Abstract
Objective: The aim of the study to elicit effects of pure quercetin in pentylenetetrazole (PTZ) and picrotoxin induced seizures. Materials and methods: Each animal group was divided into six groups and composed of six rats. Rats were assigned to the following experiments and groups (G): (G1) PTZ 45 mg/kg + DMSO; (G2) PTZ 45 mg/kg + 5 mg/kg quercetin; (G3) PTZ 45 mg/kg + 10 mg/kg quercetin; (G4) PTZ 45 mg/kg + 20 mg/kg quercetin; (G5) PTZ 45 mg/kg + 40 mg/kg quercetin; (G6) Picrotoxin 5 mg/kg + DMSO; (G7) Picrotoxin 5 mg/kg + 10 mg/kg quercetin; (G8) Picrotoxin 5 mg/kg + 20 mg/kg quercetin. In all groups quercetin were injected 30 min before PTZ and picrotoxin applications. Results: Compared to PTZ, quercetin significantly prolonged onset of the seizure in 10 mg/kg (P < 0.05) and reduced the seizure stage in 10 mg/kg quercetin injected group (P < 0.01). Compared to PTZ, quercetin also declined the generalized seizure duration at 10 mg/kg (P < 0.01) and 20 mg/kg (P < 0.05) doses. At the doses of 5 mg/kg and 40 mg/kg quercetin there were no significant changes in seizure parameters. Development of picrotoxin induced seizures is slower than in PTZ. Quercetin was found to be unable to prevent seizure in picrotoxin induced seizures. Surprisingly, quercetin also significantly reduced the onset of seizures at the dose of 20 mg/kg (P < 0.05). Conclusion: quercetin (at doses of 10 and 20 mg/kg i.p) prevented seizures in PTZ (45 mg/kg i.p) induced seizures. Especially, 10 mg/kg PTZ prolonged onset of seizures, reduced the seizure duration and seizure severity score in comparison with control group. At a higher (40 mg/kg) dose quercetin failed to prevent PTZ induced seizures. In addition 20 mg/kg quercetin significantly reduced the onset of seizures that suggest a preconvulsive effect. 20 mg/kg quercetin reduced the onset of picrotoxin induced seizures. In picrotoxin model, it may be claimed that quercetin at higher doses accelerate the epileptic activity owing to its antagonistic effect on GABAA. Further investigations are needed to explore the mechanisms of the antiepileptic and preconvulsant effects of quercetin.
Keywords: Epileptic seizure, pentylenetetrazole, picrotoxin, quercetin, flavonoids
Introduction
Epilepsy is a one of the most common chronic neurological disorder that influences 1-3% of mankind [1]. Despite the availability of new antiepileptic drugs, 30% of the epileptic patients still have uncontrolled seizures. For this rationale, several experimental seizure models have been performed in order to understand the crucial mechanisms of epileptic disorders and to discover new drugs for seizure management [2,3]. Pentylenetetrazole (PTZ) [4] and picrotoxin [5] are commonly used compounds to establish seizure models.
Flavonoids have been found to have favorable effects in cardiovascular diseases and cancer patients [6]. Moreover these compounds have antioxidant, anti-inflammatory, antitumoral and antiviral effects [7,8].
Quercetin is one of the most widely existing flavonoids in fruits and vegetables [9]. Quercetin is a an antioxidant [10] and free radical scavenger [11] and has been reported to have anti-inflammatory [12], anticoagulant [13], anti-ischemic [14], neuroprotective [15] and anticonvulsant effects [16]. Quercetin also improved the spatial memory impairment and neuronal death induced by repeated cerebral ischemia [17].
One of the pathophysiological mechanisms of epileptic seizures is an imbalance between excitatory and inhibitory amino acids in the brain. Therefore many of the antiepileptic drugs are used to ameliorate this imbalance. Glutamate and γ-amino butyric acid (GABA) are the major excitatory and inhibitory neurotransmitters in the central nervous system, respectively [18]. It is suggested that quercetin may affect γ-amino butyric acid (GABA) [19] and glutamate receptors [20]. Pentylenetetrazole and picrotoxin have excitatory effects associated GABA antagonism that induces seizures in rodents [4,5].
