Abstract
Objective: To investigate the core proteins (integrin subunits β1, β2 and β3) in the acute venous thrombi and validate the specificity and sensitivity of increased expression of integrin subunits β1, β2 and β3 in patients with venous thromboembolism. Methods: A total of 120 patients (73 females) with clinically proven acute VTE and aged between 24-90 years, and 120 non-VTE patients and healthy controls receiving physical examination matched in the sex and age were recruited. Flow cytometry was done to measure the expressions of blood integrin β1, β2 and β3. The receiver-operator characteristic (ROC) curve analysis was conducted to evaluate the diagnostic accuracy of integrin β1, β2 and β3. Results: The median levels of integrin β1, β2 and β3 were significantly higher in VTE patients than in non-VTE patients (P=0.000, 0.000 and 0.000, respectively) and healthy controls (P=0.000, 0.000 and 0.000, respectively). The ROC curves showed that integrin β1, β2 and β3 were specific diagnostic predictors of VTE with an area under the curve (AUC) of 0.870, 0.821, and 0.731, respectively. When three integrins were combined for diagnosis, the AUC of ROC curve was 0.916, and the sensitivity, specificity, positive and negative predictive values were 84.6%, 90.8%, 81.7% and 92.0%, respectively. Conclusion: The increased integrin β1, β2 and β3, as the core protein of venous thrombosis, have relatively high specificity and sensitivity for VTE and thus may serve as useful new biomarkers for the diagnoses of VTE.
Keywords: Antigens, CD29, antigens, CD18, integrin beta3, venous thromboembolism, biomarker
Introduction
Venous thromboembolism is a common disease with high prevalence. VTE includes pulmonary embolism (PE) and deep venous thrombosis (DVT). PE has a high misdiagnosis rate and high mortality and has been a healthy problem worldwide [1,2]. In acute phase of VTE, there are red thrombi, which are pathologically present with red blood cells, platelets, white blood cells and plasma proteins. In our previous study [3], results showed acute PE thrombi were mainly composed of fibrinogens, with a small amount of serum proteins and cytoskeletal proteins. Fibrinogenic thrombi are soluble, which explains a wide time window for the thrombolytic therapy of VTE (effectiveness at 2 weeks or longer after thrombosis), the effectiveness of catheter thrombectomy and the effectiveness of anticoagulant therapy with low molecular weight heparin for massive PE.
Acute venous red thrombi are mainly composed of fibrinogens, but the relationship between blood fibrinogens and receptors on cell membrane is still unclear. This has involvement of molecular mechanisms underlying the acute venous thrombosis. In our previous studies [4,5], genomics analysis, proteomics analysis and bioinformatics analysis of acute venous thrombi of PE patients confirmed that integrin β1, β2 and β3 were the core proteins of acute venous thrombi. In addition, thrombi collected from the pulmonary artery of acute PE patients were subjected to immunohistochemistry, and results showed integrin β1 mainly localized on lymphocytes, integrin β2 on neutrophils and integrin β3 on platelets [5]. Moreover, receptors of integrin β2 and β3 bound to fibrinogens to form the biofilter-like grid structure of thrombi in which red blood cells filled, forming red thrombi. Integrin β1, β2 and β3 are the core proteins of venous thrombi and their expressions increase in the active status of venous thrombosis. Thus, integrin β1, β2 and β3 may be promising markers for the early diagnosis of acute venous thrombosis. In the present study, a total of 120 patients with acute VTE, 120 patients without VTE and 120 healthy controls were recruited. VTE was diagnosed by imaging examinations. The expressions of integrin β1, β2 and β3 were detected in the peripheral blood cells and the sensitivity and specificity of integrin β1, β2 and β3 in the diagnosis of acute VTE were evaluated.
