Abstract
Several lines of evidence have demonstrated that leptin is probably involved in the cognitive impairment which induced by a single injection of streptozocin (STZ). However, there is little literature reporting the relationship between cognitive impairment and cardiopulmonary bypass (CPB). This study aimed to investigate the role of leptin in the cognitive impairment of STZ-induced diabetic rats undergoing CPB. Wistar rats received 2 h of CPB exposure 1 month after a single intraperitoneal injection of 60 mg/kg of STZ or the vehicle. Behavioral results of rats in Morris water maze were recorded. After that, rat hippocampi were harvested for measuring leptin, tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β). Besides, we observed intracerebroventricular injection of leptin on the cognitive impairment of diabetic-rats undergoing CPB and measured behavioral performance and hippocampal TNF-α and IL-1β levels. Rats undergoing CPB significantly aggravates STZ-induced an increase of the latency to the platform and a decrease of the proportion of time spent in the target quadrant of rats in Morris water maze test. Additionally, the expression of leptin significantly decreased, while TNF-α and IL-1β levels significantly increased. Moreover, intracerebroventricular injection of leptin has a therapeutic effect for cognitive impairment of diabetic rats undergoing CPB. Leptin deficiency in hippocampus is probably involved in the cognitive impairment of streptozocin-induced diabetic rats undergoing cardiopulmonary bypass.
Keywords: Leptin, cognitive impairment, streptozocin, cardiopulmonary bypass
Introduction
Cognitive impairment has become an important disease affecting human health and quality of life. Its clinical manifestations include memory impairment, aphasia, agnosia, apraxia and visuospatial disorders, and can be also accompanied by anxiety, depression, agitation, impulsive behavior and other affective disorders [1,2]. Moreover, it is also the cause of disability, which may be a heavy burden to the society.
Diabetes is a common condition in older people, which affects about 20% of elderly persons [3]. Mounting studies indicate that the incidence of cognitive dysfunction in diabetic patients is much higher than those in the control group [4,5]. A previous study by Arvanitakis et al. [6] demonstrates that diabetes may be associated with an increased risk of developing cognitive impairment. Meanwhile, our previous results show that streptozocin (STZ)-induced diabetic rats are accompanied by cognitive impairment [7].
Cardiopulmonary bypass (CPB) is a life-supporting technology that has been widely used in the cardiac surgeries [8]. However, patients undergoing CPB are often accompanied by cognitive impairment. Bruggemans et al. [8] summarized that embolism, hypoperfusion, and the inflammatory response during the CPB are probably three risk factors for inducing cognitive impairment.
It is widely known that diabetes is an important inducing factor for the emergence of cardiovascular diseases [9]. On the other hand, our clinical experience told us that patients with diabetes undergoing cardiac surgeries will show a higher incidence of cognitive impairment postoperatively. Therefore, investigating the pathogenesis of cognitive impairment of patients with diabetes undergoing CPB is particularly important. The present study utilized STZ-induced diabetic rats to observe its cognitive function after undergoing CPB, and try to elucidate its probable pathogenesis.
Materials and methods
Animals and treatment
Male Wistar rats weighing 220-300 g were purchased from Shanghai Animal Center, Shanghai, China. Six rats were housed per cage with food and water available ad libitum and maintained on a 12-h light/dark cycle (lights on at 07:00 AM). Animal care was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and was approved by the Animal Use and Protection Committee of Soochow University.
Rats were randomly divided into 4 groups (n = 12 each). Rats were intraperitoneally pretreated with either a single injection of saline or STZ (Sigma, St. Louis, MO, USA) at a dose of 60 mg/kg. One month later, rats in the control and CPB and STZ+CPB group were exposed to CPB for 2 h under anesthesia state.
Moreover, after the first part of this experiment, we enrolled another 24 rats to observe intracerebroventricular injection of 10 μg leptin (Sigma-Aldrich) which dissolved 5 μl Tris-HCl on the cognitive function in rats receiving STZ plus CPB, while the sham group received the same volume saline. The protocol was in accordance with the first part.
