Abstract
The effects of connective tissue growth factor (CTGF) gene silencing on the radiosensitivity of glioblastoma cells (GBM) were investigated. The lentivirus-mediated short hairpin RNA (shRNA) expression vector targeting CTGF was constructed and transinfected into U87MG human GBM cell line. The CTGF gene expression in U87MG cells was significantly down-regulated. After irradiation with 6 MV X-rays at a dose rate of 2.5 Gy/min, the clonogenicity, proliferation and migration of U87MG cells were assayed in vitro. The survival, proliferation and migration of U87MG cells were all remarkably inhibited by CTGF silencing (p < 0.05 vs control). Our results demonstrate that CTGF is important for GBM and CTGF gene silencing can be a potential tool to enhance the sensitivity of GBM to radiotherapy.
Keywords: Connective tissue growth factor, glioblastoma, radiosensitivity
Introduction
Glioblastoma (GBM) is the most common intracranial tumor in adults with high malignancy and poor prognosis [1]. Due to diffuse infiltrative growth of tumor cells, it is difficult to completely resect tumor and postoperative local radiotherapy is usually administrated. Unfortunately almost all patients will relapse after combined surgery, radiotherapy and chemotherapy [2]. Therefore, the development of new therapeutic approaches and strategies is urgent. One strategy to improve treatment outcome is to add specific signaling inhibitors to the chemoradiotherapy. A promising target candidate is the inhibition of connective tissue growth factor (CTGF) signaling.
CTGF, also known as CCN2, is secreted primarily by the endothelial cells, fibroblast, chondrocytes, smooth muscle cells and cancer cells. As a member of the CCN-protein-family, it has a variety of physiological and pathological functions [3,4]. Overexpression of CTGF has been reported in various solid tumors [5] and is associated with growth, migration and vascularization of GBM [6-8]. Retrospective studies showed that high level of CTGF expression was correlated with tumor grade and patient survival. Thus, CTGF may be a potential therapeutic target for GBM [9].
As radiotherapy is the mainstay treatment for GBM, we investigated whether down-regulation of CTGF expression would render human GBM cells more sensitive to radiation.
Materials and methods
Materials
The pGCL-GFP/pHelper 1.0/pHelper 2.0 (Gene Technology Co. Ltd., Shanghai, China) was used to construct lentiviral vector for the expression of shRNA targeting CTGF and mock control. Rabbit anti-human monoclonal antibody against CTGF and secondary goat anti-rabbit antibody conjugated to horseradish peroxidase were purchased from Santa Cruz Biotechnology Inc (Santa Cruz, CA, USA). Lipofectamine 2000 was bought from Invitrogen (Carlsbad, USA). The human GBM cell line U87MG was purchased from the American Type Culture Collection (ATCC; Manassas, VA) and were cultured in Dulbecco’s modified Eagle’s medium (DMEM. Gibco, USA) supplemented with 10% fetal calf serum (FCS) and 50 mg/ml penicillin/streptomycin at 37°C with 5% CO2 and 95% humidity.
Construct the lentivirus-mediated shRNA expression vector
The sequences of the CTGF-targeting and mock control shRNA were described in previous study [10], which are as follows: CTGF shRNA, 5’-GTT ACC GTG GTT GGG CCT GCC CTT TCA AGA GAA GGG CAG GCC CAA CCA CGG TTT TTT TC-3’; mock control shRNA, 5’-GTT TTC TCC GAA CGT GTC ACG TTT CAA GAG AAC GTG ACA CGT TCG GAG AAT TTT TTC-3’. These sequences were flanked by 5’ Hpa I restriction site and 3’ RNA Pol III termination signal site followed by XhoI restriction site. The shRNA sequences were cloned into the shRNA-expressing lentivirus vector, pGCL-GFP, downstream of the U6 promoter.
