Abstract
Aims: The aim of the present study was to observe changes in the temporomandibular joint (TMJ) of rats that had been subjected to chronic sleep restriction and to investigate whether Akt, Bad and Caspase3 play a role in the mechanism underlying the changes. Main methods: One hundred and eighty male Wistar rats were randomly divided into three groups (n = 60 in each): cage control group, large-platform control group, and sleep restriction group. Each group was divided into three subgroups (n = 20 in each) of three different time points (7, 14 and 21 days), respectively. The modified multiple platform method was used to induce chronic sleep restriction. The TMJ tissue histology was studied by staining with haematoxylin and eosin. The expression of Akt, p-Aktser473, Bad, p-Badser136 and Caspase3 proteins was detected by immunohistochemistry and western blotting. The expression of Akt, Bad and Caspase3 mRNAs was measured by real-time quantitative polymerase chain reaction (RT-qPCR). Key findings: Compared with the large-platform and cage control groups, condylar cartilage pathological alterations were found in the sleep restriction group. There were significantly decreased expression levels of Akt, p-Aktser473 and p-Badser136 and significantly increased expression levels of Bad and Caspase3 after sleep restriction. Significance: These data suggest that sleep restriction may induce pathological alterations in the condylar cartilage of rats. Alterations in Akt, Bad and Caspase3 may be associated with the potential mechanism by which chronic sleep restriction influences the condylar cartilage.
Keywords: Chronic sleep restriction, condylar cartilage, Akt, Bad, Caspase3
Introduction
Temporomandibular disorders (TMDs) can be characterised by positional, structural or functional abnormalities in the temporomandibular joint (TMJ). The aetiology of TMDs is regarded as multi-factorial and controversial [1,2]. Sleep disturbance appears to be quite common in TMDs [3,4], several studies have demonstrated that sleep disorder, sleep loss or other sleep problems are frequently found in patients with TMDs [5,6]. Although sleep problems may play a role in initiating or persistent symptoms in patients with TMDs, the cause-and-effect relationship remains unclear.
One important tissue pathological change of TMD is the destruction of cartilage and bone. Chondrocyte apoptosis has been described in studies of cartilage destruction and matrix depletion [7]. Alterations in intracellular signalling pathways seem to play an important role in chondrocyte dysfunction [8]. In particular, the phosphatidylinositol 3-kinase (PI3K)/Akt signalling pathway is positively correlated with the regulation of chondrocyte differentiation and apoptosis [9] or proliferation [10]. Akt is the major downstream effector of PI3K, and as a potent inhibitory signal for apoptosis, it is a prominent factor in regulating chondrocyte apoptosis and survival [11]. In addition, apoptosis is actively regulated by several members of the Caspase and Bcl-2 families. Following activation, Akt inhibits pro-apoptotic mediators such as Caspase3 and Bad. The inhibition of Caspase3 activity could reduce the occurrence of cartilage apoptosis and enhance resistance to the apoptosis inducer while retaining the capacity of type II collagen secretion [12]. Phosphorylation of Bad on serine 136 or serine 112 is necessary to promote cell survival by growth factors. Phosphorylation of Bad on serine 136 is one of the potential mechanisms through which Akt inhibits Bad activation. Considering the important role of apoptosis in the condylar cartilaginous changes, the increased apoptosis may be involved in the pathogenic mechanisms of TMDs.
Our study was designed to observe the changes in the TMJ of rats that had been subjected to chronic sleep restriction and to explore the possible mechanism underlying the changes. To pursue these issues, we focused on Akt, Caspase3 and Bad, which are predicted to play key roles in the promotion of survival or stimulation of apoptosis.
Materials and methods
Animals and treatment
Male Wistar rats (weight 220 ± 20 g) were obtained from the Animal Centre of Shan-dong University (Jinan, China) and were raised in the Laboratory Animal Center, Jinan General Military Hospital. Rats were exposed to a 12:12-h light-dark cycle and received food and water without rein for 2 weeks before the experiment. All animal handling and utilisation were in accordance with the Shandong University Animal Care and Utilisation Committee, and all efforts were made to minimise animal suffering.
