Abstract
Purpose: Ketamine is currently the N-methyl-D-aspartate receptor channel blocker in clinical use. Morphine in pain management is usually limited by adverse effect such as nausea and vomiting. Adjuvant treatment with ketamine may be value in giving better analgesia with fewer adverse effects. The purpose of this meta-analysis was to evaluate the differences when patients received morphine with adjuvant ketamine (MK) compared with higher dose of morphine (MO) for acute pain. Methods: The PubMed, EMBASE and the Cochrane Library databases were searched (Last search performed on July 1, 2014) by two reviewers independently. Data were extracted independently by the same two individuals who searched the studies. Results: A total of 7 trials involving 492 patients were included in the current analysis. We found pain scores were lower in the MK group compared to the MO group [MD 2.19, 95% CI (1.24, 3.13) P<0.00001]. And more patients in the MO required diclofenac [OR 1.97, 95% CI (1.06, 3.67) P=0.03]. Furthermore, morphine plus ketamine can reduced post-operative nausea and vomiting (PONV) [OR 3.71, 95% CI (2.37, 5.80) P<0.00001]. Importantly, the wakefulness scores for the MK group were consistently and significantly better than those for the MO group [MD -1.53, 95% CI (-2.67, -0.40) P=0.008]. Conclusion: The use of ketamine plus 1/4~2/3 the dose of morphine is better than higher dose of morphine alone in reducing pain scores, and rescuing analgesic requirement. It also improved PONV and wakefulness.
Keywords: Morphine, ketamine, acute pain, meta-analysis
Introduction
Acute pain is not satisfactory controlled despite the administration of morphine in clinical. The administration of large amounts of morphine to the awakening patient may cause respiratory and hemodynamic depression [1,2]. Also, higher dose of morphine can cause serious nausea and vomiting. Recent research indicates that morphine not only analgesia, but also hyperalgesia [3]. Consequently, perioperative morphine may increase postoperative pain and requirement [4]. For these reasons, supplementation of morphine with adjuvant agents may be a preferred way of effective controlling pain while reducing the incidence of adverse events [5,6].
Ketamine, a noncompetitive N-methy-D-aspartate (NMDA)-receptor antagonist was shown to enhance opioid-induced antinociception [7], to reduce hyperalgesia and when combined with morphine to lower morphine consumption [8]. But, the role of opioid mechanisms in ketamine analgesia in man is still undecided [9]. Anti-inflammatory effects of ketamine have been consistently reported in recent years [10,11]. Ketamine can release adenosine leading to inhibition of proinflammatory cytokine secretion. This antiinflammation effect might play a role in pain conditions. Thus, we hypothesize that morphine with adjuvant ketamine (MK) is better in reducing pain scores, rescuing analgesic requirement and improving PONV and wakefulness when compared with higher dose of morphine alone (MO).
Methods
Search strategy
We identified randomized controlled trials (RCTs) by electronically searching the following database: PubMed, EMBASE and the Cochrane Library. Briefly, the following medical subject headings (MeSH) were included: morphine, ketamine, human and randomized controlled trial, the last search performed on July 1, 2014. Alternative spellings were considered when searching. We removed duplicates that were identified in multiple database searches.
Inclusion criteria
Randomized controlled trials (RCTs) that compared morphine plus ketamine with morphine alone for acute pain were included. The dose of morphine must be reduced to 1/4~2/3 in MK group when compared with that of MO group. English language but no publication date limits were set.
Selection of studies
Two reviewers (Xibing DING, Shuqing JIN) used the pre-specified criteria to screen for relevant titles, abstracts and full papers. An article was removed if it was determined not meet the inclusion criteria. If these two reviewers reached different final selection decisions, a third reviewer (Quan Li) was consulted.
Data extraction
We extracted the following data from the included articles: First author name, publication date, number of patients, study design, description of interventions between MK group and MO group, pain scores (Visual Analogue Scale), wakefulness scores (Visual Analogue Scale), number of PONV and number of diclofenac. The above definition of indicators is in accordance with the definition of the original. These data were then compiled into a standard table. The two reviewers (Xibing Ding, Shuqing Jin) who selected the appropriate studies also extracted the data and evaluated the risk of bias. It was necessary to consult an arbiter (Quan Li) to reconcile any disagreement.