Some quercetin containing plant extracts have antiseizure effects in experimental epilepsy models [21-23]. Moreover, There are some studies investigating influence of pure quercetin on epileptic activity [15,16,24-26]. However, to the best of our knowledge, there is no study investigating the effect of quercetin on picrotoxin induced seizure in English literature. There are studies on PTZ induced seizures; however they are induced by higher doses of PTZ [16,26] or PTZ kindling [27]. Therefore, the present study is designed to elicit effects of pure quercetin in pentylenetetrazole (PTZ) and picrotoxin induced seizures.
Materials and methods
The animals were provided by the Experimental Research Center of Mustafa Kemal University at Hatay. Prior to the experiments, this study was permitted by the Ethical Committee for Animal Experiments at the Mustafa Kemal University (2012-08/15-7), all the experiments were performed in agreement with the guidelines of the European Community Council for experimental animal care. Male albino rats of the Wistar strain (11-15 weeks of age, weighing 220 ± 30 g) were used in the study. Rats were housed separately in a 12-h light: 12 h dark cycle (lights on at 08:00 am-08:00 pm), at a temperature of 22 ± 1°C and 50-55% humidity. Water and food were given ad libitum.
Rats were assigned to the following experiments and groups (G): (G1) PTZ 45 mg/kg + DMSO; (G2) PTZ 45 mg/kg + 5 mg/kg quercetin; (G3) PTZ 45 mg/kg + 10 mg/kg quercetin; (G4) PTZ 45 mg/kg + 20 mg/kg quercetin; (G5) PTZ 45 mg/kg + 40 mg/kg quercetin; (G6) Picrotoxin 5 mg/kg + DMSO; (G7) Picrotoxin 5 mg/kg + 10 mg/kg quercetin; (G8) Picrotoxin 5 mg/kg + 20 mg/kg quercetin. In all groups quercetin were injected 30 min. before PTZ and picrotoxin applications. Each animal group was composed of six rats. Quercetin, picrotoxin and pentylenetetrazole were purchased from Sigma (St. Louis, MO, USA). PTZ was dissolved in sterile physiological saline, but quercetin and picrotoxin were dissolved in DMSO. PTZ, picrotoxin and quercetin injected intraperitoneally.
The severity of the behavioral PTZ induced seizures was scored in accord with Racine scale [28]: 0-No behavioral changes; 1-Facial movements, ear and whisker twitching; 2-Myoclonic convulsions without rearing; 3-Myoclonic convulsions with rearing; 4-Clonic convulsion with loss of posture; 5-Generalized clonic-tonic seizures.
Picrotoxin-induced seizure severity scored as 1-Facial and body tremors; 2-Myoclonic jerk of the whole body; 3-Clonic convulsions of the whole body; 4-Generalized clonic-tonic convulsions with flexion of hind limbs; 5-Generalized clonic-tonic convulsions with extension of hind [5].
Behavioral activity was observed for 30 min after PTZ injection, for 60 min after picrotoxin injection. Onset of seizure, seizure stage and generalized seizure duration were recorded.
Results
Antiepileptic effects of quercetin on PTZ induced seizures
Compared to PTZ (114.4 ± 33.91), Quercetin significantly prolonged onset of the seizure in 10 mg/kg (292.50 ± 167.10) (P < 0.05) (Figure 1). Compared to PTZ (3.83 ± 0.47), Quercetin significantly reduced the seizure stage in 10 mg/kg (2.16 ± 0.54) quercetin injected group (P < 0.01) (Figure 2). Compared to PTZ (246.66 ± 122.16), Quercetin also declined the generalized seizure duration at 10 mg/kg (18.0 ± 6.34) (P < 0.01) and 20 mg/kg (107.5 ± 59.55) (P < 0.05) doses (Figure 3). At the doses of 5 mg/kg (227.0 ± 100.02) and 40 mg/kg (213.0 ± 94.69) quercetin there were no significant changes in seizure parameters (Figure 3).
Figure 1.