Lentiviral production and titration
Lentiviral vectors were generated by transient transfection of HEK293T cells with the pHelper 1.0, pHelper 2.0 packaging plasmids and the relevant transfer plasmid pGCL-CTGF. The harvested HEK293T cell medium was centrifuged at 690 g for 5 min at room temperature. The medium was then harvested and transferred to high-speed centrifuged at 50,000 g for 2 h at 4°C. The vector was harvested and resuspended in DMEM, centrifuged at 1400 g for 10 min and incubated with 5 U/ml Dnase I (Promega, Madison, USA) and 10 mM MgCl2 (Sigma) for 30 min. The vector was then aliquoted and stored at -80°C. The lentiviral titre was determined by serial dilution. Before use, all lentiviral vectors were titre maStched to 1 × 108 transducing units/ml.
Transfection
The U87MG cells were plated in 24-well plates at a density of 1 × 105 cells per well. When the cells reached 40%-50% confluence, they were transfected with the lentiviral vectors. GFP expression of cells was observed under a fluorescence microscope 72 h after transfection. Cells were used in the following experiments if the percentage of GFP-positive cells was above 90%.
RT-PCR assay
The expression of CTGF mRNA was detected by RT-PCR. The CTGF primers were 5’-GAC CGC AAC AAC GCA ATC-3’ (sense) and 5’-TAT TCC GTC TCC TTG GTT CAG-3’ (antisense). The β-actin primers were 5’-CGG CAT TGT CAC CAA CTG-3’ (sense) and 5’-CGC TCG GTC AGG ATC TTC-3’ (antisense). DNA amplification was performed for 28 cycles following an initial denaturation step at 94°C for 2 minutes in thermo cycler by using the following program: Denaturation at 94°C for 45 s, annealing at 57°C for 45 s, extension at 72°C for 45 s and final Extension: 72°C for 10 minutes. The PCR products were separated by agarose-gel electrophoresis.
Western blot analysis
Protein expression of CTGF was detected by Western blot. Total cells were collected and lysed with cell lysis buffer. Total protein concentration was determined by the Bradford method. Equivalent amounts of protein (30 ug) were separated on 10% of SDS-PAGE gels and transferred to polyvinylidene difluoride membranes. After being blocked for 2 hours in PBS with 0.1% Tween (PBS-T) containing 5% non-fat dried milk, the membranes were incubated with rabbit anti-human primary antibody overnight and washed three times with PBS-T. The membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:5,000) for 1 hour, then washed three times with PBS-T. The signals were developed with the ECL kit.
Clonogenic assay
U87MG cells were plated in 25 cm² flasks at different concentrations (10³ to 8 × 10³/ml?) and irradiated with 6 MV X-ray at a dose rate of 2.5 Gy/min. After 10 to 14 days’ culture, colonies formed were stained with crystal violet (Sigma) and those with at least 50 cells were counted under a microscopy. The linear quadratic equation was fitted to data sets to generate survival curves, and dose enhancement factor was calculated at 10% surviving fraction (DEF0.1, control radiation dose divided by the treated radiation dose). DEF values greater than 1.0 indicate enhancement of radiosensitivity.
Proliferation assay
50,000 cells were seeded in a 25 cm² flask over night and received a single 6 MV X-ray irradiation of 4 Gy. After incubatin for additional 72 h and stained with trypan blue, cells were counted.
Migration assay
Transwell migration assay was performed through a collagen IV coated membrane (invitrogen). After irradiation (4 Gy), U87MG cells were placed in the upper chamber of the transwells (104 cells in 50 μl/well). The lower chamber had been filled with DMEM with 10% BSA. After 12 hours of incubation, cells that had invaded the membrane were fixed and stained with a Diff-quick staining system. Representative photos were taken and migrated cells were counted in 6 random high-power fields per chamber under a light microscope.
