ||
| Histological type * | 0.598 | 0.097 | |||
| WHO I+II | 279 (7.1) | 27 (7.8) | 131 (8.4) | ||
| WHO III | 3670 (92.9) | 318 (92.2) | 1435 (91.6) | ||
| T-stage | 0.804 | 0.070 | |||
| T1+T2 | 1450 | 129 | 616 | ||
| T3+T4 | 2499 | 216 | 950 | ||
| N-stage | 0.246 | 0.124 | |||
| N0+N1 | 3017 | 254 | 1205 | ||
| N2+N3 | 932 | 91 | 333 | ||
| Clinical stage | 0.139 | 0.221 | |||
| I | 223 (5.6) | 12 (3.5) | 102 (6.5) | ||
| II | 918 (23.2) | 81 (23.5) | 395 (25.2) | ||
| III | 1569 (39.7) | 146 (42.3) | 618 (39.5) | ||
| IVa | 1047 (26.5) | 82 (23.8) | 382 (24.4) | ||
| IVb | 192 (4.9) | 24 (7.0) | 69 (4.4) | ||
| Chemotherapy | 0.003 | 0.005 | |||
| No | 725 (18.4) | 86 (24.9) | 339 (21.6) | ||
| Yes | 3224 (81.6) | 259 (75.1) | 1227 (78.4) | ||
| Radiotherapy | 0.632 | 0.084 | |||
| IMRT | 1161 (29.4) | 109 (31.6) | 456 (29.1) | ||
| 3DCRT | 59 (1.5) | 4 (1.2) | 37 (2.4) | ||
| 2DCRT | 2729 (69.1) | 232 (67.2) | 1073 (68.5) |
Note: BMI = body mass index, CHO = total cholesterol, TG = triglycerides, HDL-C = high density lipoprotein cholesterol, LDL-C = low density lipoprotein cholesterol, VCA = viral capsid antigen, EA = early antigen, IgA = immunoglobulin A, IMRT = intensity-modulated radiotherapy, 3DCRT = three-dimensional conformal radiotherapy, 2DCRT = two-dimensional conventional radiotherapy.
P1 – diabetes vs normoglycemia; P2 – prediabetes vs normoglycemia.
According to the World Health Organization classifications for Asian populations.
Stratified into normal and abnormal group.
In accordance with the criteria adopted in the previous study.