CPB model
According to previous studies [10,11], rats were anesthetized by chloral hydrate and were intubated with a 16-G intravenous catheter which was connected to ventilator (Buxco Company, US). The ventilator was adjusted to maintain an arterial carbon dioxide tension between 36 to 42 mmHg. The right carotid artery was dissected and cannulated with a 22-G intravenous catheter for arterial inflow in the CPB circuit. Heparin (250 IU/kg) was administered to the animals before CPB. The right external jugular vein was cannulated with a 14-G catheter for venous return to the CPB circuit. A 5-mL cylinder syringe was used to collect blood from the venous return tubing in the CPB circuit. The venous return blood was pumped through a membrane oxygenator (Dongguan Kewei Medical Instrument Co. Ltd., China) by a peristaltic pump (Cole-Parmer Instrument Co., Vernon Hills, IL) via silicone tubing. The CPB circuit was pre-filled with 8 mL of hydroxyethyl starch (130/0.4), 0.5 mL of 5% NaHCO3, and 4 mL of lactated Ringer’s solution. When the flow rate reached the target CPB rate of 120 to 140 mL/kg/min (70%-80% of the normal cardiac output), it was maintained at this level for 2 h. At the end of 1 hour, the rat was eased from CPB through stepwise decreases in the flow rate and then disconnected from the circuit. When the rats recovered from anesthesia, the ventilator and the endotracheal tube were removed.
Morris water maze test
Cognitive function was performed by using Morris water maze test system between 09:00 h and 15:00 h. According to a previous study [12], the size of maze is 80 cm deep and 100 cm in diameter, which is divided into four quadrants of equal size on the monitor screen of a computer, filled with water to a depth of 30 cm. The water temperature was maintained at 23-24°C. The swimming paths of the rats were recorded by a video camera and analyzed by Videomot software (Huaibei Zhenghua Biologic Apparatus Facilities Co., Ltd., Huaibei, China). The trials were conducted for 4 consecutive days to observe escape latency and time spent in the quadrant of rats. Rats were placed in the maze from four random points of the tank and were allowed to search for the platform for 120 sec. However, if this was not achieved, the rat was gently placed on the platform and left for 20 sec. The escape latency and the proportion of time spent in the target quadrant were recorded.
Leptin, tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) measurement
TNF-α and IL-1β levels were determined by using ELISA kits. According to the manufacturer’s instructions, the ELISA was performed by adding 100 μl of each sample to each well in a 96-well plate of a commercially available rat ELISA kit (Wuhan Huamei Bioengineering Company, Wuhan, China). Microtiter plates were coated for overnight with the samples diluted 1:2 in sample diluent. The plates were then washed three times with sample diluent and a monoclonal anti-leptin, TNF-α and IL-1β antibodies diluted 1:1000 in sample diluent was added to each well and incubated for 3 h at room temperature. After washing, a peroxidase conjugated anti-rabbit antibody (diluted 1:1000) was added to each well and incubated at room temperature for 1 h. After adding streptavidin-enzyme, substrate and stop solution, the leptin, TNF-α and IL-1β levels were determined by absorbance in 450 nm respectively. Meanwhile, total protein was measured by Lowry’s method using bovine serum albumin as a standard.
Amyloid-β (Aβ) measurement
Animals were sacrificed immediately by decapitation. Protein concentrations were determined by using BCA method assay kit (Beyotime P0012S, Haimen, Jiangsu, China). After that, samples were centrifuged at 3000G at 4°C for 30 min to obtain the supernatants. Protein was separated by SDS-PAGE. The proteins were then transferred onto polyvinylidene difluoride membrane. After blocking with 5% non-fat milk, membranes were incubated with the primary antibodies: rabbit anti-Aβ (1:200, Sigma, USA). Subsequently, membranes were incubated for 1 hour at room temperature with secondary antibody of anti-rabbit HRP-conjugated IgG (1:20000, CWBIO, Beijing, China). Labeled protein was detected using chemiluminescence reagents (ECL; Amersham Bio-sciences, Little Chalfont, Buckinghamshire, UK) and the band intensity was analyzed (Image J software).
