Abstract
Polyethylene terephthalate LARS ligament were the remnant of LARS ligament used for repairing posterior cruciate ligament obtained from operation. We want to study histological characteristics and ultrastructure of polyethylene terephthalate LARS ligament after the reconstruction of anterior cruciate ligament in rabbits. Therefore, we replaced the original ACL with polyethylene terephthalate LARS ligament which was covering with the remnant of ACL in 9 rabbits (L-LARS group), while just only polyethylene terephthalate LARS ligament were transplanted in 3 rabbits (LARS group) with the remnant of ACL. Compared with group LARS, inflammatory cell reaction and foreign body reaction were more significant in group L-LARS. Moreover, electron microscopy investigation showed the tissue near LARS fibers was highly cellular with a matrix of thin collagen fibrils (50-100 nm) in group L-LARS. These above findings suggest the polyethylene terephthalate LARS ligament possess the high biocompatibility, which contributes to the polyethylene terephthalate LARS covered with recipient connective tissues.
Keywords: Anterior cruciate ligament, polyethylene terephthalate LARS ligament, biocompatibility
Introduction
Several artificial ligaments were developed and used in the 1980s as alternatives to biological material to reduce the long rehabilitation period and to overcome the problems of autografts or allografts. The results of anterior cruciate ligament (ACL) prostheses were encouraging in the short-term follow-up, but they presented a high failure rate over the long term, leading to a recurrence of instability [1-6]. Improved surgical techniques and new designs providing more anatomical form of reconstruction may offer better results. The LARS artificial ligament (Ligament Advanced Reinforcement System; Surgical Implants and Devices, Arc-sur-Tille, France) has recently been reported to be a suitable material [7]. At the same time, the current literature supports the use of LARS in the short to medium term [8,9]. However, high-quality studies with long-term follow-up are required to determine whether the use of LARS is preferable to autograft for ACL reconstruction over the longer term. Synovitis appears to be a rare complication closely related to imperfect graft positioning [10]. The LARS ligament designed as a “scaffold”-type of prosthesis, and combined the use of the ruptured ACL remnant and the synthetic ligament scaffold guarantee the patient to return to the same preoperative level of sport and/or profession in the shortest period of time [7-9]. Despite these promising results, reports about the biological properties of the LARS ligament and their possible tissue in-growth capacity are still lacking [11,12,14], even though, as poly (sodium styrene sulfonate) (polyNaSS) were grafted onto LARS prosthesis surface, it enhances cell organization and favours collagen and decorin deposits around fibers [13].
With the aim of clarifying the real capacity of host tissue to “re-grow” inside the ligament, we evaluated histological characteristics and ultrastructure of LARS ligament graft after implanted into the knees in rabbits.
Materials and methods
“LARS ligament prosthesis” preparation
The LARS ligament consists of two types: longitudinal fibers along the entire length of the ligament with either two knitted bundles in the intra-articular portion united by transverse fibers, ending in the letters CK for “continuous knit”, or with open longitudinal fibers in the intra-articular portion type ligaments, described as “open fibers” [7]. The “open fibers” are thought to allow far more in-growth clinically than the closed fibers, to reproduce the natural orientation of the anatomical fibers, and to reduce fatigue under torsion when the knee goes from flexion to extension, and to mimics the movement of cruciate ligament better. We obtained a total of 3 strips the remaining stump of LARS ligament (PPLY 110). Each stump of LARS ligament was manually divided into 5 “LARS ligament prosthesis”. Each “LARS ligament prosthesis” made up of five cords fiber of LARS ligament. Using the raw material, we suture cords fiber continuous into a cylindrical “LARS ligament prosthesis”. By the end, a total of 12 “LARS ligament prosthesis” of the length 80 mm, diameter 2 mm were obtained (Figure 1A, 1B). After the process, each “LARS ligament prosthesis” was packed with a sterile plastic packaging, sterilization by γ-rays and stored at room temperature.
Figure 1.