Material and methods
Study population
Inpatients or outpatients (n=120) with acute VTE were recruited from the Affiliated Tongji Hospital of Tongji University from April 2011 to December 2012. There were 47 males and 73 females with the mean age of 67.84±16.09 years (range: 24-90 years). Acute DVT was diagnosed by vascular ultrasonography or selective radionuclide venography (RNV); acute PE was confirmed by pulmonary angiography or CT pulmonary angiography; malignancies, autoimmune diseases, oral medication of immunosuppressant and pregnancy were excluded. In addition, age and gender matched patients (n=120) without VTE (mean age: 68.16±12.17 years; range: 25-90 years) were also recruited as controls. Non-VTE controls were inpatients in the same period and had no clinical symptoms and signs of VTE, and VTE was excluded by venous ultrasonography or pulmonary angiography. Healthy controls who received routine physical examinations (n=120; mean age: 65.43±10.30 years; range: 20-91 years) were also included in the present study. This study was approved by the Ethics Committee of Affiliated Tongji Hospital of Tongji University, and informed consent was obtained before study.
Blood collection and measurements
Medical record was reviewed in all the patients. Fasting venous blood (2 ml) was collected from the cubital vein in the morning and anti-coagulated with EDTA. Two hours later, the anti-coagulated blood was processed as follows.
Monoclonal antibodies against integrin β1 (CD29), β2 (CD18) and β3 (CD61) (BD company) were used to detect the integrin β1, β2 and β3, respectively. In brief, 100 μl of EDTA treated blood was added to each tube and control tube was also included. Then, 20 μl of mouse IgG1-PC5, IgG1-FITC or IgG1-PE was added (20 μl of IgG2-PE was mixed with CD29), followed by addition of corresponding fluorescence antibodies (20 μl). Following vortexing, incubation was done in dark for 30 min at room temperature. Then, 500 μl of hemolysin (BECKMAN-COULTER) was added, followed by incubation at 37°C for 30 min. Following washing, 500 μl of sheath fluid was added to each tube, followed by flow cytometry (EPICS XL-4; BECKMAN-COULTER). The PMT voltage, fluorescence compensation and sensitivity of standard fluorescent microspheres (EPICS XL-4; BECKMAN-COULTER) were used to adjust the flow cytometer and a total of 10000 cells were counted for each tube. The corresponding cell population in the scatterplot of isotype controls was used to set the gate, and the proportion of positive cells was determined in each quadrant (%). SYSTEM-II was used to process the data obtained after flow cytometry.
Statistical analysis
Statistical analyses were performed using SPSS 18.0 software. According to a Kolmogorov-Smirnov analysis, the variables of integrins showed a skewed distribution. Thus, these variables are presented as medians (1st, 3rd quartiles). The medians and interquartile ranges are plotted in the figures as a box and whisker plot. In addition, differences in the variables between patients and healthy controls were examined statistically using student’s t-test or a two-tailed Mann-Whitney U-test. The chi-square test and Fisher’s exact probabilities were used for the comparison between the observed and expected frequencies. Furthermore, the receiver operating characteristic (ROC) curves for predicting survival were plotted and analyzed to compare diagnostic performance. Youden’s index [6] was calculated and optimum diagnostic cutoff levels, sensitivity, specificity, positive and negative predictive values were analyzed according to the maximum of Youden’s index. Values of p < 0.05 were considered statistically significant.
Results
Patients’ characteristics
A total of 120 VTE patients and 120 non-VTE patients matched in age and sex were enrolled into this study. Among 120 VTE patients, 72 (60%) were diagnosed with DVT and 48 (40%) with PE. There were 8 (6.67%) patients suffering from both DVT and PE. Patients’ demographics, type of episodes, disease history and integrin and D-Dimer levels are shown in Table 1.
Table 1.