Experimental groups
A total of 180 rats were randomly divided into three groups (60 in each group): cage control (CC), large-platform control (LC) and sleep restriction (SR) groups. The three groups were divided in equal numbers (n = 20 in each) into three subgroups according to the different time points at which rats were sacrificed (7, 14 and 21 days). Rats of the LC and SR groups were placed on the wide and narrow platforms, respectively. Rats of the CC group were raised in cages in the same room.
Animal model for sleep restriction
According to the modified multiple platform method [13], a sleep restriction model tank was designed. Fifteen platforms (6.3 cm in diameter) or two wide platforms (18.0 cm × 24.0 cm) were placed inside the tank, which was made of organic glass (110.0 cm long × 40.0 cm wide × 70 cm high). The narrow platforms were set 15 cm apart, so that the rats could only stand on them. On the wide platforms, the rats were allowed to lie down. During the experiment, the tanks were filled with water until 1.0 cm of the upper surface of the platforms. On the narrow platforms, when the rat entered sleep, which was characterised by muscle atonia, it touched the water and woke up. The rats were placed on the platforms for 18 hours per day (16:00-10:00), under controlled room (20 ± 2°C) and water (18 ± 2°C) temperature. All the rats had free access to food and water.
Tissue collection
Rats were killed after 7, 14 or 21 days according to their subgroups. Animals were anesthetised with pentobarbital sodium (50 mg/kg body weight), and their bilateral TMJs were rapidly excised and rinsed in cold (4°C) saline. In each subgroup, the right TMJs of 10 rats were chosen randomly for haematoxylin-eosin (HE) staining and immunohistochemistry. The TMJs of the remaining 10 rats were prepared for the detection of protein expressions by western blotting.
Haematoxylin and eosin staining
The isolated right TMJs were fixed in 10% buffered paraformaldehyde for 24 h, incubated with 10% EDTA at 4°C for 4 weeks and then embedded in paraffin. Sections were cut into 5-mm sagittal sections and stained with haematoxylin and eosin (H&E) for histological studies.
Immunohistochemistry
Immunohistochemical staining was carried out using the streptavidin-peroxidase (S-P) method. Tissue sections were prepared as described above. Endogenous peroxidase activity was inhibited by 3% hydrogen peroxide. Antigen retrieval was performed by autoclaving at 120°C for 15 min in 0.01 M citrate buffer (pH 6.0). The sections were reacted overnight with the following rabbit polyclonal antibodies (purchased from Bioworld, China): anti-Akt (diluted 1:50), anti-Bad (diluted 1:65) and anti-Caspase3 (diluted 1:65) at 4°C. The secondary antibody, biotinylated anti-rabbit IgG, was applied for 30 min at room temperature. The sections were visualised by 3, 30-diaminobenzidine-tetrahydrochloride (DAB). Digital images were further analysed via Image-Pro Plus (Media Cybernetics, USA) software.
Western blot analysis
Western blot analysis was performed according to the modified Lowry method. Briefly, the condylar cartilage tissue was homogenised in a gentle MACSTM Dissociator in ice-cold radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime, China; 1 mmol/l PMSF included) and centrifuged at 12000 rpm for 10 min at 4°C. Equal amounts of total protein for each group (40 μg protein per lane) were separated on sodium dodecyl sulphate-polyacrylamide gel electrophoresis (10-12%) and then electro-transferred onto a polyvinylidene fluoride membrane (Millipore, USA) using the Bio-Rad protein assay system (Bio-Rad, USA). Each membrane was blocked with 5% BSA in TBS-T buffer (Tris buffered saline and 0.1% T ween20) at room temperature for 2 h and then incubated overnight at 4°C with the following primary antibodies: anti-Akt (diluted 1:500), anti-p-Aktser473 (diluted 1:500), anti-Bad (diluted 1:1,000), anti-P-Badser136 (diluted 1:1,000), anti-Caspase3 (diluted 1:1000) and anti-β-actin (diluted 1:5,000, Bioworld, China). The membranes were washed extensively with TBS-T and incubated with secondary antibodies (diluted 1:1,000, Beyotime, China) for 1 h at room temperature. After extensive washing with TBS-T, immunoreactive proteins were visualised with an enhanced chemiluminescence kit (Beyotime, China) and captured on X-ray films.