Results
Lentivirus-mediated RNAi efficiently suppressed CTGF protein and mRNA expression in U87MG cells
To investigate the role of CTGF in U87MG cell’s growth and metastasis, we constructed lentivirus vector with CTGF shRNA and infected U87MG cells. After viral infection, more than 95% of the cells were GFP-positive, indicating a high efficiency of transfection. CTGF shRNA efficiently suppressed the CTGF mRNA level conformed by qRT-PCR and CTGF protein level conformed by Western blot in U87MG cells as compared to normal control and non-silencing group (p < 0.05 for all) (Figure 1A, 1B).
Figure 1.
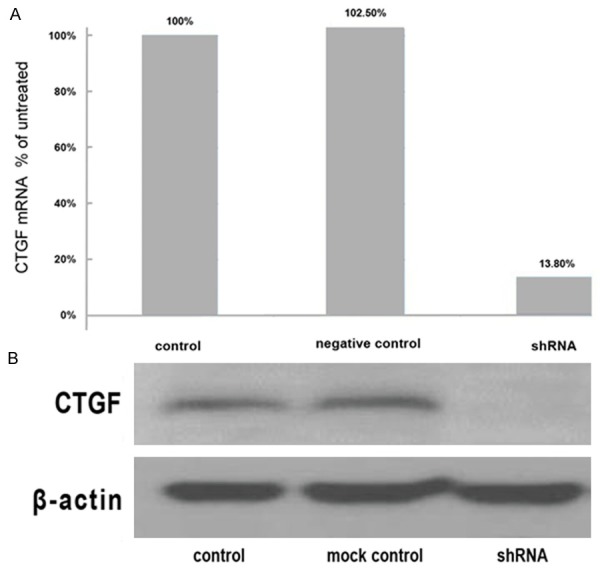
The expression levels of CTGF mRNA and protein. A. The mRNA expression of CTGF was significantly decreased in the shRNA group compared to the mock group (p < 0.05). No significant difference was observed between mock control and negative control. B. CTGF protein levels were determined by Western blotting. Weak expression of CTGF protein in shRNA group but strong expression in control and mock control group was shown.
Clonogenic survival analysis
To determine the effects of CTGF gene silencing on GBM tumor cell radiosensitivity, clonogenic survival analysis was conducted. CTGF shRNA interference reduced clonogenic survival of U87MG (Figure 2) following radiation, resulting in an increase in the radiosensitivity with a DEF0.1 of 1.28.
Figure 2.
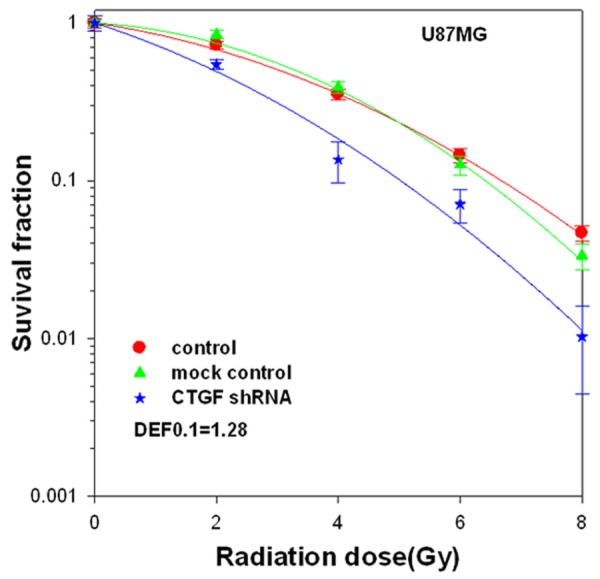
The effect of CTGF shRNA on radiosensitivity of U87MG cells. U87MG cells transfected with CTGF shRNA or mock control were plated in 25 cm² flasks, irradiated with 6 MV X-rays at a dose rate of 2.5 Gy/min. Colony-forming efficiency was determined 10 to 14 days later. Survival curve was generated and linear quadratic equation was fitted to data sets. The survival fraction of U87MG cells was significantly decreased in shRNA group as compared with control or mock control at 2 Gy, 4 Gy, 6 Gy and 8 Gy dose points (p < 0.05). DEF, dose enhancement factor.