The characteristics of VTE patients and non-VTE patients at baseline
| Parameters | VTE Patients (n=120) | Non-VTE Patients (n=120) | P |
|---|---|---|---|
| Demographics | |||
| Age mean (SD) | 67.84 (16.09) | 68.16 (12.17) | 0.864 |
| Female (n, %) | 73 (60.83%) | 68 (56.67%) | 0.600 |
| Type of episode, n (%) | |||
| DVT | 72 (60.00%) | ||
| PE | 48 (40.00%) | ||
| DVT+PE | 8 (6.67%) | ||
| Comorbidities (%) | |||
| COPD | 6 (5.00%) | 6 (5.00%) | 1.000 |
| CAD | 35 (29.17%) | 39 (32.50%) | 0.675 |
| Diabetes mellitus | 18 (15.00%) | 16 (13.33%) | 0.853 |
| Hypertension | 47 (39.17%) | 40 (33.33%) | 0.421 |
| CI | 19 (15.83%) | 16 (13.33%) | 0.715 |
| Blood levels (pg/ml) | |||
| Integrin β1 | 14.50 (10.60, 18.80) | 7.85 (5.80, 9.28) | 0.000 |
| Integrin β2 | 94.90 (91.35, 97.00) | 88.95 (83.58, 91.48) | 0.000 |
| Integrin β3 | 11.50 (9.77, 15.65) | 8.90 (7.80, 10.40) | 0.000 |
| D-Dimer (ng/ml) | 0.72 (0.12, 4.63) | 0.06 (0.05, 0.18) | 0.000 |
Footnotes: Ages are shown as mean (SD), integrins and D-Dimer level as median (1st, 3rd quartiles), and categorical data as the number and percentage to the sample group. Age was compared with student’s t test. Gender was compared with chi-square test. Integrin and D-Dimer level was compared with Mann-Whitney U test. Abbreviations: DVT, deep venous thrombosis; PE, pulmonary embolism; COPD, chronic obstructive pulmonary disease; CAD, coronary artery disease; CI, cerebral infarction.
Blood integrin levels
Blood Integrin levels were quantified by flow cytometry. The median levels of integrin β1, β2 and β3 were all significantly higher in VTE patients when compared with non-VTE patients (P=0.000, 0.000 and 0.000, respectively) and healthy controls (P=0.000, 0.000 and 0.000, respectively). When compared between non-VTE patients and healthy controls, there was no statistical significance in the blood levels of integrin β1, β2 and β3 (P=0.572, 0.544 and 0.547, respectively) (Figure 1).
Figure 1.
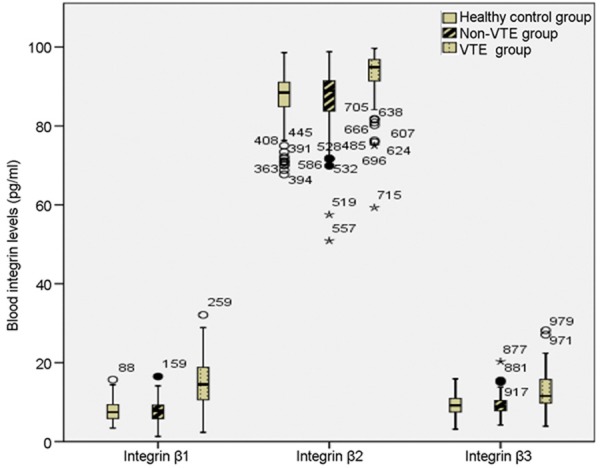
Blood integrin β1, β2 and β3 levels in VTE patients, non-VTE patients and healthy controls. Integrin levels were compared with Mann-Whitney U test. Significant differences in blood integrin β1, β2 and β3 levels were observed between VTE patients and non-VTE patients (P=0.000, 0.000 and 0.000, respectively), and between VTE patients and healthy controls (P=0.000, 0.000, and 0.000, respectively). When compared between non-VTE patients and healthy controls, there were no significant differences (P=0.572, 0.544 and 0.547, respectively).