*Based on the criteria of WHO histological type (1991): I - Squamous-cell carcinomas, II - Differentiated non-keratinising carcinoma, III - Undifferentiated non-keratinising carcinoma.
Diabetes, prediabetes and survival
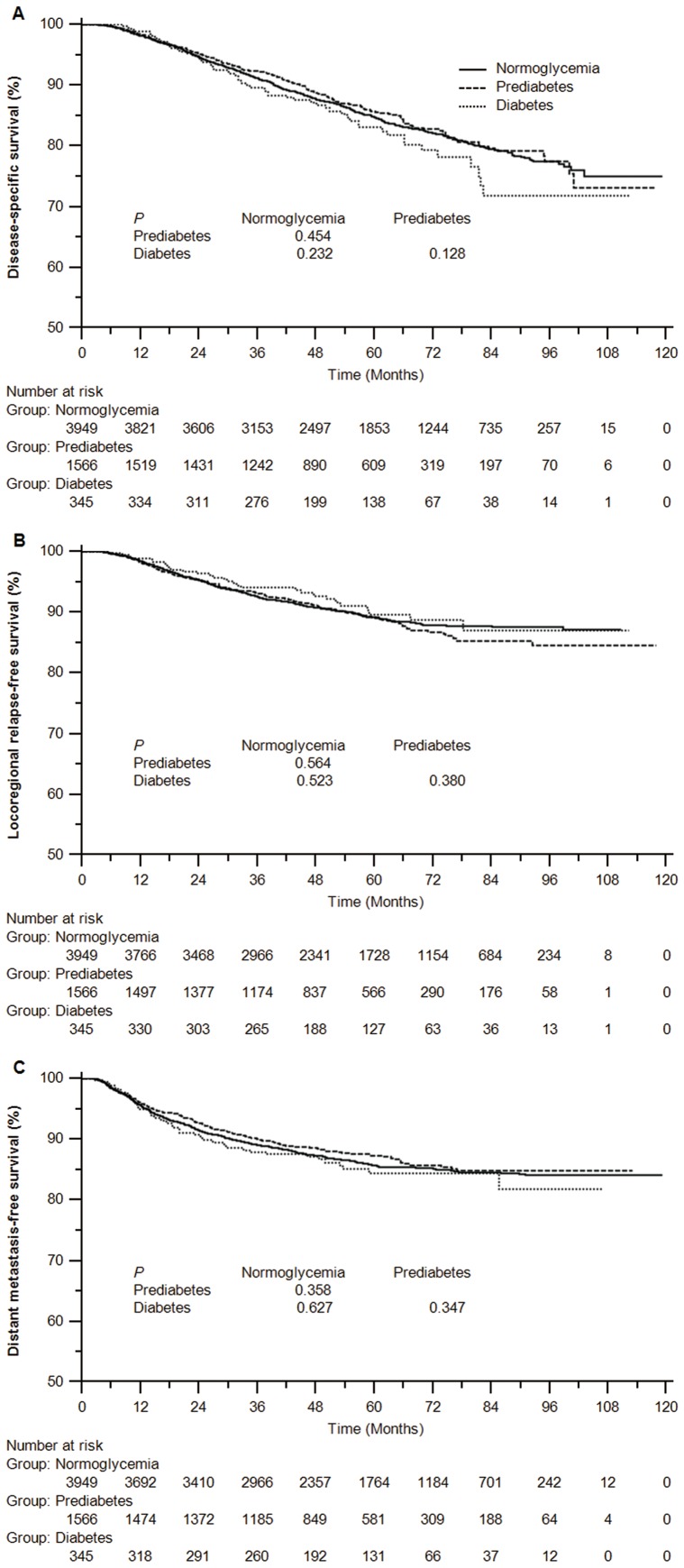
In contrast with normoglycemic patients, Kaplan-Meier curves displayed the non-significant differences of DSS, LRFS and DMFS rates for patients with diabetes or prediabetes. (Figure 1)
Figure 1. Kaplan-Meier curves of disease specific survival (A), locoregional relapse-free survival (B) and distant metastasis-free survival (C) for patients with normoglycemia, prediabetes and diabetes mellitus defined by fasting plasma glucose.
Since diabetes or prediabetes was usually accompanied with age, obesity, smoking, hypertension, heart diseases and hyperlipaemia, the actual survival differences between diabetic, prediabetic and normoglycemic NPC patients cannot be disclosed exactly without excluding the influence of these covariates. However, after adjusting for age, gender, smoking, drinking, hypertension, heart diseases, BMI, levels of CHO, TG, HDL-C and LDL-C, titer of VCA-IgA and EA-IgA, histological type, T-stage, N-stage, chemotherapy and radiotherapy, we still found no significant differences of DSS, LRFS and DMFS when comparing patients with diabetes to those with normoglycemia (P = 0.894 for DSS, P = 0.351 for LRFS and P = 0.530 for DMFS) and comparing patients with prediabetes to those with normoglycemia (P = 0.335 for DSS, P = 0.613 for LRFS and P = 0.671 for DMFS). (Table 2)
Table 2. Multivariate analysis for disease specific survival (DSS), locoregional relapse-free survival (LRFS) and distant metastasis-free survival (DMFS) *.