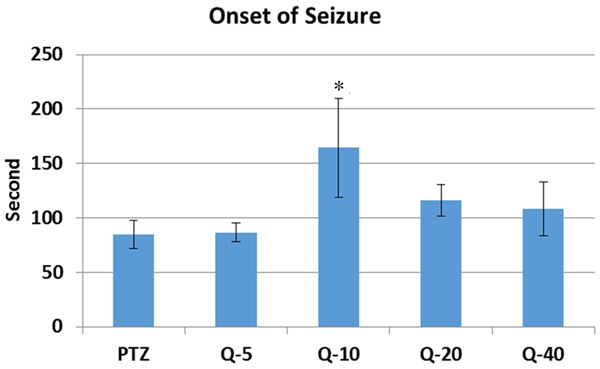
Onset of the seizure of quercetin on PTZ induced seizures. Quercetin significantly prolonged onset of the seizure (p < 0.05) in 10 mg/kg quercetin injected group. PTZ = Pentylenetetrazole, Q = Quercetin, *: p < 0.05.
Figure 2.
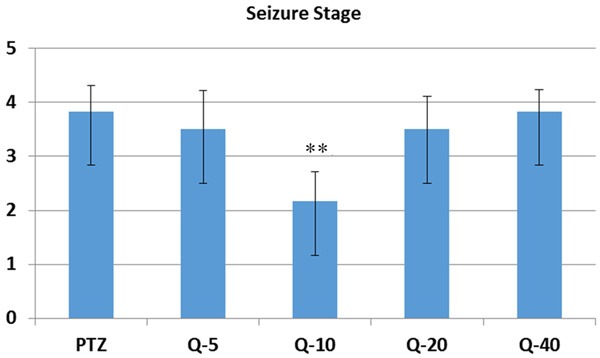
Seizure stage of quercetin on PTZ induced seizures. Quercetin significantly reduced the seizure stage (p < 0.01) in 10 mg/kg quercetin injected group. PTZ = Pentylenetetrazole, Q = Quercetin, **: p < 0.01.
Figure 3.
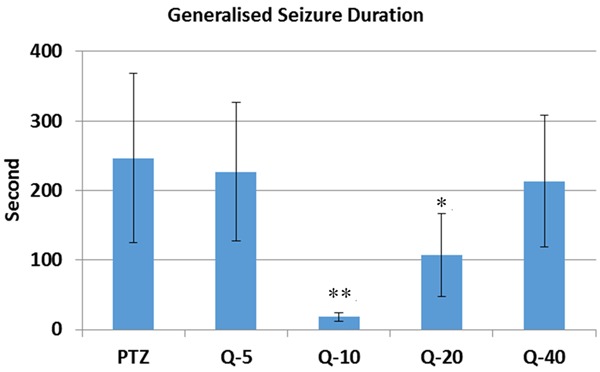
Generalized seizure duration of quercetin on PTZ induced seizures. Quercetin also declined the generalized seizure duration at 10 mg/kg (p < 0.01) and 20 mg/kg (P < 0.05) doses (Figure 3). At the doses of 5 mg/kg and 40 mg/kg quercetin there were no significant changes in seizure parameters. PTZ = Pentylenetetrazole, Q = Quercetin, *: p < 0.05 and **: p < 0.01.
Effects of quercetin on picrotoxin induced seizures
Development of picrotoxin induced seizures is slower than in PTZ (Figures 1, 4). Quercetin was found to be unable to prevent seizure in picrotoxin induced seizures. Surprisingly, quercetin also significantly reduced the onset of seizures at the dose of 20 mg/kg (441.83 ± 59.96) (P < 0.05) (Figure 4). There was no significant change in 10 mg/kg (716.66 ± 110.96) quercetin injected group in comparison with picrotoxin group (761.66 ± 12.23), according to onset of seizures (Figure 4). There was no significant change in 10 mg/kg (999.33 ± 198.30) and 20 mg/kg (894.66 ± 135.96) quercetin injected group in comparison with picrotoxin group (1225.83 ± 539.81), according to seizure duration (Figure 5). There was no significant change in 10 mg/kg (4.5 ± 0.54) and 20 mg/kg (5.0 ± 0.0) quercetin injected group in comparison with picrotoxin group according to seizure score (5.0 ± 0.0) (Figure 6).
Figure 4.