ROC curve analysis
ROC curve analysis was utilized to assess diagnostic performance of these proteins. When a comparison was made between VTE patients and non-VTE patients, the AUC of integrin β1, integrin β2 and integrin β3 was 0.869 (P=0.000, 95% CI: 0.821-0.916), 0.809 (P=0.000, 95% CI: 0.752-0.867) and 0.742 (P=0.000, 95% CI: 0.676-0.809), respectively, and that of combined three integrins and D-Dimer was 0.917 (P=0.000, 95% CI: 0.878-0.956), and 0.811 (P=0.000, 95% CI: 0.754-0.868), respectively (Figure 2).
Figure 2.
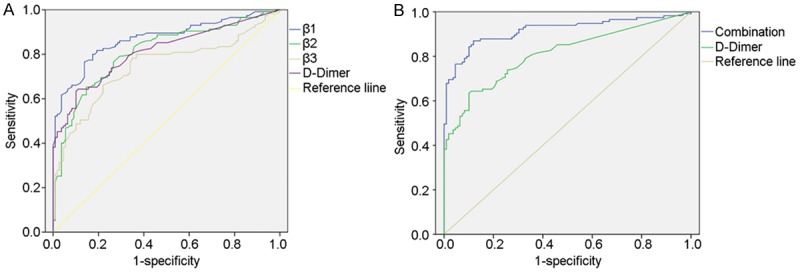
Receiver Operating Characteristic (ROC) curves for distinguishing VTE patients from non-VTE patients. The comparative ROC curves for all the three integrins (left), combination of three integrins (right) and D-Dimer are provided. The area under the curve (AUC) of integrin β1, integrin β2 and integrinβ3 was 0.869 (P=0.000, 95% CI: 0.821-0.916), 0.809 (P=0.000, 95% CI: 0.752-0.867) and 0.742 (P=0.000, 95% CI: 0.676-0.809), respectively, and that of combined three integrins and D-Dimer was 0.917 (P=0.000, 95% CI: 0.878-0.956), and 0.811 (P=0.000, 95% CI: 0.754-0.868), respectively.
When a comparison was made between VTE patients and healthy controls, the AUC of integrin β1 integrin β2 and integrin β3 was 0.875 (P=0.000, 95% CI: 0.829-0.922), 0.828 (P=0.000, 95% CI: 0.774-0.882), and 0.721 (P=0.000, 95% CI: 0.655-0.786), and that of combined three integrins was 0.915 (P=0.000, 95% CI: 0.876-0.954) (Figure 3).
Figure 3.
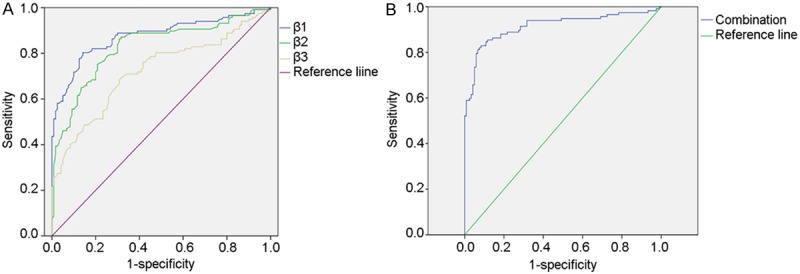
Receiver Operating Characteristic (ROC) curves for distinguishing VTE patients from healthy controls. The comparative ROC curves for all the three integrins (left) and t he combination of integrins (right) are provided. The area under the curve (AUC) of integrin β1, integrin β2 and integrin β3 was 0.875 (P=0.000, 95% CI: 0.829-0.922), 0.828 (P=0.000, 95% CI: 0.774-0.882), and 0.721 (P=0.000, 95% CI: 0.655-0.786), respectively, and that of combined three integrins was 0.915 (P=0.000, 95% CI: 0.876-0.954).
When a comparison was made between VTE patients and non-VTE patients plus healthy controls, the AUC of integrin β1, integrin β2 and integrin β3 was 0.870 (P=0.000, 95% CI: 0.825-0.915), 0.821 (P=0.000, 95% CI: 0.771-0.871) and 0.731 (P=0.000, 95% CI: 0.671-0.792), and that of combined three integrins was 0.916 (P=0.000, 95% CI: 0.878-0.953) (Figure 4).