| Factor | DSS | LRFS | DMFS | ||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Normoglycemia | 1.00 | 1.00 | 1.00 | ||||||
| Diabetes | 0.98 | 0.75–1.29 | 0.894 | 0.83 | 0.57–1.22 | 0.351 | 1.10 | 0.81–1.49 | 0.530 |
| Prediabetes | 0.93 | 0.79–1.08 | 0.335 | 1.05 | 0.87–1.27 | 0.613 | 0.96 | 0.82–1.14 | 0.671 |
| Gender | 0.64 | 0.54–0.76 | <0.001 | 0.65 | 0.53–0.80 | <0.001 | 0.64 | 0.54–0.77 | <0.001 |
| Age | 1.47 | 1.38–1.57 | <0.001 | 1.09 | 1.01–1.18 | 0.030 | 1.09 | 1.02–1.16 | 0.017 |
| T-stage | 1.53 | 1.42–1.64 | <0.001 | 1.28 | 1.18–1.39 | <0.001 | 1.45 | 1.35–1.57 | <0.001 |
| N-stage | 1.61 | 1.51–1.72 | <0.001 | 1.27 | 1.16–1.39 | <0.001 | 1.72 | 1.61–1.85 | <0.001 |
| BMI | 0.81 | 0.74–0.88 | <0.001 | NS | 0.84 | 0.76–0.92 | <0.001 |
NOTE: HR = hazard ratio, CI = confidence interval, BMI = body mass index.
*Adjusting for age, gender, smoking, drinking, hypertension, heart diseases, BMI, levels of total cholesterol, triglycerides, high density lipoprotein cholesterol and low density lipoprotein cholesterol, titer of VCA-IgA and EA-IgA, histological type, T-stage, N-stage, chemotherapy and radiotherapy with forward selection method.
To fully eliminate the effect of the discrepancies as a result of the normal or prediabetic FPG level for the 121 patients with known diabetes history, we did sensitivity analysis by excluding them. Consequently, the above results remained unchanged, as shown in Table S1.
In addition, we performed second analyses stratified by several important subgroups. (Table 3) Resultantly, multivariate analyses indicated that neither diabetes nor prediabetes was significantly associated with DSS in subgroups of age (≤45 and>45 y), gender, smoking, drinking, hypertension, heart diseases, BMI (<25 and ≥25 kg/m2), CHO, TG, HDL-C, LDL-C, T-stage (T1+T2 and T3+T4), N-stage (N0+N1 and N2+N3), clinical stage (I+II and III+IV), chemotherapy and radiotherapy (2DCRT and IMRT + 3DCRT).
Table 3. Subgroup analysis of disease specific survival by patients' characteristics*.
| Factor | Diabetes | Prediabetes | ||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (year) § | ||||||
| ≤45 | 1.51 | 0.86–2.64 | 0.155 | 1.06 | 0.80–1.40 | 0.691 |
| >45 | 0.98 | 0.72–1.33 | 0.877 | 0.88 | 0.73–1.06 | 0.184 |
| Gender | ||||||
| Male | 1.13 | 0.85–1.51 | 0.388 | 0.90 | 0.75–1.07 | 0.215 |
| Female | 0.83 | 0.57–1.22 | 0.351 | 1.07 | 0.75–1.54 | 0.701 |
| Smoking | ||||||
| Yes | 0.98 | 0.69–1.39 | 0.893 | 0.93 | 0.75–1.16 | 0.535 |
| No | 0.96 | −.62–1.48 | 0.835 | 0.90 | 0.71–1.13 | 0.364 |
| Drinking | ||||||
| Yes | 0.97 | 0.49–1.94 | 0.934 | 1.19 | 0.81–1.76 | 0.377 |
| No | 1.00 | 0.74–1.34 | 0.986 | 0.89 | 0.75–1.06 | 0.180 |
| Hypertension | ||||||
| Yes | 0.73 | 0.34–1.58 | 0.428 | 0.93 | 0.48–1.80 | 0.824 |
| No | 1.02 | 0.76–1.38 | 0.872 | 0.94 | 0.79–1.10 | 0.415 |
| Heart diseases | ||||||