Statistical analysis
Data are expressed as mean ± SD. Statistical analyses were performed by one-way analysis of variance and post hoc analyses were performed using Fisher’s least significant difference tests. Statistical analyses were conducted using Statistical Product for Social Sciences (SPSS), version17.0 (SPSS, Inc., Chicago, IL, USA). Percentages of time spent in quadrant of rats in Morris water maze were evaluated by using X 2 tests. P < 0.05 was considered to indicate a statistically significant difference.
Results
Effects of CPB on escape latency and percent of time spent in quadrant of diabetic rats in Morris water maze test
Rats undergoing CPB or STZ administration significantly increased the escape latency as compared to the control group (Figure 1A), and decreased the percentage of time spent in the 4th quadrant (Figure 1B). Additionally, rats undergoing CPB plus STZ administration further increased the escape latency as compared to the CPB group (Figure 1A), and further decreased the percentage of time spent in the 4th quadrant (Figure 1A).
Figure 1.
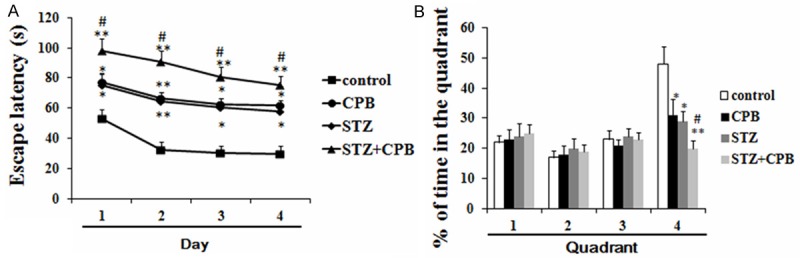
Effects of CPB on escape latency and percent of time spent in quadrant of diabetic rats in Morris water maze test (A, B); *P < 0.05 vs. saline, **P < 0.01 vs. control, #P < 0.05 vs. CPB. CPB, cardiopulmonary bypass; STZ, streptozocin.
Effects of CPB on the expression of leptin, TNF-α and IL-1β levels in STZ-induced diabetic rat hippocampus
CPB administration could increase the levels of TNF-α and IL-1β, but did not affect the leptin levels in hippocampus. Moreover, STZ administration could induce a significant increase of leptin, TNF-α and IL-1β levels in rat hippocampus. Besides, our results also showed that rats undergoing CPB plus STZ significantly increased the leptin, TNF-α and IL-1β levels in STZ-induced diabetic rat hippocampus as compared with CPB group (Figure 2A, 2B).
Figure 2.
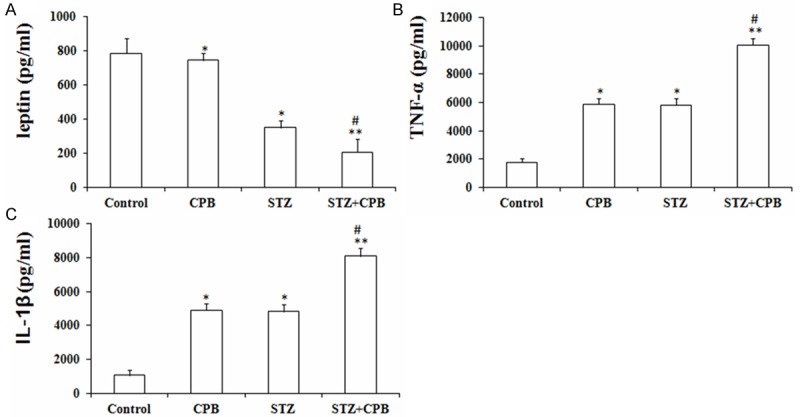
Effects of CPB on the expression of the TNF-α (A) and IL-1β (B) levels in STZ-induced diabetic rat hippocampus (A-C). *P < 0.05 and **P < 0.01 vs. control, #P < 0.05 vs. CPB. TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; CPB, cardiopulmonary bypass; STZ, streptozotocin.