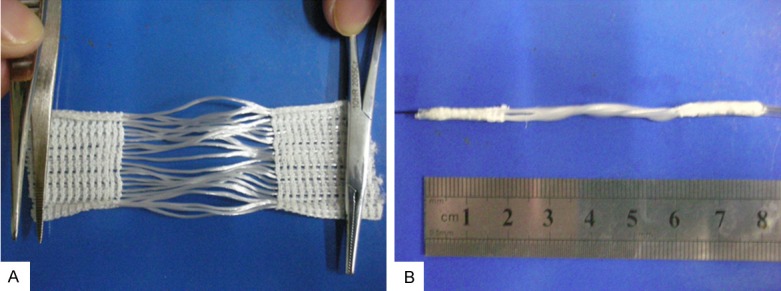
Preparation of “LARS ligament prosthesis” using the remnant of LARS ligament. A. The unfolded remnant of “LARS ligament prosthesis”; B. The prepared “LARS ligament prosthesis”.
Animal experiments
Twelve skeletally matured New Zealand white rabbits aged four months weighing 2,500-3,000 g were used for this study. Animal intervention met the animal ethical standard [15]. These rabbits were housed in the animal center of Suzhou University at a constant temperature of 23 and 60% humidity. They had free access to pellet food and water. Rabbits were anesthetized by intravenous injection of pentobarbital sodium 30 mg/Kg. All right knee were shaved, and the surgical fields were disinfected and sterilized with povidone iodine. Lateral knee incision was selected. When the ACL were removed, the tunnels were created using a 2 mm drill just along the direction from the tibia and femur as the direction of normal ACL. The “LARS ligament prosthesis” was positioned through the bone tunnels. The animals were divided into two groups according the purposes of this study regardless of body weight, and male or female. One group was named as L-LARS group, the other group, as a control, was named as LARS group. In L-LARS group, the tibial attachment point of ACL was retained. After the “LARS ligament prosthesis” were introduce into the knee, the retained remnant of ACL was used to cover “LARS ligament prosthesis” and sutured with “LARS ligament prosthesis” used 3-0 absorbable thread (Shanghai, China). This process same as clinical LARS ligament transplantation [7]. In LARS group, the ACL of right knee were completely resected, no retaining the remnant of ACL. After the introduction of “LARS ligament prosthesis”, all knee joint of animal flexion were maintained at 30 degrees, a diameter 2 mm of bone tunnel was drilled parallel to the right femoral condyle joint line, the stump of “LARS ligament prosthesis” were introduced the bone tunnel, and sutured with surrounding tissue. On the proximal tibial the stump of “LARS ligament prosthesis” were sutured with surrounding tissue immediately. Every time when implants was successful, the inspection was executed in order to ensure the free fibers bundle of “LARS ligament prosthesis” just lie in the knee of rabbits. Search the drawer test negative after washing the wound with normal saline, layered wound closure, sterile bandage. All limbs are unfixed, cage farmed, and activities limited. Each rabbit were intramuscular injected with 400,000 unit penicillin for 3 days.
HE and Masson-trichrome staining
Four rabbits were sacrificed at 1, 3, 6 months respectively after the operation in which 1 LARS group and 3 L-LARS group. Each time, neoligament were cut off from the point of the attachment of tibia and femur. The neoligament was dissected into two parts along longitudinal axis in the middle of 1/3. One part was used to observe under light microscopy, and the other was used to observe under scanning electron microscope (SEM).
For light microscopy the sample was fixed in 10% buffered formalin, dehydrated and embedded in paraffin. Serial transvers sections 5 mm thick were stained with haematoxylin and eosin to reveal connective tissue.
For Masson-trichrome staining [16] was performed in accordance with the standard protocol using a reagent kit (Sigma, USA). Ten separate fields of each section were observed under light microscopy at 400× magnification.
For SEM [17], all specimens were fixed with 2% glutaraldehyde solution, washed with 0.1 M sodium cacodylate buffer, and post-fixed with 1% osmium tetroxide. After dehydrating with an alcohol gradient series, different protocol was performed for SEM procedures. After dehydrating with isoamyl acetate again, the specimen was dried using a critical point dryer with HCP-2. After being coated with a layer of gold, all specimens were studied under a scanning electron microscope (QUANTA-200, Philips, Eindhoven, The Netherlands).