| Yes | 0.49 | 0.20–1.20 | 0.118 | 0.41 | 0.14–1.22 | 0.109 |
| No | 0.99 | 0.74–1.33 | 0.937 | 0.94 | 0.80–1.10 | 0.422 |
| BMI (kg/m2) § | ||||||
| <25 | 0.91 | 0.64–1.29 | 0.600 | 0.87 | 0.72–1.05 | 0.152 |
| ≥25 | 1.03 | 0.66–1.62 | 0.884 | 0.98 | 0.74–1.32 | 0.914 |
| CHO (mmol/L) | ||||||
| ≤6.47 | 0.97 | 0.72–1.30 | 0.833 | 0.93 | .79–1.10 | 0.394 |
| >6.47 | 0.92 | 0.43–2.00 | 0.837 | 0.78 | 0.48–1.26 | 0.309 |
| TG (mmol/L) | ||||||
| ≤1.7 | 1.23 | 0.88–1.72 | 0.230 | 0.96 | 0.79–1.16 | 0.669 |
| >1.7 | 0.66 | 0.41–1.06 | 0.084 | 0.87 | 0.65–1.15 | 0.324 |
| HDL-C mmol/L) | ||||||
| ≥0.78 | 0.93 | 0.70–1.23 | 0.595 | 0.91 | 0.78–1.07 | 0.912 |
| <0.78 | 1.39 | 0.44–4.36 | 0.577 | 1.30 | 0.59–2.83 | 0.518 |
| LDL-C (mmol/L) | ||||||
| ≤3.4 | 0.92 | 0.62–1.36 | 0.666 | 1.03 | 0.84–1.28 | 0.759 |
| >3.4 | 1.03 | 0.71–1.51 | 0.873 | 0.83 | 0.65–1.05 | 0.114 |
| T-stage | ||||||
| T1+T2 | 1.10 | 0.65–1.84 | 0.730 | 0.80 | 0.58–1.13 | 0.203 |
| T3+T4 | 0.89 | 0.64–1.22 | 0.464 | 0.95 | 0.79–1.13 | 0.534 |
| N-stage | ||||||
| N0+N1 | 0.72 | 0.49–1.06 | 0.093 | 0.89 | 0.73–1.09 | 0.269 |
| N2+N3 | 1.08 | 0.72–1.63 | 0.699 | 0.91 | 0.70–1.17 | 0.465 |
| Clinical stage | ||||||
| I+II | 1.07 | 0.55–2.09 | 0.846 | 0.70 | 0.46–1.08 | 0.106 |
| III+ IV | 0.89 | 0.66–1.20 | 0.440 | 0.95 | 0.80–1.12 | 0.512 |
| Chemotherapy | ||||||
| Yes | 1.04 | 0.77–1.42 | 0.784 | 0.98 | 0.83–1.16 | 0.811 |
| No | 0.63 | 0.34–1.14 | 0.124 | 0.68 | 0.45–1.02 | 0.060 |
| Radiotherapy | ||||||
| 2DCRT | 1.11 | 0.82–1.51 | 0.498 | 0.90 | 0.75–1.08 | 0.256 |
| IMRT + 3DCRT | 0.68 | 0.37–1.28 | 0.231 | 1.03 | 0.76–1.40 | 0.860 |
NOTE: HR = hazard ratio, CI = confidence interval, BMI = body mass index, CHO = total cholesterol, TG = triglycerides, HDL-C = high density lipoprotein cholesterol, LDL-C = low density lipoprotein cholesterol, VCA = viral capsid antigen, EA = early antigen, IgA = immunoglobulin A, 2DCRT = two-dimensional conventional radiotherapy, IMRT = intensity-modulated radiotherapy, 3DCRT = three-dimensional conformal radiotherapy.
*Adjusting for age, gender, smoking, drinking, hypertension, heart diseases, BMI, levels of total cholesterol, triglycerides, high density lipoprotein cholesterol and low density lipoprotein cholesterol, titer of VCA-IgA and EA-IgA, histological type, T-stage, N-stage, chemotherapy and radiotherapy.
According to the stratification criteria for the risk factor of age and BMI mentioned in the 2014 diagnosis and classification of diabetes mellitus by American Diabetes Association (ADA).
Discussion
Based on 5860 patients and thoroughly adjusting for the influence of age, obesity, smoking, drinking, hypertension, heart diseases, hyperlipaemia, tumor stage and treatment modality, our study concluded that the diabetic and prediabetic NPC patients had similar survival to the normoglycemic NPC patients.