Real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted in 1.0 ml of TRIzol reagent (Invitrogen, USA) and then homogenised in a gentle MACSTM Dissociator (Miltenyl Biotech, Germany). Total RNA was quantified with spectrophotometry. For RT-PCR, we used the Ultra SYBR Two Step RT-qPCR Kit (with ROX; CWbiotech, China) following the manufacturer’s instructions. The cycles were initial denaturation at 95°C for 10 min, followed by 40 amplification cycles of 95°C for 20 s and 55°C for 60 s. Every real-time PCR assay contained 10 μm of the forward and reverse primers and cDNA template in a 25-μl reaction mixture. Results were normalised to GAPDH. The primer sequences were as follows: Akt (forward: 5’-AGGAGGAGGAGACGATGGAC-3’ and reverse: 5’-AGGGCTGTAAGGAAGGGATG-3’); Bad (forward: 5’-CCGAAGAATGAGCGATGAAT-3’ and reverse: 5’-GATAATGCGCGTCCAACTG-3’); Caspase3 (forward: 5’-ACGGGACTTGGAAAGCATC-3’ and reverse: 5’-TAAGGAAGCCTGGAGCACAG-3’); GAPDH (forward: 5’-CAGTGCCAGCCTCGTCTCAT-3’ and reverse: 5’-AGGGGCCATCCACAGTCTTC-3’). The 2-ΔΔCT method was used to analyse the relative intensity of the mRNA expression.
Statistical analysis
Data analyses were performed with SPSS 13.0 software (SPSS, Inc., USA). All data were presented as the mean ± standard error. Differences between groups were analysed NOVA and the Student-Newman-Keuls post-test. The level of significance was set at P < 0.05.
Results
Histological observations
The HE staining results of the TMJs are shown in Figure 1. In the CC and LC groups, no obvious histological changes were found, the condylar cartilage appeared normal, and the fibrous, proliferative, mature and hypertrophic layer could be distinguished clearly (Figure 1A). In contrast, there were some histological alterations in the SR group (Figure 1B). The surface of condylar cartilage became rough and uneven after 7 days of sleep restriction (6/10 rats), small cracks appeared, and then the integrity disappeared at 14 (7/10 rats) and 21 (8/10 rats) days of sleep restriction. Additionally, the most obvious changes were found in the group with 14 days of sleep restriction. Thus, these data demonstrated that sleep restriction can cause abnormal changes in the rat TMJ.
Figure 1.
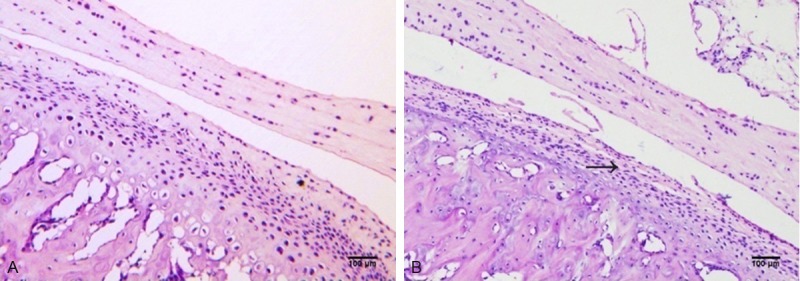
Sagittal section of the rat TMJ stained with hematoxylin and eosin. Representative stained condylar cartilage in the caged control group after 14 days (A) and in the group deprived of sleep for 14 days (B). Haematoxylin and eosin, scale bar = 100 μm.
Protein expression of Akt, p-Akt, Bad, p-Bad and Caspase3
Immunohistochemistry results are shown in Figure 2. Compared with the CC and LC groups, the integrated optical density (IOD) and the positive expression areas of Bad (Figure 2A) and Caspase3 (Figure 2B) were significantly higher in the SR group (P < 0.05). To confirm the expression quantity in the mandibular condyle, the above protein were quantitated by western blot analysis. As shown in Figures 3, 4, there were significantly decreased levels of total Akt (Figure 3A), p-Aktser473 (Figure 4A) and p-Badser136 (Figure 4B) expressions were noted in the SR groups compared with the CC and LC groups. It was important to note that total levels of Bad (Figure 3B) and Caspase3 (Figure 3C) were significantly increased in the SR group.
Figure 2.