Knockdown of CTGF inhibited U87MG cell growth in vitro
Frequent up-regulation of CTGF mRNA and protein in a variety of cancers suggests a potential oncogenic role of this gene. To investigate the possible antiproliferative effects of CTGF knockdown on U87MG cells, trypan blue staining assay was performed. CTGF shRNA transfected cells showed significantly reduced viability when compared with the control group (p < 0.05, Figure 3). Radiation treatment also inhibited the cell survival (p < 0.05, Figure 3). When radiation treatment was combined with CTGF knockdown, a further reduction of the cell number was observed (p < 0.05).
Figure 3.
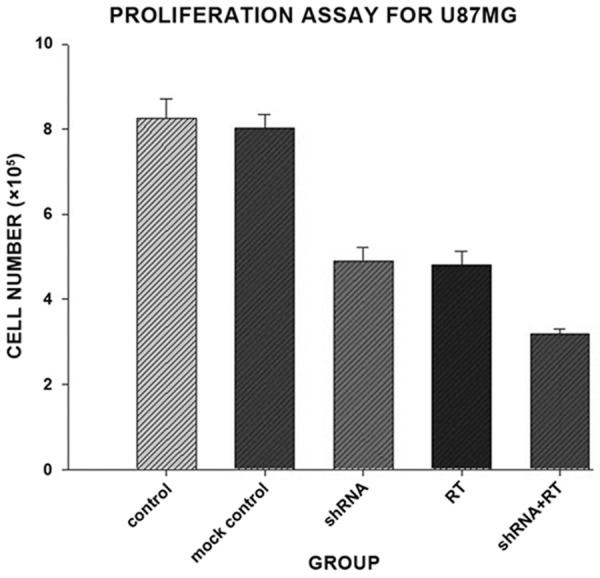
The effects of CTGF knockdown on tumor cell proliferation. U87MG cells transfected with CTGF shRNA or mock control received a single 6 MV X-ray irradiation of 4 Gy. The numbers of living cells were counted with trypan blue staining after incubation for 72 h. The proliferation of U87MG cells was significantly decreased in shRNA group as compared with control or mock control (p < 0.05). No significant difference was observed between shRNA group and RT group.
Knockdown of CTGF expression inhibited migration of U87MG cells
To evaluate the effects of CTGF knockdown on U87MG cell migration, transwell system was used. The number of migrated cells was significantly reduced from 249.67 ± 8.76 in the mock control group to 154.00 ± 12.49 in the shRNA group. However, irradiation could markedly enhanced migration capability of U87MG cells in vitro (309.33 ± 12.47) compared with mock control (p < 0.05), which could be counteracted by knockdown of CTGF (114.33 ± 11.05, p < 0.05). These data suggested that CTGF shRNA could suppress radiation-induced migration of U87MG cells (Figure 4A, 4B).
Figure 4.