In contrast to our present study, Liu et al [17] detected a lower disease-free survival in patients with diabetes (n = 37) than those without diabetes (n = 897); nevertheless, this study did not account for all the various potential confounders, such as obesity, smoking, hypertension, heart diseases and hyperlipaemia. Similar studies also found the significant association between hyperglycemia and the survival of patients with extranodal natural killer (NK)/T-cell lymphoma (nasal type) [4] or acute lymphocytic leukemia [10], and between DM and the survival of patients with lung cancer [5], pancreatic cancer [6], breast cancer [7], [8] or colorectal cancer [11], [12]. But this is hardly convincing as the small sample size of these studies [4]–[6], [10] is very likely to cause the skewed results.
Actually, Zhou et al [26] recruited 26,460 men and 18,195 women aged 25–90 years from 17 European population-based or occupational cohorts and found that diabetes was not significantly associated with the mortality of male patients with cancers of pancreas, bronchus/lung, prostate and kidney/bladder, or the mortality of female patients with cancers of stomach or colon – rectum, bronchus/lung, breast and kidney/bladder. Also, Höfner et al [27] enrolled 1140 patients with localized renal cell carcinoma and revealed that type 2 diabetes at the time of surgery had no significant impact on cancer-specific and recurrence-free survival. In the study by Kiderlen et al [8], relapse-free period was better in elderly breast cancer patients with diabetes compared with patients without diabetes if taking competing mortality into account; patients with diabetes without other comorbidity had a similar overall survival as patients without any comorbidity. Additionally, the ORIGIN trial found no evidence for increased cancer incidence or mortality in patients with impaired glucose metabolism or early type 2 diabetes [28]. Overall, despite of absence of straight evidence regarding the impact of FPG on survival of other types of head and neck cancer, our findings of neutral impact in NPC patients were not unreasonable. Finally, the non-significant association between diabetes and risk of head and neck cancer from the prior pooled analysis [13] and meta-analysis [14], along with the inverse relationship between diabetes and development of larynx cancer in another cohort study [3], at least indirectly suggested no impact of FPG on the survival of NPC.
Previous research showed that cancer patients with diabetes may have increased tumor cell proliferation and metastatic capacity as a consequence of the high insulin or increased free insulin-like growth factor (IGF-1) levels in hyperinsulinemic states [29]. But this was denied by a recent study [30], in which incident insulin users, exposure to insulin and glargine insulin in particular was not associated with any deleterious effect on overall and site specific cancer mortality of lung, colorectal, female genital, liver and urinary tract cancer. What is more, Margel et al [31] discovered that increased cumulative duration of metformin exposure after prostate cancer diagnosis was associated with decreases in both all-cause and cancer-specific mortality among diabetic men. But this similar protective effect from metformin exposure was not supported in NPC patients with diabetes in our study.
Certainly, the pooled analysis by Stott-Miller et al [13] showed a modest association between diabetes and the incidence of head and neck cancer among never smokers. And Atchison et al [3] assumed that smoking and BMI were two important factors potentially contributed to the inverse relationship between diabetes and development of larynx cancer. As observed in our study, a higher percentage of diabetic patients were indeed smokers and overweight or obese. Additionally, according to recent studies, NPC patients with smoking history had poorer survival [32] whereas those with higher BMI had favorable survival [33]. Therefore, the contradictory effect of smoking and BMI maybe just right principally confounded the impact of FPG on survival of NPC. However, in the stratum of patients who had normal BMI and never smoked (n = 1461), multivariate analysis showed that both diabetic and prediabetic patients had similar DSS, LRFS and DMFS rates to euglycemic patients (P = 0.298, P = 0.613 and P = 0.433 for DSS; P = 0.554, P = 0.315 and P = 0.693 for LRFS; P = 0.434, P = 0.747 and P = 0.458 for DMFS, respectively).
Considering the influence of mortality from such hyperglycemia-related complications as hypertension, heart diseases and various hyperlipaemia, we set DSS as the primary endpoint, adjusted for these covariates and conducted subgroup analyses; finally, diabetes or prediabetes still had null influence to NPC survival. Moreover, there were no significant differences with respect to the distribution of tumor stage and radiotherapy, and diabetes or prediabetes remained to be irrelevant to the survival in these subgroups. Particularly, patients with diabetes or prediabetes usually received radiotherapy alone with a higher percentage than that of patients with normoglycemia. But this rarely affected the DSS of the diabetic or prediabetic subgroups.