Assessing the risk of bias
We used the Cochrane Handbook V5.0.2 [12] to assess the risk of bias for all articles. The following information was evaluated: random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting and other bias. Two reviewers (Xiaoyin Niu, Ting-ting Wang) evaluated the methodological quality of all articles examined in the current study. An arbiter (Quan Li) was consulted to reconcile any disagreements.
Statistical analysis
Review Manager Software (Revman 5.0, The Cochrane Collaboration, Oxford, United Kingdom) was used for the meta-analysis. Heterogeneity among the studies was evaluated using I2 statistic and chi-squared test. The fixed effects model was used if the heterogeneity test did not reveal statistical significance (I2<50%; P>0.1). Otherwise, we adopted the random effects model. The variables of pain scores and wakefulness in the studies included in this meta-analysis were continuous, so we used the mean difference (MD) and 95% confidence interval (95% CI). Other variables, such as No. of PONV and diclofenac were dichotomous data, so we used the Odds Ratio (OR) and 95% CI. All tests of statistical significance were two-sided [13].
Results
Search results
The process of indentifying eligible studies was outlined in Figure 1. 167 records were initially identified through the PubMed, Embase and the Cochrane Library. Of these, 40 potentially eligible articles were included based on their titles and abstracts. After reviewing these 40 potentially eligible articles, only 7 articles fulfilled the inclusion criteria [14-20]. The remaining 33 articles were removed because the dose of morphine didn’t reduced to 1/4~2/3 in MK group when compared with that of MO group. A detailed explanation of the full electronic search strategy for PubMed is presented in Figure 1. Among these the dose of morphine and ketamine are not the same in each paper. The characteristics of each included study are described in Table 1.
Figure 1.
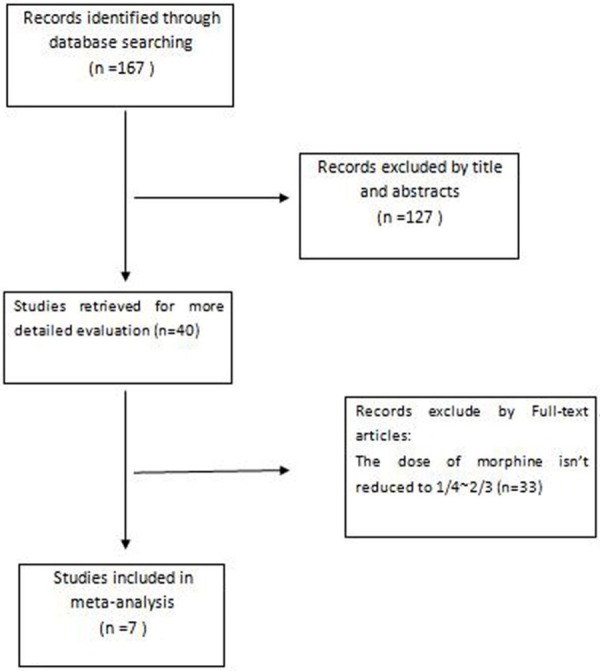
Flow chart of the study selection procedure.
Table 1.