Figure 4.
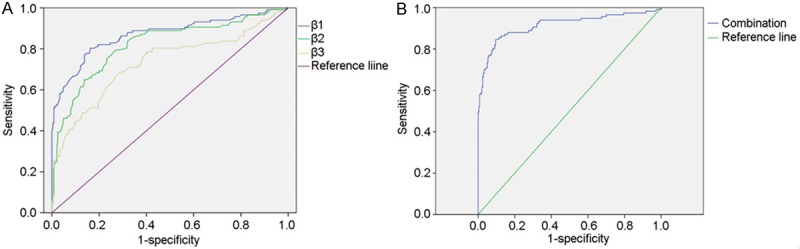
Receiver Operating Characteristic (ROC) curves for distinguishing VTE patients from non-VTE patients plus healthy controls. The comparative ROC curves for all the three integrins (left) and the combination of integrins (right) are provided. The area under the curve (AUC) of integrin β, integrin β2 and integrin β3 was 0.870 (P=0.000, 95% CI: 0.825-0.915), 0.821 (P=0.000, 95% CI: 0.771-0.871) and 0.731 (P=0.000, 95% CI: 0.671-0.792), respectively, and that of combined three integrins was 0.916 (P=0.000, 95% CI: 0.878-0.953).
Diagnostic performance of three integrins and combination of them were shown in Tables 2 and 3.
Table 2.
Diagnostic performance of three integrins for VTE
| Integrin β1 | Integrin β2 | Integrin β3 | |
|---|---|---|---|
| AUC | 0.870 | 0.821 | 0.731 |
| Optimum cutoff (pg/ml) | 10.29 | 91.10 | 10.35 |
| Sensitivity | 80.3% | 78.6% | 68.4% |
| Specificity | 83.7% | 73.7% | 71.2% |
| Positive predictive value | 71.1% | 59.4% | 54.3% |
| Negative predictive value | 89.3% | 87.6% | 81.8% |
Table 3.
Diagnostic performance of combined three integrins for VTE
| Integrin β1+β2 | Integrin β1+β3 | Integrin β2+β3 | β1+β2+β3 | |
|---|---|---|---|---|
| AUC | 0.892 | 0.903 | 0.834 | 0.916 |
| Sensitivity | 82.9% | 82.5% | 76.9% | 84.6% |
| Specificity | 85.4% | 87.1% | 75.8% | 90.8% |
| Positive predictive value | 73.5% | 76.2% | 60.1% | 81.7% |
| Negative predictive value | 91.1% | 90.9% | 87.1% | 92.0% |
Discussion
Integrins are cell adhesion receptors, and they play an important role in the interaction between cells and extracellular matrix (ECM), and cell-cell interactions [7]. Integrins are heterodimers consisting of noncovalently linked α and β transmembrane glycoprotein subunits. They consist of at least 18 α and 8 β subunits, producing 24 different heterodimers [8]. The α and β subunits separate from each other once the integrin is activated, and then the α subunit binds the ligand. The β1 subunit is expressed mainly on cell surface of lymphocytes, and its ligands consist of laminins, collagens, thrombospondin, vascular cell adhesion molecule 1 and fibronectin [8,9]. The β2 subunit is distributed on cell surface of neutrophils and monocytes, and ligands for this subunit include fibrinogen, complement component iC3b, intracellular adhesion molecule-1, factor X and so on [10,11]. The β3 subunit is observed on platelets, and this subunit binds fibrinogen, fibronectin, vitronectin v-on Willebrand factor (vWF) and thrombospondin [12,13].
Integrin β1 is mainly expressed on lymphocytes, and increased integrin β1 expression is related to the inflammation, thrombosis, homing of lymphocytes and metastasis of cancer cells. Integrin β2 is mainly distributed on neutrophils and monocytes, and increased integrin β2 expression is associated with inflammation. Integrin β3 is mainly expressed on platelets, and elevated integrin β3 expression suggests the platelet activation which is associated with platelet aggregation and thrombosis.