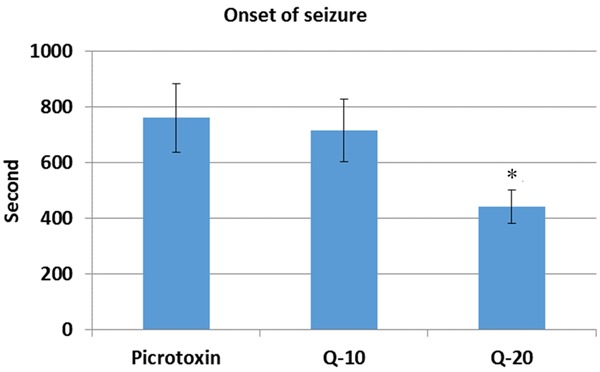
Onset of the seizure of quercetin on picrotoxin induced seizures. Quercetin significantly reduced the onset of seizures at the dose of 20 mg/kg .p. (p < 0.05). Q = Quercetin, *: p < 0.05.
Figure 5.
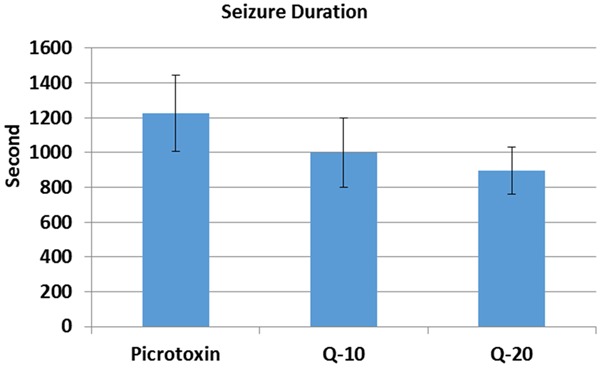
The seizure duration of quercetin on picrotoxin induced seizures. There was no significant change in 10 mg/kg and 20 mg/kg quercetin injected group in comparison with picrotoxin group. Q = Quercetin.
Figure 6.
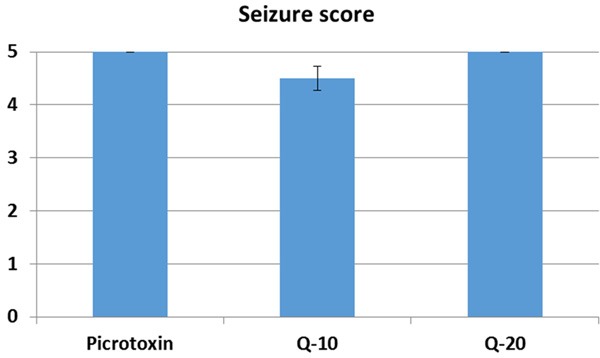
The seizure score of quercetin on picrotoxin induced seizures. There was no significant change in 10 mg/kg and 20 mg/kg quercetin injected group in comparison with picrotoxin group. Q = Quercetin.
Discussion
The present study showed that quercetin administration (10 and 20 mg/kg i.p.) reduced generalized seizure duration in PTZ induced seizures. Quercetin also significantly decreased the seizure severity score and prolonged the onset of seizure at 10 mg/kg dose in PTZ induced seizure. However, quercetin did not any change of the seizure severity in picrotoxin induced seizures. Contrary to other previous studies [16,26] we used an intermediate dose of PTZ (45 mg/kg) to induce seizure in this study, and we found significant antiepileptic effects of quercetin at lower doses in comparison these previous studies. Moreover, to our knowledge, this is the first study investigating the effect of quercetin on picrotoxin induced seizure.
In vitro and in vivo studies indicated that flavonoids may pass the blood-brain barrier and have many effects on the central nervous system [29]. It has been also reported that quercetin can pass the blood brain barrier [30].