Results
General observation
At 1st month after the reconstruction knee joints was swelling significantly, joint effusion, synovial fluid was yellow. At 3rd month after surgery the effusion of reconstructed knee joints gradually alleviation. The synovial fluid also gradually became clearer and predominant in L-LARS group. After surgery 6 month, outer fibers “LARS ligament prosthesis” was wrapped connective tissue in L-LARS group, generally observed in all intra-articular neoligament showed a completely covered ligament with regenerated collagen and synovial tissue on which there was a relatively sparse vascular network similar to a normal ACL in L-LARS group. But in LARS group, there is no connective tissue wrapped LARS, neoligament just as pre-transplantation. All rabbit knee joint capsule hypertrophy were shown, no significant difference between the two groups, two groups of articular cartilage and meniscus damage were not clear.
HE staining of “LARS ligament prosthesis”
After 1 month surgery, we found “LARS ligament prosthesis” were surrounded by granulation tissue in L-LARS group. These granulation tissue enveloping the “LARS ligament prosthesis”, subdivided the fibers of “LARS ligament prosthesis” from surrounding and gradually formed “blind spot” (no organization long into the area), as the time past, these “blind spot” gradually reduced (Figure 2A). After 3 month surgery, the fibers of “LARS ligament prosthesis” were separated into “island-like” structure, the fibers were surrounding fibrous connective tissue within “LARS ligament prosthesis”. The fibers of “LARS ligament prosthesis” and surrounding tissue clearly boundaries. With the time past, among the fibers of “LARS ligament prosthesis” were gradually filling fibrous tissue, the fibrous tissue-like mutual connection, around the fibers of “LARS ligament prosthesis”. Overall these collagen fibers appeared confusion. After 6 months surgery, “LARS ligament prosthesis” and the fibrous tissue-like communicate with each other, but the collagen fibers with new artificial materials there are still gaps between, and the fibers bundle can’t be arranged along the stress direction (Figure 2B) in L-LARS group. In contrast to L-LARS group, in LARS group there no invasive fibrous tissue and no obvious covered by any fibrous tissue-like in 6month after surgery (Figure 2C).
Figure 2.
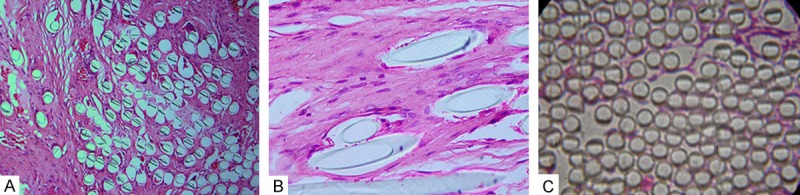
Histology of “LARS ligament prosthesis” (Hematoxylin-eosin staining). A. Collagen fibers grew into the inter-fibers of LARS ligament in the group of L-LARS at one month after implantation (HE×100); B. Collagen fibers appeared to be more oriented in the group of L-LARS at six months after implantation (×200); C. No collagen fibers ingrew into the inter-fiber of LARS ligament in the group of LARS at six months after implantation (×100).
Masson-trichrome staining of “LARS ligament prosthesis”
At 3rd month after the operation, in L-LARS group, in the internal of “LARS ligament prosthesis” can be seen shallow collagen fibers, collagen fibers appear disorder, can’t along the stress distribution, these collagen fibers appear more sparse in the center of “LARS ligament prosthesis” (Figure 3A). Elastic fibers are mainly located in peripheral of neolighment, just between the fibers of “LARS ligament prosthesis” and vascular. Among the fibers bundles of “LARS ligament prosthesis” can be seen a large number of new vascular tissue, neo-vascular more and more can be found the entrance of bone tunnel (Figure 3B). At the entrance of bone tunnel can observe the wearing or fracture of artificial materials, such wear debris was wrapped thick granulation tissue (Figure 3C). In LARS group no any fibrous tissue formation can be seen.
Figure 3.

Photograph of “LARS ligament prosthesis” stained by Masson. A. Collagen fibers grew into the fibers bundles of “LARS ligament prosthesis” at one month after operation in the group of L-LARS (×100); B. New vessels appeared among the fibers of “LARS ligament prosthesis” at three months after operation in the group of L-LARS (×100); C. The detritus were noted among the fibers of “LARS ligament prosthesis” in the bone tunnel at six months after operation in the group of LARS (×100).
Intra-articular synovial tissue HE staining
At 1 month after surgery, there was obvious inflammatory reaction in intra-articular synovial tissue of both LARS and L-LARS groups, cell proliferating significantly. Interstitial infiltration of the organizations were round or polygonal, multinucleated giant was seen every here and there, and enriching in blood vessels. 6 months after surgery, the inflammatory reaction began to decrease, but in shallow synovial tissue of both LARS and L-LARS group still can be seen clearly mononuclear cells, especially in group (Figure 4).