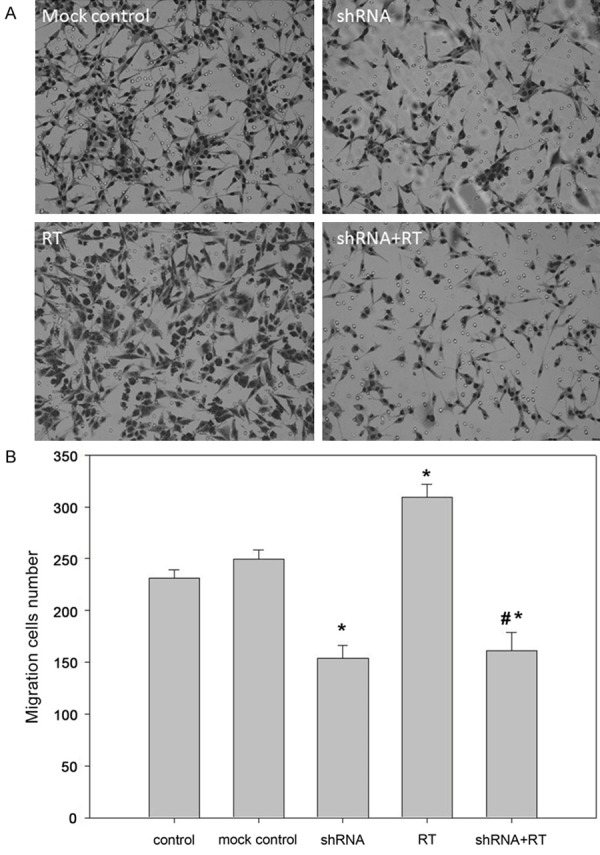
The effect of CTGF knockdown on tumor cell migration. U87MG cell migration was determined by counting cells that had migrated to the lower chamber 12 h after treatment. A. Stained cells seen under a microscope (200 ×). B. The number of migrating cells in each group. *, P < 0.05 versus control (or mock control); #*, P > 0.05 versus shRNA group and P < 0.05 versus other group.
Discussions
In the present study we showed that CTGF gene silencing can decrease the clonogenicity, proliferation and migration of human GBM cells and enhance their radiosensitivity. Despite intensive conventional treatment protocols, the prognosis of GBM is still dismal. The median survival is only 14.6 months [2]. The application of classic chemotherapeutic agent and the recent addition of anti-angiogenic drug bevacizumab has only prolonged the median survival for a few months [2,11]. Thus, more effective treatment strategies are urgently needed.
Studies have shown that the expression level of CTGF correlates with the migration ability of GBM cell lines in vitro and CTGF overexpression is associated with tumor growth and vascularization in vivo. Several retrospective studies reported that CTGF expression was correlated with tumor grade and survival of GBM patients. These indicate that CTGF may be an oncogene and down-regulation of CTGF gene expression may inhibit GBM and sensitize GBM cells to radiotherapy. We thus used lentivirus-mediated shRNA expression vector to knockdown CTGF and detected the effect of CTGF silencing on U87MG cells. Lentiviral vector has the advantage of infecting both dividing and non-dividing cells and the inhibition of target gene is stable and lasting. Despite there are some concerns with using lentiviral vectors and shRNAs for RNA interference, they still present a powerful tool that can be effective as long as appropriate designing, modifications and controls are applied [5,12].
In our study, we demonstrated markedly attenuation of clonogenicity, proliferation and migration of U87MG cells after CTGF knockdown. CTGF can activate adhesion and migration by binding integrins and a FAK-mediated actin-skeleton reorganization [6,13]. CTGF activates expression of MMPs and inhibit expression of their inhibitors, the TIMPs, which degrade ECM and facilitate migration of GBM cells [14]. Moreover, CTGF is also a downstream modulator of TGF-β [15-17]. There is evidence that overexpression of TGF-β is associated with proliferation, invasion/migration, and angiogenesis of glioma and blockade of TGF-β by the TGFβR-I kinase inhibitor LY2109761 can enhance radiation re-sponse and prolong survival in GBM [18]. TGF-β can induce expression of CTGF through TGF-β/Smads and cAMP/PKA/MAPK signal pathways [19].
Radiotherapy is an essential treatment for GBM and much effort has been paid to improve the radiosensitivity. In this study, we observed that CTGF silencing combined with radiotherapy was more effective than radiotherapy alone. In the clonogenic assay, CTGF silencing obviously improved radiosensitivity of U87MG cells (DEF0.1 = 1.28). CTGF gene silencing and radiotherapy both markedly decreased proliferation ability of U87MG cells as compared with control (p < 0.05) and no significant difference was ob-served between these two groups.
Surprisingly, migration of U87MG cells was promoted by radiotherapy. Sublethal doses of photon irradiation have been reported to be able to promote tumor invasion/migration [20,21]. The underlying mechanisms are unclear and may at least be caused by increased MMP-2 and MMP-9 gene expression [20,22]. MMPs can degrade collagen IV in the basement membrane, gelatin, laminin and ECM, enhance tumor invasion and metastasis.