Effects of intracerebroventricular injection of leptin on escape latency and percent of time spent in quadrant of diabetic rats in Morris water maze test
Intracerebroventricular injection of leptin significantly decreased the escape latency as compared to the sham group (Figure 3A), and increased the percentage of time spent in the 4th quadrant as compared with sham group (Figure 3B).
Figure 3.
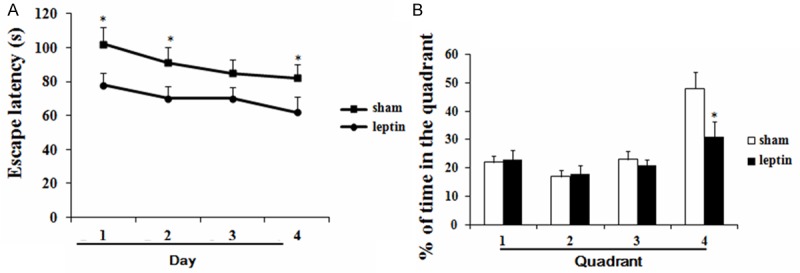
Effects of leptin on escape latency and percent of time spent in quadrant of diabetic rats undergoing CPB in Morris water maze test (A, B); *P < 0.05 vs. saline, **P < 0.05 vs. sham. CPB, cardiopulmonary bypass.
Effects of intracerebroventricular injection of leptin on Aβ, TNF-α and IL-1β levels in STZ-induced diabetic rat hippocampus
Intracerebroventricular injection of leptin showed significantly decreased levels of Aβ, TNF-α and IL-1β in STZ-induced diabetic rat hippocampus as compared with sham group (Figure 4A, 4B).
Figure 4.
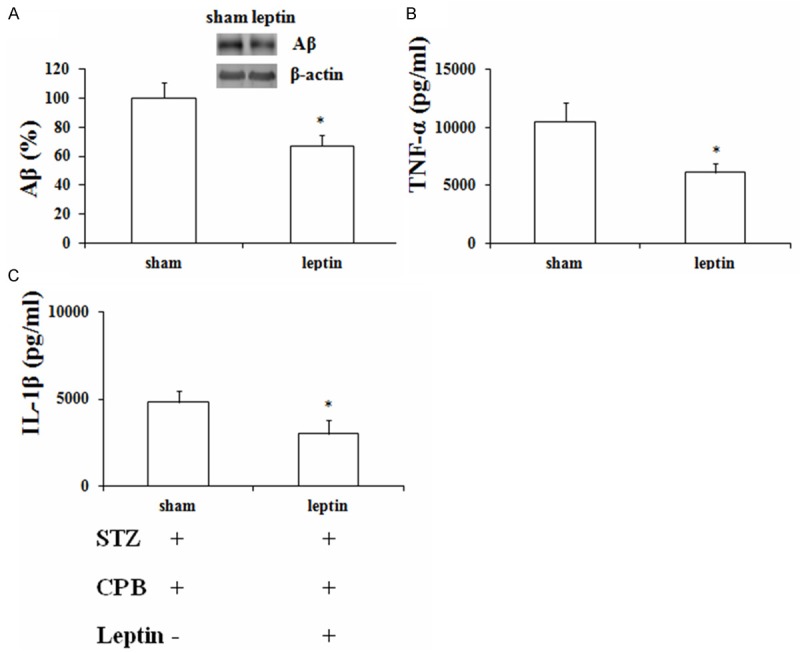
Effects of leptin on the expression of the Aβ, TNF-α (A) and IL-1β (B) levels in STZ-induced diabetic rat hippocampus (A-C). *P < 0.05 vs. sham. TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; CPB, cardiopulmonary bypass; Aβ, amyloid β.
Discussion
The results of this study demonstrate that STZ-induced diabetic rats undergoing CPB will show a decreased expression of leptin in hippocampus. Besides, intracerebroventricular injection of leptin will improve the cognitive impairment of diabetic rats undergoing CPB. Collectively, our results suggest that hippocampal leptin deficiency is probably involved in the cognitive impairment of STZ-induced diabetic rats undergoing CPB.