Characteristics of included studies
| Article | Year | No. of patients | MK Group | MO Group |
|---|---|---|---|---|
| Weinbroum AA | 2003 | 245 | 15 μg/kg of morphine plus 250 μg/kg of ketamine intravenously | 30 μg/kg of morphine plus saline intravenously |
| Arroyo-Novoa CM | 2011 | 22 | 50 μg/kg of morphine plus ketamine 250 μg/kg intravenously | 100 μg/kg of morphine plus saline intravenously |
| Wong CS | 1996 | 20 | 0.5 mg morphine plus 10.0 mg ketamine epidural catheter | 2.0 mg morphine epidural catheter |
| Nesher N | 2008 | 58 | 1.0 mg morphine plus 5.0 mg ketamine by IV-PCA | 1.5 mg bolus by IV-PCA |
| Chazan S | 2010 | 46 | 1.0 mg morphine plus 5.0 mg ketamine/bolus by PCA | 2.0 mg/bolus morphine by PCA |
| Nesher N | 2009 | 41 | 1.0 mg morphine plus 5.0 mg ketamine/bolus by IV-PCA | 1.5 mg morphine plus saline by IV-PCA |
| Kollender Y | 2008 | 60 | 1.0 mg morphine plus 5.0 mg ketamine/bolus by IV-PCA | 1.5 mg morphine/bolus by IV-PCA |
MK: morphine plus ketamine; MO: morphine alone.
Risk of bias of included studies
According to the Cochrane Handbook V5.0.2, each article was at a high risk of bias. Thus, the evidence involved in this meta-analysis had a high overall risk of bias. Each article was described as randomized. Only 1 article used the allocation concealment method. Most articles were blinded except 1. Incomplete outcome data were at a low risk of bias in all articles. Selecting reporting bias was considered low for with no access to each trial’s original protocol. The risk of bias assessment of all included studies is described in Table 2.
Table 2.
Risk of bias assessment of included studies
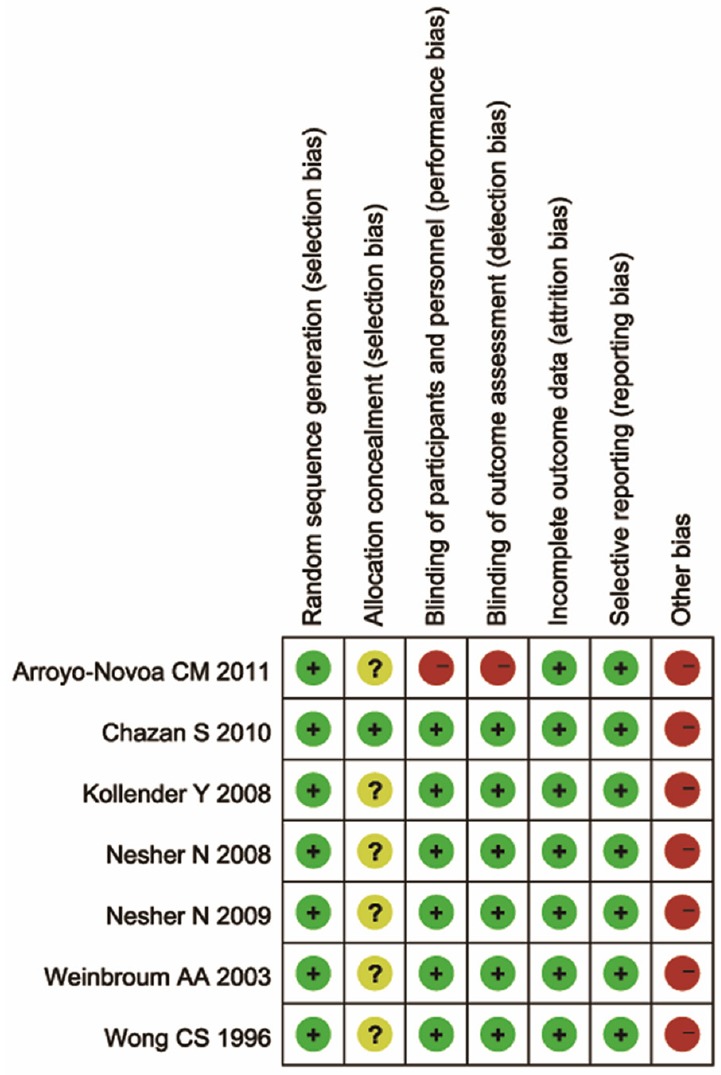
|
The primary outcomes: MK versus MO on the analgesic efficacy
Trials assessed pain intensity using a visual analog scale. There was statistically significant difference in pain scores at 24h between MK group and MO group [MD (2.19), 95% CI (1.24, 3.13), P<0.00001] (Figure 2). If pain was not attenuated within 30 min of initial activation, a rescue dose of diclofenac 75 mg was available in four articles. Significant difference in No. of diclofenac was observed between the two groups. [MD (1.97), 95% CI (1.06, 3.67), P=0.03] (Figure 3). These data show MK group is better than MO group in reducing pain scores, rescuing analgesic requirement.