Several quercetin containing plant extracts including Abelmoschus manihot, Pongamia pinnata, Hypericum montbretti, Argyreia speciosa, Anisomeles malabarica and Drosera burmannii have anticonvulsant effect in experimental epilepsy models [21-23,31-33]. Hypericum montbretti [23] and Argyreia speciosa [31] extracts have been reported to be effective in PTZ (80 mg/kg, i.p.) induced seizures in mice. It has been suggested that quercetin is one of the phytochemical responsible for the antiseizure effect of Pongamia pinnata PTZ (80 mg/kg, i.p.) induced seizures in rats [22]. In addition, it is reported that Drosera burmannii alcoholic extract also delayed the onset of convulsions in PTZ induced (70 mg/kg, i.p.) seizures [33]. Moreover, it was found that Anisomeles malabarica extract is effective in PTZ induced seizures. However, in that study pure quercetin at doses (at the doses 25 and 50 mg/kg, i.p.) have been reported failed to prevent PTZ (80 mg/kg, i.p.) induced convulsions [32]. Similarly, Nieoczym et al. reported that quercetin (at doses ranging from 100 to 800 mg/kg) did not prevent seizures in i.v. PTZ infusion test [16]. In this context, it may be suggested that quercetin is not unique responsible for the anticonvulsant properties of mentioned plants at a single dose and quercetin is not effective against high dose PTZ (80 mg/kg) induced seizures.
Moreover, chronic administiration may be more effective that Nassiri-Asl administered quercetin (25 and 50 mg/kg, i.p.) for 7 days and 30 min before PTZ (90 mg/kg) administration on seventh day. They have showed that quercetin prolonged seizure onset and decreased generalized seizure duration [26]. However, in a study quercetin at doses of 25, 50, and 100 mg/kg unable to prevent PTZ kindling, quercetin 50 mg/kg exceptionally improved memory retrieval in the retention tests of a passive avoidance task [27]. On the other hand, it was reported that quercetin had protective effects against PTZ (60 mg/kg i.p.) induced convulsions in mice with alcohol abstinence when given repeatedly for 6 days. In that study, it was found that acute quercetin administration following ethanol withdrawal also ameliorated PTZ-induced epileptic seizures [24]. In that study, quercetin (25 and 40 mg/kg) completely prevented lower dose PTZ (40 mg/kg) induced seizures in mice with alcohol abstinence [24]. Similarly, we found that quercetin attenuated PTZ induced seizures in Wistar albino rats.
Moreover, in another study it was shown that quercetin administration (40 mg/kg/day; i.p.) decreased seizure severity and duration of amygdala electrical kindling epileptic seizures. In our study, quercetin (at doses of 10 and 20 mg/kg i.p.) prevented seizures in PTZ (45 mg/kg i.p.) induced seizures. Especially, 10 mg/kg PTZ prolonged onset of seizures, reduced the seizure duration and seizure severity score in comparison with control group. At a higher (40 mg/kg) dose quercetin failed to prevent PTZ induced seizures. In addition 20 mg/kg quercetin significantly reduced the onset of seizures that suggest a proconvulsive effect.
Growing evidence suggested that quercetin has adverse effects [34]. It was demonstrated that feeding rats with high doses of quercetin (2% or 4%) induced nephropathy [35]. Additionally, it was reported that mice with a quercetin supplemented diet (0.1%) had significantly reduced mice life expectancy [36].
It is accepted that PTZ is the most common used chemoconvulsant in order to observe the effects of antiepileptic drugs. It is confirmed that PTZ is an inhibitor of chloride channels associated with GABAA receptors and induces epileptic activity via reducing GABA [4,37].
Similarly, it is demonstrated that picrotoxin decreases inhibitory transmission via blocking the chloride channel related with the GABAA receptors [5]. Differential application methods of these two drugs can induce various seizure models. It was suggested that low dose PTZ (20-30 mg/kg) and low dose picrotoxin (1.5 mg/kg) induced seizure models mimic absence epilepsy. It was also proposed that intermediate dose of these drugs (45 mg/kg for PTZ, 1.8 mg/kg for picrotoxin) induced clonic seizures. In our study we used an intermediate dose of PTZ and a high dose of picrotoxin [38].
NMDA and AMPA receptor antagonists and GABA receptor agonists have antiepileptic effects on experimental seizure models [39,40]. In this context, Goutman et al. suggested that quercetin inhibited GABA(A), a4-h2 nicotinic, AMPA-kainate and 5-HT3 serotonin receptors [19]. It is well known that GABA(A) blockers can induce seizures. In our study 20 mg/kg quercetin reduced the onset of picrotoxin induced seizures.