Morris water maze is widely used to study the spatial learning and memory-related brain function. In the present study, we used the Morris water maze to observe the effects of CPB and STZ on cognitive dysfunction in rat model. Our results indicate that CPB and STZ used alone and jointly can significantly increase the escape latency and decreased the percentage of time spent in the 4th quadrant. This indicates that we have successfully constructed such rat model of cognitive impairment.
Leptin is a hormone-like protein secreted by fat cells which plays an important role in regulating food intake and energy metabolism [13]. It has been reported that leptin is a potential cognitive enhancer as genetically obese rodents with dysfunctional leptin receptors show impairments in hippocampal synaptic plasticity [14]. Moreover, direct administration of leptin into the hippocampus can improve memory processing in mice [15]. The results of this study shows that cognitive impairment occurred in the diabetic rats after experiencing CPB, and the hippocampal leptin also decreased. Our results are consistent with our hypothesis and previous studies. Moreover, in order to further prove hippocampal leptin deficiency playing critical role in cognitive impairment, we intracerebroventricularly injected leptin to observe whether it can improve cognitive impairment or not. The results suggest that intracerebroventricular injection of leptin could significantly improve cognitive impairment. Our results from two aspects demonstrate that leptin deficiency contributes to the cognitive impairment in this animal model that we constructed.
At present, researches regarding the relationship between the CPB and leptin have not been reported. However, as to inflammatory cytokines, Kazmierski et al. [16] have demonstrated that the increased levels of IL-2 and TNF-α are associated with delirium in patients undergoing coronary-artery bypass graft surgery. Up-regulated expression of pro-inflammatory cytokines has been considered as one of the mechanisms of cognitive dysfunction. In addition, it has been widely acknowledged that the relationship between higher expression of inflammatory cytokines and STZ-caused cognitive impairment. Previous studies have shown that cognitive function will be improved if the excessive activation of the inflammatory response has been corrected [17]. Barrientos et al. [18] demonstrates that IL-1 receptor antagonist prevents cognitive decline and neuroinflammatory response. Thus, our results show that increased TNF-α and IL-1β may be involved in the cause of cognitive dysfunction in the model, which are consistent with previous studies.
Aβ is crucially involved in Alzheimer’s disease as the main component of the amyloid plaques found in the brains of Alzheimer patient [19]. A previous study by Greco et al. [20] has shown that leptin reduces pathology and improves memory in a mouse model of Alzheimer’s disease by inhibiting Aβ expression. Additionally, another study by Martins et al. [21] also demonstrates that leptin acts as a neuroprotective agent which is able to rescue hippocampal neurons from Aβ toxicity. In the present study, our results demonstrate that intracerebroventricular injection of leptin has an inhibitory effect on the Aβ.
In Conclusion, STZ-induced diabetic rats experiencing CPB can induce cognitive impairment, and its pathogenesis is probably related to the leptin deficiency in hippocampus.
Acknowledgements
We acknowledge Dr. Liang-Cai Ding for his technical assistance.
Disclosure of conflict of interest
None.