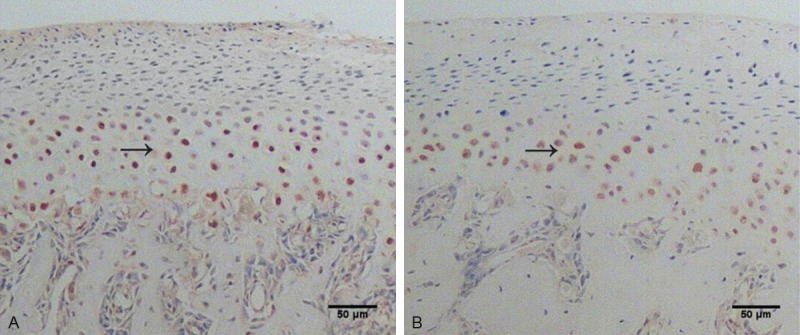
Sagittal section of the rat TMJ stained with immunohistochemistry. Representative immunohistochemically stained mandibular condylar cartilage for Bad (A) and Caspase3 (B) in the group deprived of sleep for 14 days. Scale bar = 50 μm.
Figure 3.
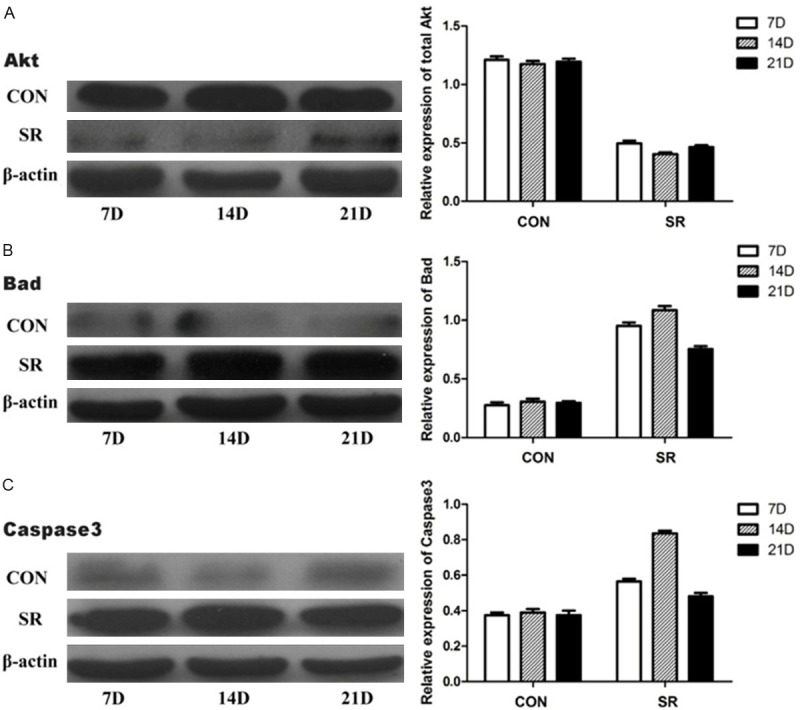
Relative protein expression levels of total Akt, Bad and Caspase3. Relative protein expression levels of total Akt, Bad and Caspase3 in different groups. CON, caged controls; SR, sleep restriction group; and D, days. *P < 0.05, #P < 0.05, §P < 0.05, compared with the matching caged control group. Data are representative of three separate experiments performed in triplicates. Results are presented as the mean (SEM).
Figure 4.