It has been found that radiotherapy can promote the occurrence of epithelial-mesenchymal transition (EMT) of human colorectal cancer cells, human breast cancer cells and human lung cancer cell. EMT is the biological processes of the epithelial cells being transformed to the cells with mesenchymal phenotype. TGF-β plays an important role in this process [16]. As CTGF is a downstream modulator of TGF-β signaling pathway, CTGF may also be involved in the EMT process. Thus CTGF silencing may suppress EMT process, and reduce tumor invasion and migration.
In summary, through the lentivirus-mediated shRNA expression vector targeting CTGF we demonstrated CTGF knockdown may inhibit clonogenicity, proliferation and migration ability of U87MG cells and enhance the efficacy of radiation in vitro. Based on our findings CTGF may be a potential therapeutic target of GBM and warrants further study.
Acknowledgements
This work was supported in part by grants from National Nature Science Foundation of China (No. 81272780/H1618) and Huazhong University of Science and Technology frontier exploration fund (2013TS151).
Disclosure of conflict of interest
None declared.
References
- 1.Perry A, Louis D, Scheithauer B. WHO classification of tumours of the central nervous system. Lyon: IARC; 2007. pp. 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Bradham DM, Igarashi A, Potter RL, Grotendorst GR. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol. 1991;114:1285–1294. doi: 10.1083/jcb.114.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- 5.Zuo GW, Kohls CD, He BC, Chen L, Zhang W, Shi Q, Zhang BQ, Kang Q, Luo J, Luo X, Wagner ER, Kim SH, Restegar F, Haydon RC, Deng ZL, Luu HH, He TC, Luo Q. The CCN proteins: important signaling mediators in stem cell differentiation and tumorigenesis. Histol Histopathol. 2010;25:795–806. doi: 10.14670/hh-25.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang T, Zhang B, Pat BK, Wei MQ, Gobe GC. Lentiviral-mediated RNA interference against TGF-beta receptor type II in renal epithelial and fibroblast cell populations in vitro demonstrates regulated renal fibrogenesis that is more efficient than a nonlentiviral vector. J Biomed Biotechnol. 2010;2010:859240. doi: 10.1155/2010/859240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cicha I, Goppelt-Struebe M. Connective tissue growth factor: context-dependent functions and mechanisms of regulation. Biofactors. 2009;35:200–208. doi: 10.1002/biof.30. [DOI] [PubMed] [Google Scholar]
- 8.Cordes N, Hansmeier B, Beinke C, Meineke V, van Beuningen D. Irradiation differentially affects substratum-dependent survival, adhesion, and invasion of glioblastoma cell lines. Br J Cancer. 2003;89:2122–2132. doi: 10.1038/sj.bjc.6601429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie D, Yin D, Wang HJ, Liu GT, Elashoff R, Black K, Koeffler HP. Levels of expression of CYR61 and CTGF are prognostic for tumor progression and survival of individuals with gliomas. Clin Cancer Res. 2004;10:2072–2081. doi: 10.1158/1078-0432.ccr-0659-03. [DOI] [PubMed] [Google Scholar]