References
- 1.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 2.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 3.Meneilly GS, Tessier D. Diabetes in elderly adults. J Gerontol A Biol Sci Med Sci. 2001;56:M5–M13. doi: 10.1093/gerona/56.1.m5. [DOI] [PubMed] [Google Scholar]
- 4.Strachan MW, Price JF, Frier BM. Diabetes, cognitive impairment, and dementia. BMJ. 2008;336:6. doi: 10.1136/bmj.39386.664016.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van den Berg E, Kessels RP, Kappelle LJ, de Haan EH, Biessels GJ Utrecht Diabetic Encephalopathy Study Group. Type 2 diabetes, cognitive function and dementia: vascular and metabolic determinants. Drugs Today (Barc) 2006;42:741–754. doi: 10.1358/dot.2006.42.11.1003542. [DOI] [PubMed] [Google Scholar]
- 6.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 7.Yang C, Zhu B, Ding J, Wang ZG. Isoflurane anesthesia aggravates cognitive impairment in streptozotocin-induced diabetic rats. Int J Clin Exp Med. 2014;7:903–910. [PMC free article] [PubMed] [Google Scholar]
- 8.Bruggemans EF, Van Dijk JG, Huysmans HA. Residual cognitive dysfunctioning at 6 months following coronary artery bypass graft surgery. Eur J Cardiothorac Surg. 1994;9:636–643. doi: 10.1016/s1010-7940(05)80110-1. [DOI] [PubMed] [Google Scholar]
- 9.Harasym J, Oledzki R. Effect of fruit and vegetable antioxidants on total antioxidant capacity of blood plasma. Nutrition. 2014;30:511–517. doi: 10.1016/j.nut.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Jungwirth B, Eckel B, Blobner M, Kellermann K, Kochs EF, Mackensen GB. The impact of cardiopulmonary bypass on systemic interleukin-6 release, cerebral nuclear factor-kappa B expression, and neurocognitive outcome in rats. Anesth Analg. 2010;110:312–320. doi: 10.1213/ANE.0b013e3181bbc42e. [DOI] [PubMed] [Google Scholar]
- 11.Grocott HP, Mackensen GB, Newman MF, Warner DS. Neurological injury during cardiopulmonary bypass in the rat. Perfusion. 2001;16:75–81. doi: 10.1177/026765910101600111. [DOI] [PubMed] [Google Scholar]
- 12.Szyndler J, Piechal A, Blecharz-klin K, Skórzewska A, Maciejak P, Walkowiak J, Turzyńska D, Bidziński A, Płaźnik A, Widy-Tyszkiewicz E. Effect of kindled seizures on rat behavior in water Morris maze test and amino acid concentrations in brain structures. Pharmacol Rep. 2006;58:75–82. [PubMed] [Google Scholar]
- 13.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 14.Harvey J, Shanley LJ, O’Malley D, Irving AJ. Leptin: a potential cognitive enhancer? Biochem Soc Trans. 2005;33:1029–1032. doi: 10.1042/BST20051029. [DOI] [PubMed] [Google Scholar]
- 15.Harvey J, Solovyova N, Irving A. Leptin and its role in hippocampal synaptic plasticity. Prog Lipid Res. 2006;45:369–378. doi: 10.1016/j.plipres.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kazmierski J, Banys A, Latek J, Bourke J, Jaszewski R. Raised IL-2 and TNF-α concentrations are associated with postoperative delirium in patients undergoing coronary-artery bypass graft surgery. Int Psychogeriatr. 2014;26:845–855. doi: 10.1017/S1041610213002378. [DOI] [PubMed] [Google Scholar]
- 17.Dik MG, Jonker C, Hack CE, Smit JH, Comijs HC, Eikelenboom P. Serum inflammatory proteins and cognitive decline in older persons. Neurology. 2005;64:1371–1377. doi: 10.1212/01.WNL.0000158281.08946.68. [DOI] [PubMed] [Google Scholar]
- 18.Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, Rudy JW, Maier SF. Time course of hippocampal IL-1β and memory consolidation impairments in aging rats following peripheral infection. Brain Behav Immun. 2009;23:46–54. doi: 10.1016/j.bbi.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 20.Greco SJ, Hamzelou A, Johnston JM, Smith MA, Ashford JW, Tezapsidis N. Leptin boosts cellular metabolism by activating AMPK and the sirtuins to reduce tau phosphorylation and β-amyloid in neurons. Biochem Biophys Res Commun. 2011;414:170–174. doi: 10.1016/j.bbrc.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martins I, Gomes S, Costa RO, Otvos L, Oliveira CR, Resende R, Pereira CM. Leptin and ghrelin prevent hippocampal dysfunction induced by Aβ oligomers. Neuroscience. 2013;241:41–51. doi: 10.1016/j.neuroscience.2013.02.062. [DOI] [PubMed] [Google Scholar]