Our results showed the expressions of integrin β1, β2 and β3 in the peripheral blood of VTE patients were significantly higher than those in non-VTE patients and healthy controls. ROC analysis was employed to evaluate the effectiveness of these proteins in the diagnosis of PE. Results showed the AUC of integrin β1, β2 and β3 was 0.870, 0.821 and 0.731, respectively, in the diagnosis of acute VTE. According to the Youden index, the optimum cutoff of integrin β1, β2 and β3 was 10.29 pg/ml, 91.10 pg/ml and 10.35 pg/ml, at which the sensitivity, specificity, positive predictive value and negative predictive value was 80.3%, 83.7%, 71.1% and 89.3%, respectively for integrin β1; 78.6%, 73.7%, 59.4% and 87.6%, respectively for integrin β2; 68.4%, 71.2%, 54.3% and 81.8%, respectively for integrin β3. When all of integrin β1, β2 and β3 were used for the diagnosis of acute VTE, the AUC was 0.916.
Our findings also revealed that the D-Dimer level in VTE patients was markedly higher than that in non-VTE patients. D-Dimer is the most common indicator used in the diagnosis of VTE.
It is a degradation product of cross-linked fibrin that is formed immediately after thrombin-generated fibrin clots are degraded by plasmin and reflects a global activation of blood coagulation and fibrinolysis. Being the best-recognized biomarker for the initial assessment of suspected VTE, a negative value of D-Dimer may safely rule out both DVT and PE with a high sensitivity of 83%-96% and a negative predictive value of nearly 100% [14-18]. However, due to its low specificity (around 40%), D-Dimer, even combined with clinical criteria, cannot be used to diagnose the VTE.
This is explorative clinical study aiming to validate our previous findings. Findings from genomics analysis, proteomics analysis and immunohistochemistry demonstrate that the core proteins of venous thrombi are integrin β1, β2 and β3. Clinical study also confirm that integrin β1, β2 and β3 increase significantly in VTE patient, and they have high specificity and sensitivity in the diagnosis of VTE.
Acknowledgements
The study was granted by “12th Five-year” National Science & Technology Supporting Program (2011BAI11B16).
Disclosure of conflict of interest
None.
References
- 1.Cardiovascular Disease Educational and Research Trust; Cyprus Cardiovascular Disease Educational and Research Trust; European Venous Forum; International Surgical Thrombosis Forum; International Union of Angiology; Union Internationale de Phlébologie. Prevention and treatment of venous thromboembolism. International Consensus Statement (guidelines according to scientific evidence) Int Angiol. 2006;25:101–161. [PubMed] [Google Scholar]
- 2.Piazza G, Goldhaber SZ. Physician alerts to prevent venous thromboembolism. J Thromb Thrombolysis. 2010;30:1–6. doi: 10.1007/s11239-009-0404-5. [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Gong Z, Jiang J, Xu W, Duan Q, Liu J, Qin C. Confusion of wide thrombolytic time window for acute pulmonary embolism: mass spectrographic analysis for thrombus proteins. Am J Respir Crit Care Med. 2011;184:145–146. doi: 10.1164/ajrccm.184.1.145. [DOI] [PubMed] [Google Scholar]
- 4.Xie Y, Duan Q, Wang L, Gong Z, Wang Q, Song H, Wang H. Genomic characteristics of adhesion molecules in patients with symptomatic pulmonary embolism. Mol Med Rep. 2012;6:585–590. doi: 10.3892/mmr.2012.940. [DOI] [PubMed] [Google Scholar]