Conclusions
Quercetin (at doses of 10 and 20 mg/kg i.p.) prevented seizures in PTZ (45 mg/kg i.p.) induced seizures. Especially, 10 mg/kg PTZ prolonged onset of seizures, reduced the seizure duration and seizure severity score in comparison with control group. At a higher (40 mg/kg) dose quercetin failed to prevent PTZ induced seizures. In addition 20 mg/kg quercetin significantly reduced the onset of seizures that suggest a preconvulsive effect. 20 mg/kg quercetin reduced the onset of picrotoxin induced seizures. In picrotoxin model, it may be claimed that quercetin at higher doses accelerate the epileptic activity owing to its antagonistic effect on GABAA. Further investigations are needed to explore the mechanisms of the antiepileptic and preconvulsant effects of quercetin.
Acknowledgements
This project was supported by the Mustafa Kemal University Research Foundation (8500-2013).
Disclosure of conflict of interest
The authors of this paper have no conflicts of interest, including specific financial interests, relationships, and/or affiliations, relevant to the subject matter or materials included.
References
- 1.Hauser WA, Hesdorffer DC. Epilepsy: Frequency, causes and consequences. New York: Demos; 1990. [Google Scholar]
- 2.Sander JW. The epidemiology of epilepsy revisited. Curr Opin Neurol. 2003;16:165–170. doi: 10.1097/01.wco.0000063766.15877.8e. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs MP, Fischbach GD, Davis MR, Dichter MA, Dingledine R, Lowenstein DH, Morrell MJ, Noebels JL, Rogawski MA, Spencer SS, Theodore WH. Future directions for epilepsy research. Neurology. 2001;57:1536–1542. doi: 10.1212/wnl.57.9.1536. [DOI] [PubMed] [Google Scholar]
- 4.Hansen SL, Sperling BB, Sanchez C. Anticonvulsant and antiepileptogenic effects of GABAA receptor ligands in pentylenetetrazole-kindled mice. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:105–113. doi: 10.1016/j.pnpbp.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 5.Rajasekaran K, Jayakumar R, Venkatachalam K. Increased neuronal nitric oxide synthase (nNOS) activity triggers picrotoxin-induced seizures in rats and evidence for participation of nNOS mechanism in the action of antiepileptic drugs. Brain Res. 2003;979:85–97. doi: 10.1016/s0006-8993(03)02878-6. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez SP, Wasowski C, Loscalzo LM, Granger RE, Johnston GA, Paladini AC, Marder M. Central nervous system depressant action of flavonoid glycosides. Eur J Pharmacol. 2006;539:168–176. doi: 10.1016/j.ejphar.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Rahmani AH, Aly SM, Ali H, Babiker AY, Srikar S, Khan AA. Therapeutic effects of date fruits (Phoenix dactylifera) in the prevention of diseases via modulation of anti-inflammatory, anti-oxidant and anti-tumour activity. Int J Clin Exp Med. 2014;7:483–491. [PMC free article] [PubMed] [Google Scholar]
- 8.Mou X, Kesari S, Wen PY, Huang X. Crude drugs as anticancer agents. Int J Clin Exp Med. 2011;4:17–25. [PMC free article] [PubMed] [Google Scholar]
- 9.Shen Y, Croft KD, Hodgson JM, Kyle R, Lee IL, Wang Y, Stocker R, Ward NC. Quercetin and its metabolites improve vessel function by inducing eNOS activity via phosphorylation of AMPK. Biochem Pharmacol. 2012;84:1036–1044. doi: 10.1016/j.bcp.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 10.Yang T, Kong B, Gu JW, Kuang YQ, Cheng L, Yang WT, Xia X, Shu HF. Anti-apoptotic and Anti-oxidative Roles of Quercetin After Traumatic Brain Injury. Cell Mol Neurobiol. 2014;34:797–804. doi: 10.1007/s10571-014-0070-9. [DOI] [PubMed] [Google Scholar]