Figure 2.
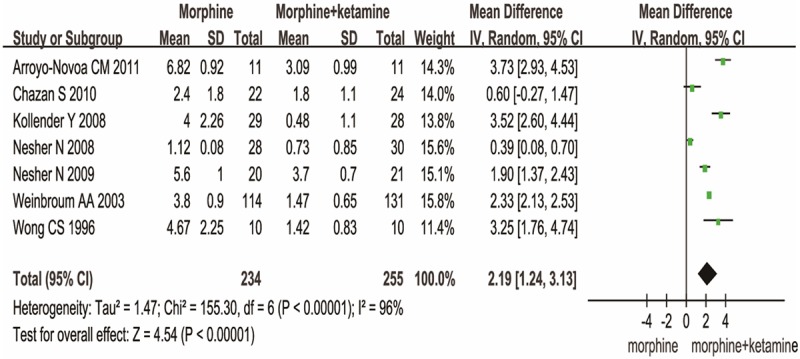
MK versus MO on the pain scores.
Figure 3.
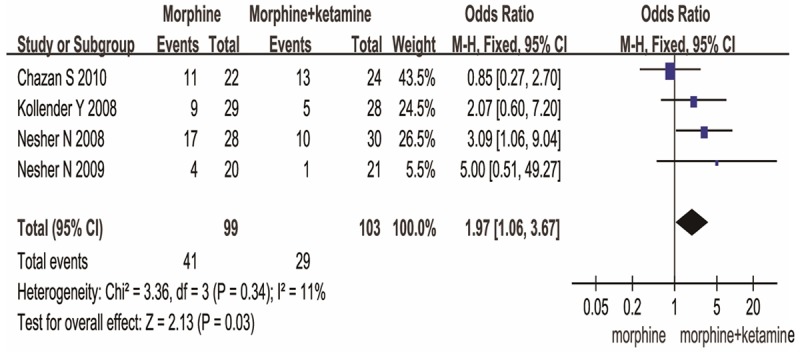
MK versus MO on the No. of diclofenac.
The secondary outcomes: MK versus MO on the side effects
The side effects consist of wakefulness and PONV. Compared to MO group, MK group resulted in a significant difference in wakefulness [MD (-1.53), 95% CI (-2.67, -0.40), P=0.008] (Figure 4) and PONV [OR (3.71), 95% CI (2.37, 5.80), P<0.00001] (Figure 5). These data show MK is better than MO group in side effects.
Figure 4.
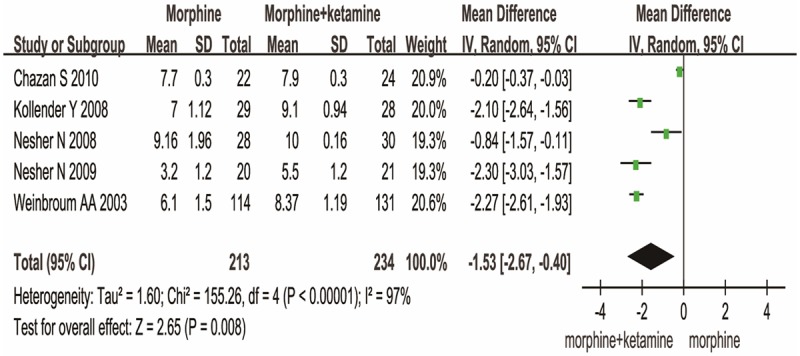
MK versus MO on the wakefulness.
Figure 5.