Figure 4.

Histocompatibility of the “LARS ligament prosthesis” (×100) Inflammatory reaction remarkably decreased in the group of LARS (Arrow indicated monocytes).
Ultrastructure of “LARS ligament prosthesis”
In Group L-LARS, the fiber bundles of “LARS ligament prosthesis” can be seen around by the elastic fibers and collagen fibers. Collagen fiber diameter are smaller, with an average diameter of 50 nm-100 nm (Figure 5A). Collagen fibers arranged around the artificial fibers, nearly irregular distribution. A large number of cells can be seen between artificial fibers and collagen fibers. Fibroblasts-like embedded along the loose connective tissue, increasing volume, riched in chromatin and apparent nucleoli, cytoplasm with expansion rough endoplasmic reticulum. Endoplasmic reticulum cavity can be seen occasionally, including the low electron density of the flocculent material. What indicated that fibroblast-like active proliferation (Figure 5B).
Figure 5.
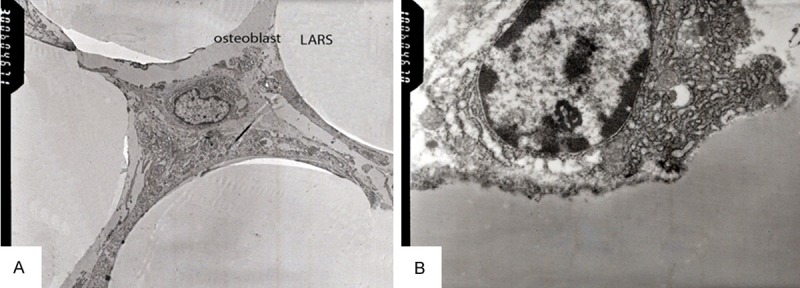
Ultrastructure of “LARS ligament prosthesis”. A. Among the artificial fiber, the collagen fibers were observed at six months after operation in the group of L-LARS (×3,000); B. Among the artificial fiber, many fibroblasts-like were observed with dilated cisterns in their granular rough endoplasmic reticulum (×10,000).
Discussion
In the past, a variety of synthetic ligament has been in clinical applications, including carbon fiber ligament [18], Leeds-Keio ligament [18] from Leeds University, UK and Japan jointly developed by Keio University, Kennedy LAD ligament [20], Gore-Tex ligament [21], Meadox ligament [22], ABC ligament [23], Trevira ligament [24] and so on. Old synthetic ligament had many problems, such as synovitis and predisposing the area to infection. Some ligaments, such as the Gore-Tex (Gore & Assoc, Flagstaff, AZ) ligament, were brittle and could not be placed around sharp corners; therefore, they had to be placed non isometrically, such as “over the top” Still, other materials in the past were coated with substances that were poorly tolerated in the joint, such as the carbon fiber ligaments in the 1960s and 1970s. Some of the newer ligaments, such as the Kennedy Ligament Augmentation Device (LAD) (3M Co, Minneapolis-St Paul, MN) [25] were marked improvements over the previous generations of synthetic ligaments because they were better tolerated and caused less problems within the joint; however, these ligaments had other problems such as eventual rupture, lack of tissue ingrowth, and low resistance to abrasion and fraying. Several artificial ligaments were developed and used in the 1980s as alternatives to biological material and to reduce the long rehabilitation period. Leeds-Keio ligament, a “scaffold”-type of prosthesis, from Leeds University, UK and Japan jointly developed by Keio University, regarding tissue ingrowth inside the ligament remains contradictory. The lack of effective organization, stress fatigue, ligament and bone surface wear artificial ligament is the main reason for the failure [26,27]. Follow-up of 40-78% over 15 years in the ligament graft failure, and failure rate increase with time [28].