- 11.Huk I, Brovkovych V, Nanobash Vili J, Weigel G, Neumayer C, Partyka L, Patton S, Malinski T. Bioflavonoid quercetin scavenges superoxide and increases nitric oxide concentration in ischaemia-reperfusion injury: an experimental study. Br J Surg. 1998;85:1080–1085. doi: 10.1046/j.1365-2168.1998.00787.x. [DOI] [PubMed] [Google Scholar]
- 12.Aguirre L, Portillo MP, Hijona E, Bujanda L. Effects of resveratrol and other polyphenols in hepatic steatosis. World J Gastroenterol. 2014;20:7366–7380. doi: 10.3748/wjg.v20.i23.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bijak M, Ponczek MB, Nowak P. Polyphenol compounds belonging to flavonoids inhibit activity of coagulation factor X. Int J Biol Macromol. 2014;65:129–135. doi: 10.1016/j.ijbiomac.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Guo X, Chu Y, Lu S. Heart protective effects and mechanism of quercetin preconditioning on anti-myocardial ischemia reperfusion (IR) injuries in rats. Gene. 2014;545:149–155. doi: 10.1016/j.gene.2014.04.043. [DOI] [PubMed] [Google Scholar]
- 15.Hu K, Li SY, Xiao B, Bi FF, Lu XQ, Wu XM. Protective effects of quercetin against status epilepticus induced hippocampal neuronal injury in rats: involvement of X-linked inhibitor of apoptosis protein. Acta Neurol Belg. 2011;111:205–212. [PubMed] [Google Scholar]
- 16.Nieoczym D, Socala K, Raszewski G, Wlaz P. Effect of quercetin and rutin in some acute seizure models in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54C:50–58. doi: 10.1016/j.pnpbp.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Pu F, Mishima K, Irie K, Motohashi K, Tanaka Y, Orito K, Egawa T, Kitamura Y, Egashira N, Iwasaki K, Fujiwara M. Neuroprotective effects of quercetin and rutin on spatial memory impairment in an 8-arm radial maze task and neuronal death induced by repeated cerebral ischemia in rats. J Pharmacol Sci. 2007;104:329–334. doi: 10.1254/jphs.fp0070247. [DOI] [PubMed] [Google Scholar]
- 18.Higuera-Matas A, Miguens M, Coria SM, Assis MA, Borcel E, del Olmo N, Ambrosio E. Sex-specific disturbances of the glutamate/GABA balance in the hippocampus of adult rats subjected to adolescent cannabinoid exposure. Neuropharmacology. 2012;62:1975–1984. doi: 10.1016/j.neuropharm.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 19.Goutman JD, Waxemberg MD, Donate-Oliver F, Pomata PE, Calvo DJ. Flavonoid modulation of ionic currents mediated by GABA(A) and GABA(C) receptors. Eur J Pharmacol. 2003;461:79–87. doi: 10.1016/s0014-2999(03)01309-8. [DOI] [PubMed] [Google Scholar]
- 20.Cheng XP, Qin S, Dong LY, Zhou JN. Inhibitory effect of Total Flavone of Abelmoschus manihot L. Medic on NMDA receptor-mediated current in cultured rat hippocampal neurons. Neurosci Res. 2006;55:142–145. doi: 10.1016/j.neures.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Guo J, Xue C, Duan JA, Qian D, Tang Y, You Y. Anticonvulsant, antidepressant-like activity of Abelmoschus manihot ethanol extract and its potential active components in vivo. Phytomedicine. 2011;18:1250–1254. doi: 10.1016/j.phymed.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Manigauha A, Patel S. Anticonvulsant study of Pongamia pinnata Linn. Against pentylenetetrazole induced convulsion in rats. International Journal of Pharma and Bio Sciences. 2010 [Google Scholar]
- 23.Can OD, Ozkay UD. Effects of Hypericum montbretti extract on the central nervous system and involvement of GABA(A)/Benzodiazepine receptors in its pharmacological activity. Phytother Res. 2012;26:1695–1700. doi: 10.1002/ptr.4629. [DOI] [PubMed] [Google Scholar]
- 24.Joshi D, Naidu PS, Singh A, Kulkarni SK. Protective effect of quercetin on alcohol abstinence-induced anxiety and convulsions. J Med Food. 2005;8:392–396. doi: 10.1089/jmf.2005.8.392. [DOI] [PubMed] [Google Scholar]