- 5.Wang LM, Duan QL, Yang F, Yi XH, Zeng Y, Tian HY, Lv W, Jin Y. Activation of circulated immune cells and inflammatory immune adherence are involved in the whole process of acute venous thrombosis. Int J Clin Exp Med. 2014;7:566–572. [PMC free article] [PubMed] [Google Scholar]
- 6.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavers M, Afzali B, Macey M, McCarthy DA, Irshad S, Brown KA. Differential expression of beta1 and beta2 integrins and L-selectin on CD4+ and CD8+ T lymphocytes in human blood: comparative analysis between isolated cells, whole blood samples and cryopreserved preparations. Clin Exp Immunol. 2002;127:60–65. doi: 10.1046/j.1365-2249.2002.01711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiorilli P, Partridge D, Staniszewska I, Wang JY, Grabacka M, So K, Marcinkiewicz C, Reiss K, Khalili K, Croul SE. Integrins mediate adhesion of medulloblastoma cells to tenascin and activate pathways associated with survival and proliferation. Lab Invest. 2008;88:1143–1156. doi: 10.1038/labinvest.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rezzonico R, Chicheportiche R, Imbert V, Dayer JM. Engagement of CD11b and CD11c beta2 integrin by antibodies or soluble CD23 induces IL-1beta production on primary human monocytes through mitogen-activated protein kinase-dependent pathways. Blood. 2000;95:3868–3877. [PubMed] [Google Scholar]
- 11.Schwarz M, Nordt T, Bode C, Peter K. The GP IIb/IIIa inhibitor abciximab (c7E3) inhibits the binding of various ligands to the leukocyte integrin Mac-1 (CD11b/CD18, alphaMbeta2) Thromb Res. 2002;107:121–128. doi: 10.1016/s0049-3848(02)00207-4. [DOI] [PubMed] [Google Scholar]
- 12.Fang J, Nurden P, North P, Nurden AT, Du LM, Valentin N, Wilcox DA. C560Rbeta3 caused platelet integrin alphaII b beta3 to bind fibrinogen continuously, but resulted in a severe bleeding syndrome and increased murine mortality. J Thromb Haemost. 2013;11:1163–1171. doi: 10.1111/jth.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coburn J, Magoun L, Bodary SC, Leong JM. Integrins alpha(v)beta3 and alpha5beta1 mediate attachment of lyme disease spirochetes to human cells. Infect Immun. 1998;66:1946–1952. doi: 10.1128/iai.66.5.1946-1952.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bounameaux H, Cirafici P, de Moerloose P, Schneider PA, Slosman D, Reber G, Unger PF. Measurement of D-dimer in plasma as diagnostic aid in suspected pulmonary embolism. Lancet. 1991;337:196–200. doi: 10.1016/0140-6736(91)92158-x. [DOI] [PubMed] [Google Scholar]
- 15.Bozic M, Blinc A, Stegnar M. D-dimer, other markers of haemostasis activation and soluble adhesion molecules in patients with different clinical probabilities of deep vein thrombosis. Thromb Res. 2002;108:107–114. doi: 10.1016/s0049-3848(03)00007-0. [DOI] [PubMed] [Google Scholar]
- 16.Wells PS, Anderson DR, Rodger M, Forgie M, Kearon C, Dreyer J, Kovacs G, Mitchell M, Lewandowski B, Kovacs MJ. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349:1227–1235. doi: 10.1056/NEJMoa023153. [DOI] [PubMed] [Google Scholar]
- 17.Karami-Djurabi R, Klok FA, Kooiman J, Velthuis SI, Nijkeuter M, Huisman MV. D-dimer testing in patients with suspected pulmonary embolism and impaired renal function. Am J Med. 2009;122:1050–1053. doi: 10.1016/j.amjmed.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 18.Di Nisio M, Squizzato A, Rutjes AW, Buller HR, Zwinderman AH, Bossuyt PM. Diagnostic accuracy of D-dimer test for exclusion of venous thromboembolism: a systematic review. J Thromb Haemost. 2007;5:296–304. doi: 10.1111/j.1538-7836.2007.02328.x. [DOI] [PubMed] [Google Scholar]