The LARS artificial ligament (Ligament Advanced Reinforcement System; Surgical Implants and Devices, Arc-sur-Tille, France) has recently been reported to be a suitable material. LARS ligament is composed of two parts, intra-articular part, also named as free fiber, by the parallel longitudinal fibers of the ligament replicable activities of the human body to LARS ligament excellent addition to biomechanical properties, post-transplant immediately so that the stability of the knee to correct the dislocation, and the restoration of motor function as soon as possible [8], the reconstruction of knee extensor function [11]; Many retrospective studies has already shown satisfying results. Talbot et al. [29], such as in 1996 to 1999 using the LARS ligament ACL injury treatment, range of motion, Lysholm score and other aspects of follow-up, short-term satisfactory. Lavoie et al. [9] of 47 cases of acceptance of the LARS ligament reconstruction in patients with follow-up of 8~47 months, and Tenger compared with the preoperative score and found a marked increase over pre-operative. Nau et al. [10] in 2002 reported that the application of a LARS artificial ligament and bone - patellar tendon - bone ACL reconstruction of the forward-control study. 2-year follow-up after acute synovitis LARS ligament is not happening, stability and bone-patellar tendon-bone. There were no differences in early recovery activities have the obvious advantage.
These clinical applications have shown that the artificial ligament material has an excellent short-term and medium-term clinical efficacy [8-10].
There is still a lack of LARS ligament graft in vivo histological and ultrastructural study of prognosis [8-11]. In this study, for the first time, we observed in vitro the histological characteristics and ultrastructure of polyethylene terephthalate LARS ligament following the reconstruction of anterior cruciate ligament in rabbits. We found that: (1) polyethylene terephthalate LARS ligament has a very good histocompatibility. Notwithstanding at 1 month after surgery all the reconstruction of knee joints swelling significantly, joint effusion, synovial fluid was yellow. The synovial fluid may gradually become clear and appear predominance in L-LARS group. After surgery 6 month, polyethylene terephthalate LARS ligament fiber outer was wrapped connective tissue in L-LARS group, generally observed in all intra-articular neoligament showed a completely covered ligament with regenerated collagen and synovial tissue on which there was a relatively sparse vascular network similar to a normal ACL. All rabbit knee joint capsule hypertrophy were shown, no significant difference between the two groups, two groups of articular cartilage and meniscus damage are not clear. (2) polyethylene terephthalate LARS ligament has a better capability of in-growth, in L-LARS group, we were surprised to find there are fibrous connective tissue formed a sheath wrapped free fibers at 1 month after surgery, as time passes, the free fibers are divided and wrapped by fibrous connective tissue, although the fibrous tissue invaded is still immature, with disorder, new cell-rich tissue, and the lack of stress distribution along the collagen fibers, especially near the LARS ligament. Furthermore, between fibers of LARS ligament fibroblasts has higher proliferative activity, smaller the diameter of collagen fibers. All cases show that the synthesis of these neoligament still can’t bear the load, per fas et nefas its mechanical functions should be deliberate. But on another point, to some extent, these invaded fibrous connective tissues to some extent, an important practical significance, or intrusion into the fibrous tissue between the fibers and LARS ligament can reduce the friction and prolong the period of use of LARS ligament. LARS group after surgery in 6 month has yet to see the connective tissue fibers, indicating that the use of autologous tissue coverage LARS artificial ligament will help “biological process”. (3) L-LARS group see masson staining, artificial fibers in a large number of inter-tissue distribution of neovascularization, neovascularization located in fibrous connective tissue to provide the necessary source of nutrition.
In term of the model, In general, because of surgical accessibility, smaller animals have been satisfactory for modeling extra-articular ligaments, larger animals have been used most commonly for modeling anterior cruciate 1igament. In our study, a rabbit model of cut off ACL were chosen because the advantages of smaller animals are cost and ease of care, although larger animals may allow for more accurate tissue manipulation, quantitative analysis, human clinical parallel, and more suit for artificial ligament study. Otherwise, the graft of “LARS ligament prosthesis” are non-human standard LARS ligament, we discuss here mainly about the histological characteristics and ultrastructure of polyethylene terephthalate LARS ligament following the reconstruction of anterior cruciate ligament. Our results coincided with in vivo and in vitro finding of LARS ligament [25]. Due to this finding one might speculate about the capacity of collagen fiber can grow into the fibers of LARS ligament, and polyethylene terephthalate LARS ligament have high biocompatibility LARS ligament. Clinical investigations of this new generation of artificial ligaments have shown satisfactory results without causing synovialitis. The ingrowth of fibroblasts, which adhere to the fibers and build a capsule, might be an explanation. However, there are still much inadequate, such as the experimental animal relatively small, the sample relatively small and so on. And a shorter observation time used in the experiment LARS artificial ligament implant by non-human standard LARS ligament, which are to be in-depth study.