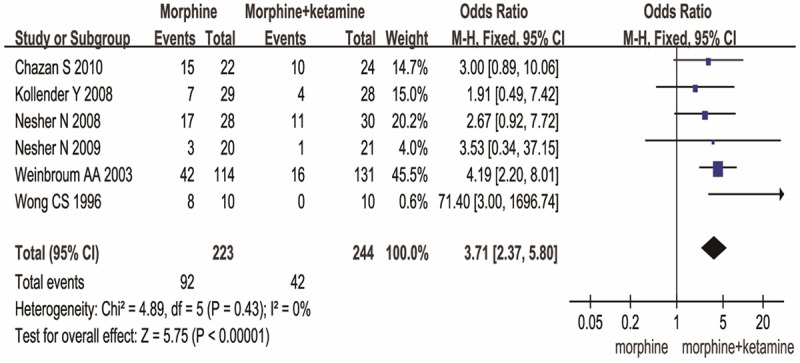
MK versus MO on PONV.
Discussion
To our knowledge, this is the first meta-analysis to evaluate the effect of ketamine plus low dose of morphine for acute pain. In this study, a small dose of ketamine with morphine not only reduced pain intensity but also improved wakefulness and PONV when compared with the higher dose of morphine alone.
This finding suggests that the combination of ketamine with morphine results in synergistic effects. These effects may be related to the different mechanisms [9]. Ketamine may produce antinociception through various mechanisms of action: interaction with μ-receptors, NMDA receptor antagonism and activation of the descending pain inhibitory monoaminergic pathway [21]. Morphine and other opioids produce antinociception through μ-receptor agonist activity, they also active NMDA receptors, resulting in hyperalgesia and the development of tolerance to opioids [22]. Thus, if this was the type of tolerance or resistance involved in the sustained and severe acute pain in patients, it could be overcome by small dose of ketamine either via central desensitization or via antagonization of NMDA activity [18].
Although our study shows MK group is better in reducing pain scores, rescuing analgesic requirement and improving PONV, wakefulness, it doesn’t mean there are no disadvantages of ketamine. Ketamine has been recognized as a potent psychedelic drug and dissociative anesthetic since its introduction into clinical practice [23]. It provokes imaginative, dissociative states, and psychotic symptoms up to schizophrenia due to its NMDA-antagonistic action [24-26] as well as severely impairing semantic and episodic memory when used in sub-anesthetic doses [26]. A dissociative effect of loss-of-self, inability to move the body, and isolation of mind from body is reported when ketamine is used as analgesic. Ketamine can cause emergence phenomena that have been variously described as a floating sensation, vivid pleasant dreams, nightmares, hallucinations and delirium [23]. Arroyo-Novoa CM et al. [14] reported 91 percent (n=10) of patients who had received MK had adverse effects compared with 0% of the patients when they were treated with MO. The most common adverse effects were hallucinations (n=4, 36%) and strange sensations (n=6, 55%). But Nesher N et al. [20] reported no patient in MK group had illusions or bad dreams. The reason most likely is the small intermittent dosing of ketamine. Weinbroum AA et al. [18] also concluded a similar result, at no time did patients report hallucinations; but 1 patient described an unpleasant dream.
Ketamine provides profound analgesia after neuraxial application, which has triggered great interest in its potential benefits during neuraxial anesthesia. However, the widespread use of ketamine has been hampered due to fear of potential neurotoxicity [27]. Neurotoxicity was reported in a patient after long-term intrathecal administration of ketamine with preserves due to chronic pain [28]. But in a recent clinical study with 24 infants concluded that there was no convincing evidence that ketamine was neurotoxic [29].
Limitation
This meta-analysis was characterized by several limitations that should be noted. Firstly, which is common in many systematic reviews, was that the findings were based on relatively low quality data that had a high risk of bias. Also the included papers just come from that written by English language. Secondly, the sample sizes of these studies were small which may lead to a small-study effect, thus we should be cautions of the application of this meta-analysis. Thirdly, we did not study the hallucinations, strange sensations and haemodynamic alterations because we can’t get enough data. At last, there were high levels of heterogeneity when evaluating the pain scores and wakefulness most likely to due to different dose of morphine and ketamine, also the different in route of drug administration and anesthesia. Most papers used the intravenous route, but Wong CS et al. [19] used the epidural catheter especially. Of course, the most influence came from the surgical procedures, because the type of surgery made a difference in the severity of pain.