- 25.Ekimova IV, Komarova TG, Nitsinskaya LE, Pastukhov YF, Guzhova IV. Effects of exogenous heat shock protein 70 and quercetin on NMDA-induced seizures. Dokl Biol Sci. 2008;418:13–15. doi: 10.1134/S0012496608010055. [DOI] [PubMed] [Google Scholar]
- 26.Nassiri-Asl M, Hajiali F, Taghiloo M, Abbasi E, Mohseni F, Yousefi F. Comparison between the effects of quercetin on seizure threshold in acute and chronic seizure models. Toxicol Ind Health. 2014 doi: 10.1177/0748233713518603. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Nassiri-Asl M, Moghbelinejad S, Abbasi E, Yonesi F, Haghighi MR, Lotfizadeh M, Bazahang P. Effects of quercetin on oxidative stress and memory retrieval in kindled rats. Epilepsy Behav. 2013;28:151–155. doi: 10.1016/j.yebeh.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 28.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 29.Jager AK, Saaby L. Flavonoids and the CNS. Molecules. 2011;16:1471–1485. doi: 10.3390/molecules16021471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Youdim KA, Qaiser MZ, Begley DJ, Rice-Evans CA, Abbott NJ. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic Biol Med. 2004;36:592–604. doi: 10.1016/j.freeradbiomed.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 31.Vyawahare NS, Bodhankar SL. Anticonvulsant Activity of Argyreia speciosa in Mice. Indian J Pharm Sci. 2009;71:131–134. doi: 10.4103/0250-474X.54277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choudhary N, Bijjem KR, Kalia AN. Antiepileptic potential of flavonoids fraction from the leaves of Anisomeles malabarica. J Ethnopharmacol. 2011;135:238–242. doi: 10.1016/j.jep.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 33.Hema B, Bhupendra S, Saleem TM, Gauthaman K. Anticonvulsant effect of Drosera burmannii Vahl. International Journal of Applied Research in Natural Products. 2009;2:1–4. [Google Scholar]
- 34.Ebrahimi A, Schluesener H. Natural polyphenols against neurodegenerative disorders: potentials and pitfalls. Ageing Res Rev. 2012;11:329–345. doi: 10.1016/j.arr.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Dunnick JK, Hailey JR. Toxicity and carcinogenicity studies of quercetin, a natural component of foods. Fundam Appl Toxicol. 1992;19:423–431. doi: 10.1016/0272-0590(92)90181-g. [DOI] [PubMed] [Google Scholar]
- 36.Jones E, Hughes RE. Quercetin, flavonoids and the life-span of mice. Exp Gerontol. 1982;17:213–217. doi: 10.1016/0531-5565(82)90027-4. [DOI] [PubMed] [Google Scholar]
- 37.Sejima H, Ito M, Kishi K, Tsuda H, Shiraishi H. Regional excitatory and inhibitory amino acid concentrations in pentylenetetrazol kindling and kindled rat brain. Brain Dev. 1997;19:171–175. doi: 10.1016/s0387-7604(96)00492-5. [DOI] [PubMed] [Google Scholar]
- 38.Cortez MA, Snead OC. Chapter 10: Pharmacologic models of generalized absence seizures in rodents. Academic Press; 2005. [Google Scholar]
- 39.Veliskova J, Velisek L, Mares P, Rokyta R. Ketamine suppresses both bicuculline- and picrotoxin-induced generalized tonic-clonic seizures during ontogenesis. Pharmacol Biochem Behav. 1990;37:667–674. doi: 10.1016/0091-3057(90)90544-r. [DOI] [PubMed] [Google Scholar]
- 40.Sierra-Paredes G, Galan-Valiente J, Vazquez-Illanes MD, Aguilar-Veiga E, Sierra-Marcuno G. Effect of ionotropic glutamate receptors antagonists on the modifications in extracellular glutamate and aspartate levels during picrotoxin seizures: a microdialysis study in freely moving rats. Neurochem Int. 2000;37:377–386. doi: 10.1016/s0197-0186(00)00038-3. [DOI] [PubMed] [Google Scholar]