To our knowledge, this is the largest and most detailed study to evaluate the relation between diabetes, prediabetes before treatment and the survival of NPC patients. Clinicopathologic and survival data were verified by review of individual patient records. Our findings were derived from complete adjustment and particular stratification of various important covariates. The conclusions are relevant to patient management and provided evidence of the effect on the disease of NPC exerted by comorbidities. Indeed, albeit that FPG is the primary routine test in clinic, further study with data on standard 2-hour oral glucose tolerance test (OGTT) and hemoglobin A1c (HbA1c) is warranted. Apart from that, the effect of glycemic control during radiotherapy and chemotherapy on the survival is essential to be studied.
Supporting Information
Sensitivity analysis by excluding the 121 patients with known diabetes history *.
(DOCX)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, et al. (2010) Diabetes and cancer: a consensus report. Diabetes Care 33: 1674–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bosetti C, Rosato V, Polesel J, Levi F, Talamini R, et al. (2012) Diabetes mellitus and cancer risk in a network of case-control studies. Nutr Cancer 64: 643–651. [DOI] [PubMed] [Google Scholar]
- 3. Atchison EA, Gridley G, Carreon JD, Leitzmann MF, McGlynn KA (2011) Risk of cancer in a large cohort of U.S. veterans with diabetes. Int J Cancer 128: 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cai Q, Luo X, Liang Y, Rao H, Fang X, et al. (2013) Fasting blood glucose is a novel prognostic indicator for extranodal natural killer/T-cell lymphoma, nasal type. Br J Cancer 108: 380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luo J, Chen YJ, Chang LJ (2012) Fasting blood glucose level and prognosis in non-small cell lung cancer (NSCLC) patients. Lung Cancer 76: 242–247. [DOI] [PubMed] [Google Scholar]
- 6. Chu CK, Mazo AE, Goodman M, Egnatashvili V, Sarmiento JM, et al. (2010) Preoperative diabetes mellitus and long-term survival after resection of pancreatic adenocarcinoma. Ann Surg Oncol 17: 502–513. [DOI] [PubMed] [Google Scholar]
- 7. Erickson K, Patterson RE, Flatt SW, Natarajan L, Parker BA, et al. (2011) Clinically defined type 2 diabetes mellitus and prognosis in early-stage breast cancer. J Clin Oncol 29: 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kiderlen M, de Glas NA, Bastiaannet E, Engels CC, van de Water W, et al. (2013) Diabetes in relation to breast cancer relapse and all-cause mortality in elderly breast cancer patients: a FOCUS study analysis. Ann Oncol 24: 3011–3016. [DOI] [PubMed] [Google Scholar]
- 9. Minicozzi P, Berrino F, Sebastiani F, Falcini F, Vattiato R, et al. (2013) High fasting blood glucose and obesity significantly and independently increase risk of breast cancer death in hormone receptor-positive disease. Eur J Cancer 49: 3881–3888. [DOI] [PubMed] [Google Scholar]
- 10. Sonabend RY, McKay SV, Okcu MF, Yan J, Haymond MW, et al. (2009) Hyperglycemia during induction therapy is associated with poorer survival in children with acute lymphocytic leukemia. J Pediatr 155: 73–78. [DOI] [PubMed] [Google Scholar]
- 11. Luo J, Lin HC, He K, Hendryx M (2014) Diabetes and prognosis in older persons with colorectal cancer. Br J Cancer 110: 1847–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Macdonald JS, et al. (2003) Impact of diabetes mellitus on outcomes in patients with colon cancer. J Clin Oncol 21: 433–440. [DOI] [PubMed] [Google Scholar]
- 13. Stott-Miller M, Chen C, Chuang SC, Lee YC, Boccia S, et al. (2012) History of diabetes and risk of head and neck cancer: a pooled analysis from the international head and neck cancer epidemiology consortium. Cancer Epidemiol Biomarkers Prev 21: 294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmid D, Behrens G, Jochem C, Keimling M, Leitzmann M (2013) Physical activity, diabetes, and risk of thyroid cancer: a systematic review and meta-analysis. Eur J Epidemiol 28: 945–958. [DOI] [PubMed] [Google Scholar]