Acknowledgements
This paper was supported by the National Natural Science Foundation of China (Grant No. 81401604).
Disclosure of conflict of interest
None.
References
- 1.Ahlfeld SK, Larson RL, Collins HR. Anterior cruciate reconstruction in the chronically unstable knee using an expanded polytetrafluoroethylene (PTFE) prosthetic ligament. Am J Sports Med. 1987;15:326–330. doi: 10.1177/036354658701500406. [DOI] [PubMed] [Google Scholar]
- 2.Andersen HN, Bruun C, Sønergåro-Petersen PE. Reconstruction of chronic insufficient anterior cruciate ligament in the knee using a synthetic Dacron prosthesis A prospective study of 57 cases. Am J Sports Med. 1992;20:20–23. doi: 10.1177/036354659202000106. [DOI] [PubMed] [Google Scholar]
- 3.Barrett GR, Line LL, Shelton WR, Manning JO, Phelps R. The Dacron ligament prosthesis in anterior cruciate ligament reconstruction. A four-year review. Am J Sports Med. 1993;21:367–373. doi: 10.1177/036354659302100307. [DOI] [PubMed] [Google Scholar]
- 4.Gacon G. Problèmes généraux posés par l’utilisation des ligaments artificiels en chirurgie du genou. J Traumatol Sport. 1986;3:3–5. [Google Scholar]
- 5.Gillquist J, Odensten M. Reconstruction of old anterior cruciate ligament tears with a Dacron prosthesis. A prospective study. Am J Sports Med. 1993;21:358–366. doi: 10.1177/036354659302100306. [DOI] [PubMed] [Google Scholar]
- 6.Indelicato PA, Pascale MS, Huegel MO. Early experience with the GORE-TEX polytetrafluoroethylene anterior cruciate ligament prosthesis. Am J Sports Med. 1989;17:55–62. doi: 10.1177/036354658901700109. [DOI] [PubMed] [Google Scholar]
- 7.Nau T, Lavoie P, Duval N. A new generation of artificial ligaments in reconstruction of the anterior cruciate ligament: two-year follow-up of a randomised trial. J Bone Joint Surg Br. 2002;84:356–360. doi: 10.1302/0301-620x.84b3.12400. [DOI] [PubMed] [Google Scholar]
- 8.Ye JX, Shen GS, Zhou HB, Xu W, Xie ZG, Dong QR, Xu YJ. Arthroscopic reconstruction of the anterior cruciate ligament with the LARS artificial ligament: thirty-six to fifty-two months follow-up study. Eur Rev Med Pharmacol Sci. 2013;17:1438–46. [PubMed] [Google Scholar]
- 9.Gao K, Chen S, Wang L, Zhang W, Kang Y, Dong Q, Li L. Anterior cruciate ligament reconstruction with LARS artificial ligament: a multicenter study with 3-to 5-year follow-up. Arthroscopy. 2010;26:515–523. doi: 10.1016/j.arthro.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Glezos CM, Waller A, Bourke HE, Salmon LJ, Pinczewski LA. Disabling synovitis associated with lars artificial ligament use in anterior cruciate ligament reconstruction: a case report. Am J Sports Med. 2012;40:1167–1171. doi: 10.1177/0363546512438510. [DOI] [PubMed] [Google Scholar]
- 11.Mulford JS, Chen D. Anterior cruciate ligament reconstruction: a systematic review of polyethylene terephthalate grafts. ANZ Surg. 2011;81:785–789. doi: 10.1111/j.1445-2197.2011.05884.x. [DOI] [PubMed] [Google Scholar]
- 12.Machotka Z, Scarborough I, Duncan W, Kumar S, Perraton L. Anterior cruciate ligament repair with LARS (ligament advanced reinforcement system): a systematic review. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:29. doi: 10.1186/1758-2555-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhahri M, Abed A, Lajimi RH, Mansour MB, Gueguen V, Abdesselem SB, Maaroufi RM. Grafting of dermatan sulfate on polyethylene terephtalate to enhance biointegration. J Biomed Mater Res A. 2011;98:114–121. doi: 10.1002/jbm.a.33077. [DOI] [PubMed] [Google Scholar]
- 14.Trieb K, Blahovec H, Brand G, Sabeti M, Dominkus M, Kotz R. In vivo and in vitro cellular ingrowth into a new generation of artificial ligaments. Eur Surg Res. 2004;36:148–151. doi: 10.1159/000077256. [DOI] [PubMed] [Google Scholar]
- 15.The Ministry of Science and Technology of the People’s Republic of China. Guidance suggestion of caring laboratory animals. [2006-09-30]. http://www.most.gov.cn/zfwj/zfwj2006/200512/t2005121454389.htm.