- 10.Fox JL, Dews M, Minn AJ, Thomas-Tikhonenko A. Targeting of TGFbeta signature and its essential component CTGF by miR-18 correlates with improved survival in glioblastoma. RNA. 2013;19:177–190. doi: 10.1261/rna.036467.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J. Clin. Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manjunath N, Wu H, Subramanya S, Shankar P. Lentiviral delivery of short hairpin RNAs. Adv Drug Deliv Rev. 2009;61:732–745. doi: 10.1016/j.addr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janouskova H, Maglott A, Leger DY, Bossert C, Noulet F, Guerin E, Guenot D, Pinel S, Chastagner P, Plenat F, Entz-Werle N, Lehmann-Che J, Godet J, Martin S, Teisinger J, Dontenwill M. Integrin alpha5beta1 plays a critical role in resistance to temozolomide by interfering with the p53 pathway in high-grade glioma. Cancer Res. 2012;72:3463–3470. doi: 10.1158/0008-5472.CAN-11-4199. [DOI] [PubMed] [Google Scholar]
- 14.Maeda A, Nishida T, Aoyama E, Kubota S, Lyons KM, Kuboki T, Takigawa M. CCN family 2/connective tissue growth factor modulates BMP signalling as a signal conductor, which action regulates the proliferation and differentiation of chondrocytes. J Biochem. 2009;145:207–216. doi: 10.1093/jb/mvn159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao L, Hantash BM. TGF-beta1 regulates differentiation of bone marrow mesenchymal stem cells. Vitam Horm. 2011;87:127–141. doi: 10.1016/B978-0-12-386015-6.00042-1. [DOI] [PubMed] [Google Scholar]
- 16.Yuan Q, Wang L, Zhang F, Wang R, Fu X, Peng Z, Ning W, Hu G, Wang Z, Tao L. Fluorofenidone suppresses epithelial-mesenchymal transition and the expression of connective tissue growth factor via inhibiting TGF-beta/Smads signaling in human proximal tubular epithelial cells. Pharmazie. 2011;66:961–967. [PubMed] [Google Scholar]
- 17.Gressner OA, Lahme B, Siluschek M, Rehbein K, Herrmann J, Weiskirchen R, Gressner AM. Activation of TGF-beta within cultured hepatocytes and in liver injury leads to intracrine signaling with expression of connective tissue growth factor. J Cell Mol Med. 2008;12:2717–2730. doi: 10.1111/j.1582-4934.2008.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M, Kleber S, Röhrich M, Timke C, Han N, Tuettenberg J, Martin-Villalba A, Debus J, Peschke P, Wirkner U, Lahn M, Huber PE. Blockade of TGF-beta signaling by the TGF-betaR-I kinase inhibitor LY2109761 enhances radiation response and prolongs survival in glioblastoma. Cancer Res. 2011;71:7155–7167. doi: 10.1158/0008-5472.CAN-11-1212. [DOI] [PubMed] [Google Scholar]
- 19.Yin D, Chen W, O’Kelly J, Lu D, Ham M, Doan NB, Xie D, Wang C, Vadgama J, Said JW, Black KL, Koeffler HP. Connective tissue growth factor associated with oncogenic activities and drug resistance in glioblastoma multiforme. Int J Cancer. 2010;127:2257–2267. doi: 10.1002/ijc.25257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park CM, Park MJ, Kwak HJ, Lee HC, Kim MS, Lee SH, Park IC, Rhee CH, Hong SI. Ionizing radiation enhances matrix metalloproteinase-2 secretion and invasion of glioma cells through Src/epidermal growth factor receptor-mediated p38/Akt and phosphatidylinositol 3-kinase/Akt signaling pathways. Cancer Res. 2006;66:8511–8519. doi: 10.1158/0008-5472.CAN-05-4340. [DOI] [PubMed] [Google Scholar]
- 21.Zhang QN, Wang DY, Wang XH, Hui TJ, Yang KH, Li Z, Li HY, Guo LY. Non-conventional radiotherapy versus conventional radiotherapy for inoperable non-small-cell lung cancer: A meta-analysis of randomized clinical trials. Thoracic Cancer. 2012;3:269–279. doi: 10.1111/j.1759-7714.2011.00094.x. [DOI] [PubMed] [Google Scholar]
- 22.Kim J, Lee SM, Yim JJ, Yoo CG, Kim YW, Han SK, Yang SC. Prognosis for non-small cell lung cancer patients with brain metastases. Thoracic Cancer. 2013;4:167–173. doi: 10.1111/j.1759-7714.2012.00164.x. [DOI] [PubMed] [Google Scholar]