- 15. Chang ET, Adami HO (2006) The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 15: 1765–1777. [DOI] [PubMed] [Google Scholar]
- 16. Wei WI, Sham JS (2005) Nasopharyngeal carcinoma. Lancet 365: 2041–2054. [DOI] [PubMed] [Google Scholar]
- 17. Liu H, Xia Y, Cui N (2006) Impact of diabetes mellitus on treatment outcomes in patients with nasopharyngeal cancer. Med Oncol 23: 341–346. [DOI] [PubMed] [Google Scholar]
- 18.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, et al.. (2010) AJCC cancer staging handbook from the AJCC cancer staging manual; Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL et al., editors. New York: Springer. [Google Scholar]
- 19. Lai SZ, Li WF, Chen L, Luo W, Chen YY, et al. (2011) How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys 80: 661–668. [DOI] [PubMed] [Google Scholar]
- 20. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 37 Suppl 1S81–90. [DOI] [PubMed] [Google Scholar]
- 21. Sun X, Su S, Chen C, Han F, Zhao C, et al. (2014) Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: An analysis of survival and treatment toxicities. Radiother Oncol 110: 398–403. [DOI] [PubMed] [Google Scholar]
- 22. Liu N, Chen NY, Cui RX, Li WF, Li Y, et al. (2012) Prognostic value of a microRNA signature in nasopharyngeal carcinoma: a microRNA expression analysis. Lancet Oncol 13: 633–641. [DOI] [PubMed] [Google Scholar]
- 23. Ouyang PY, Su Z, Mao YP, Liang XX, Liu Q, et al. (2013) Prognostic impact of family history in southern Chinese patients with undifferentiated nasopharyngeal carcinoma. Br J Cancer 109: 788–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observation. J Am Stat Assoc 53: 457–481. [Google Scholar]
- 25. Cox DR (1972) Regression models and life tables. J R Stat Soc B 34: 187–220. [Google Scholar]
- 26. Zhou XH, Qiao Q, Zethelius B, Pyorala K, Soderberg S, et al. (2010) Diabetes, prediabetes and cancer mortality. Diabetologia 53: 1867–1876. [DOI] [PubMed] [Google Scholar]
- 27.Hofner T, Zeier M, Hatiboglu G, Eisen C, Schonberg G, et al.. (2013) The impact of type 2 diabetes on the outcome of localized renal cell carcinoma. World J Urol. [DOI] [PubMed]
- 28. Gerstein HC, Bosch J, Dagenais GR, Diaz R, Jung H, et al. (2012) Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 367: 319–328. [DOI] [PubMed] [Google Scholar]
- 29. Richardson LC, Pollack LA (2005) Therapy insight: Influence of type 2 diabetes on the development, treatment and outcomes of cancer. Nat Clin Pract Oncol 2: 48–53. [DOI] [PubMed] [Google Scholar]
- 30. Ioacara S, Guja C, Ionescu-Tirgoviste C, Fica S, Roden M (2014) Cancer specific mortality in insulin-treated type 2 diabetes patients. PLoS One 9: e93132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Margel D, Urbach DR, Lipscombe LL, Bell CM, Kulkarni G, et al. (2013) Metformin use and all-cause and prostate cancer-specific mortality among men with diabetes. J Clin Oncol 31: 3069–3075. [DOI] [PubMed] [Google Scholar]
- 32. Ouyang PY, Su Z, Mao YP, Liang XX, Liu Q, et al. (2013) Prognostic impact of cigarette smoking on the survival of patients with established nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 22: 2285–2294. [DOI] [PubMed] [Google Scholar]
- 33. Huang PY, Wang CT, Cao KJ, Guo X, Guo L, et al. (2013) Pretreatment body mass index as an independent prognostic factor in patients with locoregionally advanced nasopharyngeal carcinoma treated with chemoradiotherapy: findings from a randomised trial. Eur J Cancer 49: 1923–1931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sensitivity analysis by excluding the 121 patients with known diabetes history *.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.