Conclusions
Our analysis represents a least-biased attempt to pool the results of several studies. Larger, prospective, randomized trials which compared morphine plus ketamine and morphine are necessary to confirm these findings.
In summary, this meta-analysis showed the use of ketamine plus 1/4~2/3 the dose of morphine is better than higher dose of morphine alone in reducing pain scores, rescuing analgesic requirement. It also improved PONV and wakefulness.
Acknowledgements
We are grateful for the support from the National Natural Science Foundation. We also thank all authors of the publications included in this study for contributing information as required. This study was supported financially by the National Natural Science Foundation (81270135), the Shanghai Education Committee Key Project (13ZZ024).
Disclosure of conflict of interest
None.
References
- 1.Rothbard RL, Schreiner BF, Yu PN. Hemodynamic and respiratory effects of dezocine, ciramadol, and morphine. Clin Pharmacol Ther. 1985;38:84–88. doi: 10.1038/clpt.1985.139. [DOI] [PubMed] [Google Scholar]
- 2.Pena BM, Krauss B. Adverse events of procedural sedation and analgesia in a pediatric emergency department. Ann Emerg Med. 1999;34:483–91. doi: 10.1016/s0196-0644(99)80050-x. [DOI] [PubMed] [Google Scholar]
- 3.Mao J. Opioid-induced abnormal pain sensitivity: implications in clinical opioid therapy. Pain. 2002;100:213–7. doi: 10.1016/S0304-3959(02)00422-0. [DOI] [PubMed] [Google Scholar]
- 4.Guignard B, Bossard AE, Coste C, Sessler DI, Lebrault C, Alfonsi P, Fletcher D, Chauvin M. Acute opioid tolerance: intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology. 2000;93:409–17. doi: 10.1097/00000542-200008000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Hynninen MS, Cheng DC, Hossain I, Carroll J, Aumbhagavan SS, Yue R, Karski JM. Non-steroidal anti-inflammatory drugs in treatment of postoperative pain after cardiac surgery. Can J Anaesth. 2000;47:1182–7. doi: 10.1007/BF03019866. [DOI] [PubMed] [Google Scholar]
- 6.Lahtinen P, Kokki H, Hendolin H, Hakala T, Hynynen M. Propacetamol as adjunctive treatment for postoperative pain after cardiac surgery. Anesth Analg. 2002;95:813–9. doi: 10.1097/00000539-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Oye I, Paulsen O, Maurset A. Effects of ketamine on sensory perception: evidence for a role of N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 1992;260:1209–13. [PubMed] [Google Scholar]
- 8.Laulin JP, Maurette P, Corcuff JB, Rivat C, Chauvin M, Simonnet G. The role of ketamine in preventing fentanyl-induced hyperalgesia and subsequent acute morphine tolerance. Anesth Analg. 2002;94:1263–9. doi: 10.1097/00000539-200205000-00040. [DOI] [PubMed] [Google Scholar]
- 9.Persson J. Ketamine in pain management. CNS Neurosci Ther. 2013;19:396–402. doi: 10.1111/cns.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Kock M, Loix S, Lavand’homme P. Ketamine and peripheral inflammation. CNS Neurosci Ther. 2013;19:403–10. doi: 10.1111/cns.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C, Liu F, Patterson TA, Paule MG, Slikker W Jr. Preclinical assessment of ketamine. CNS Neurosci Ther. 2013;19:448–53. doi: 10.1111/cns.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JPT, Altman DG. Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Wiley; 2008. pp. 187–241. [Google Scholar]
- 13.Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. Br Med J. 1997;315:1533–7. doi: 10.1136/bmj.315.7121.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arroyo-Novoa CM, Figueroa-Ramos MI, Miaskowski C, Padilla G, Paul SM, Rodríguez-Ortiz P, Stotts NA, Puntillo KA. Efficacy of small doses of ketamine with morphine to decrease procedural pain responses during open wound care. Clin J Pain. 2011;27:561–6. doi: 10.1097/AJP.0b013e318211936a. [DOI] [PubMed] [Google Scholar]