- 16.Yamakado K, Kitaoka K, Nakamura T, Yamada H, Hashiba K, Nakamura R, Tomita K. Histologic analysis of the tibial bone tunnel after anterior cruciate ligament reconstruction using solvent-dried and gamma-irradiated fascia lata allograft. Arthroscopy. 2001;17:1–5. doi: 10.1053/jars.2001.24694. [DOI] [PubMed] [Google Scholar]
- 17.Danylchuk KD, Finlay JB, Krcek JP. Microstructural organization of human and bovine cruciate ligaments. Clin Orthop Relat Res. 1978;131:294–298. [PubMed] [Google Scholar]
- 18.Foster IW, Rális ZA, McKibbin B, Jenkins DH. Biological reaction to carbon fiber implants: the formation and structure of a carbon-induced “Neotendon”. Clin Orthop Relat Res. 1978;131:299–307. [PubMed] [Google Scholar]
- 19.Murray AW, Macnicol MF. 10-16 year results of Leeds-Keio anterior cruciate ligament reconstruction. Knee. 2004;11:9–14. doi: 10.1016/S0968-0160(03)00076-0. [DOI] [PubMed] [Google Scholar]
- 20.Miles S. The Kennedy LAD ligament augmentation device. NAT News. 1988;25:18–20. [PubMed] [Google Scholar]
- 21.Bolton W, Bruchman B. Biological properties of expanded GORE-TEX--polyfluoroethylene (PTFE) prosthesis ligament. Aktuelle Probleme in Chirurgie und Orthopädie. 1983;25:76. [PubMed] [Google Scholar]
- 22.Barrett GR, Savoie FH. Operative management of acute PCL injuries with associated pathology: long-term results. Orthopedics. 1991;14:687–692. doi: 10.3928/0147-7447-19910601-10. [DOI] [PubMed] [Google Scholar]
- 23.Mody BS, Howard L, Harding ML, Parmar HV, Learmonth DJ. The ABC carbon and polyester prosthetic ligament for ACL-deficient knees. Early results in 31 cases. J Bone Joint Surg Br. 1993;75:818–821. doi: 10.1302/0301-620X.75B5.8376448. [DOI] [PubMed] [Google Scholar]
- 24.Boszotta H, Helperstorfer W. Long-term results of arthroscopic implantation of a Trevira prosthesis for replacement of the anterior cruciate ligament. Aktuelle Traumatol. 1994;24:91–94. [PubMed] [Google Scholar]
- 25.Kdolsky R, Kwasny O, Schabus R. Synthetic augmented repair of proximal ruptures of the anterior cruciate ligament. Long-term results of 66 patients. Clin Orthop Relat Res. 1993;295:183–189. [PubMed] [Google Scholar]
- 26.Mascarenhas R, MacDonald PB. Anterior cruciate ligament reconstruction: a look at prosthetics-past, present and possible future. McGill J Med. 2008;11:29. [PMC free article] [PubMed] [Google Scholar]
- 27.Poddevin N, King MW, Guidoin RG. Failure mechanisms of anterior cruciate ligament prostheses: in vitro wear study. J Biomed Mater Res. 1997;38:370–381. doi: 10.1002/(sici)1097-4636(199724)38:4<370::aid-jbm10>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 28.Amis AA, Kempson SA. Failure mechanisms of polyester fiber anterior cruciate ligament implants: A human retrieval and laboratory study. J Biomed Mater Res. 1999;48:534–539. doi: 10.1002/(sici)1097-4636(1999)48:4<534::aid-jbm20>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Talbot M, Berry G, Fernandes J, Ranger P. Knee dislocations: experience at the Hopital du Sacre-Coeur de Montreal. Can J Surg. 2004;47:20–24. [PMC free article] [PubMed] [Google Scholar]