- 15.Nesher N, Ekstein MP, Paz Y, Marouani N, Chazan S, Weinbroum AA. Morphine with adjuvant ketamine vs higher dose of morphine alone for immediate postthoracotomy analgesia. Chest. 2009;136:245–52. doi: 10.1378/chest.08-0246. [DOI] [PubMed] [Google Scholar]
- 16.Chazan S, Buda I, Nesher N, Paz J, Weinbroum AA. Low-dose ketamine via intravenous patient-controlled analgesia device after various transthoracic procedures improves analgesia and patient and family satisfaction. Pain Manag Nurs. 2010;11:169–76. doi: 10.1016/j.pmn.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Kollender Y, Bickels J, Stocki D, Maruoani N, Chazan S, Nirkin A, Meller I, Weinbroum AA. Subanaesthetic ketamine spares postoperative morphine and controls pain better than standard morphine does alone in orthopaedic-oncological patients. Eur J Cancer. 2008;44:954–62. doi: 10.1016/j.ejca.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Weinbroum AA. A single small dose of postoperative ketamine provides rapid and sustained improvement in morphine analgesia in the presence of morphine-resistant pain. Anesth Analg. 2003;96:789–95. doi: 10.1213/01.ANE.0000048088.17761.B4. [DOI] [PubMed] [Google Scholar]
- 19.Wong CS, Liaw WJ, Tung CS, Su YF, Ho ST. Ketamine potentiates analgesic effect of morphine in postoperative epidural pain control. Reg Anesth. 1996;21:534–41. [PubMed] [Google Scholar]
- 20.Nesher N, Serovian I, Marouani N, Chazan S, Weinbroum AA. Ketamine spares morphine consumption after transthoracic lung and heart surgery without adverse hemodynamic effects. Pharmacol Res. 2008;58:38–44. doi: 10.1016/j.phrs.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Pekoe GM, Smith DJ. The involvement of opiate and monoaminergic neuronal systems in the analgesic effects of ketamine. Pain. 1982;12:57–73. doi: 10.1016/0304-3959(82)90170-1. [DOI] [PubMed] [Google Scholar]
- 22.Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain. 1995;62:259–74. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- 23.Marland S, Ellerton J, Andolfatto G, Strapazzon G, Thomassen O, Brandner B, Weatherall A, Paal P. Ketamine: use in anesthesia. CNS Neurosci Ther. 2013;19:81–9. doi: 10.1111/cns.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fletcher PC, Honey GD. Schizophrenia, ketamine and cannabis: Evidence of overlapping memory defi cits. Trends Cogn Sci. 2006;10:167–74. doi: 10.1016/j.tics.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 26.Morgan CJ, Mofeez A, Brandner B, Bromley L, Curran HV. Ketamine impairs response inhibition and is positively reinforcing in healthy volunteers: a dose-response study. Psychopharmacology. 2004;172:298–308. doi: 10.1007/s00213-003-1656-y. [DOI] [PubMed] [Google Scholar]
- 27.Wang C, Liu F, Patterson TA, Paule MG, Slikker W Jr. Preclinical assessment of ketamine. CNS Neurosci Ther. 2013;19:448–53. doi: 10.1111/cns.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stotz M, Oehen HP, Gerber H. Histological fi ndings after long-term infusion of intrathecal ketamine for chronic pain: A case report. J Pain Symptom Manage. 1999;18:223–8. doi: 10.1016/s0885-3924(99)00069-x. [DOI] [PubMed] [Google Scholar]
- 29.Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Zhang X, Dissen GA, Creeley CE, Olney JW. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–4. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]