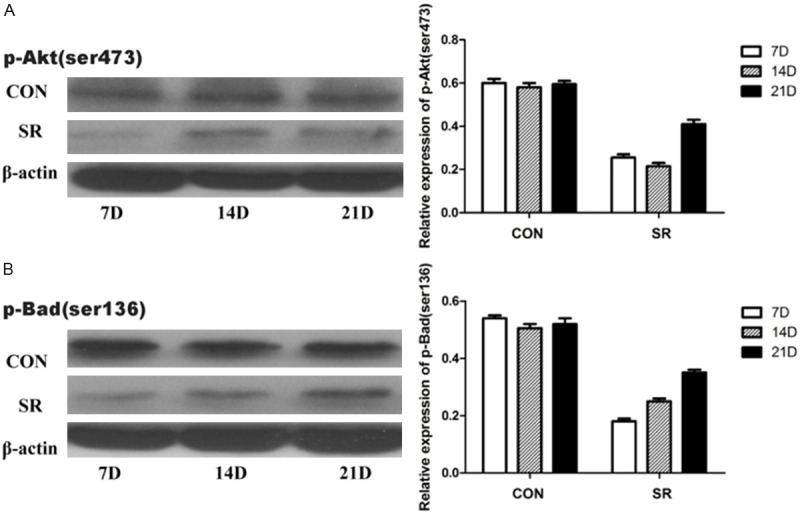
Relative protein expression levels of p-Aktser473 and p-Badser136. Relative protein expression levels of p-Aktser473 and p-Badser136 in different groups. CON, caged controls; SR, sleep restriction group; and D, days. *P < 0.05, #P < 0.05, compared with the matching caged control group. Data are representative of three separate experiments performed in triplicates. Results are presented as the mean (SEM).
mRNA expression of Akt, Bad and Caspase3
There were no significant differences in mRNA concentrations of total Akt, Bad and Caspase3 between the CC and LC groups. As shown in Table 1, there were significantly decreased in mRNA expression of total Akt in the SR groups. In contrast, the mRNA expression of Bad and Caspase3 increased significantly in the SR groups (P < 0.05).
Table 1.
Relative mRNA expression levels of total Akt, Bad and Caspase3
| Total Akt | Bad | Caspase3 | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| CON | SR | CON | SR | CON | SR | |
| 7D | 1.32 ± 0.09 | 0.58 ± 0.07* | 0.23 ± 0.04 | 1.12 ± 0.01# | 0.36 ± 0.05 | 0.69 ± 0.01 |
| 14D | 1.29 ± 0.05 | 0.36 ± 0.12* | 0.27 ± 0.02 | 1.34 ± 0.04# | 0.42 ± 0.06 | 0.92 ± 0.03§ |
| 21D | 1.36 ± 0.03 | 0.42 ± 0.06* | 0.21 ± 0.01 | 0.96 ± 0.02# | 0.33 ± 0.04 | 0.62 ± 0.04 |
CON, caged controls; SR, sleep restriction group; and D, days.
P < 0.05;
P < 0.05;
P < 0.05, compared with the matching caged control group.
Data are representative of three separate experiments performed in triplicates. Results are mean ± SEM.
Discussion
Temporomandibular joint disorders can be caused by many factors, such as abnormal occlusion, parafunctional habits (e.g., bruxism, teeth clenching, lip biting), stress, anxiety, or abnormalities of the intra-articular disc [14]. Because sleep problems commonly exist in patients with TMD, their role in the aetiology of TMD is receiving increasing attention. Although the exact mechanism is incompletely understood, it has been widely acknowledged that a bidirectional relationship exists between sleep problems and TMD [3,15,16].
In a previous study, we showed that total sleep deprivation for 8 days can cause pathological changes in the TMJs of rats [17]. Since the body’s responses to chronic sleep restriction and acute total sleep deprivation are very different [18] and sleep disorders in modern life are mostly characterised by chronic sleep problems, chronic sleep restriction is more modest and more similar to human sleep disorders in comparison with total sleep deprivation.
In our current study, we proposed two experimental hypotheses: firstly, that chronic sleep restriction might lead to pathological alterations in the TMJs of rats; and secondly, that activation of protein factors associated with the PI3K/Akt signalling pathway is possibly behind the effect of sleep restriction on these changes of TMJs.
In order to achieve the purpose of chronic sleep restriction, we referred to the modified multiple platform method. The SR group rats were subjected to sleep restriction for 18 h per day, and then were allowed to sleep for 6 h from 10:00 to 16:00. Meanwhile, we set up an LC group to rule out the influence of a water environment. The results showed that there was no significant difference between the CC and LC groups, and no obvious histological changes in the TMJs of both groups. However, in the SR group, there were changes in the TMJs in the form of tough and uneven areas and loss of cartilage surface integrity. Based on these observations, we have confirmed that chronic sleep restriction can cause pathological alterations in rat condylar cartilage.
Apoptosis plays an important role in temporomandibular joint disc degeneration, cartilage degradation and bone resorption. It is well documented that apoptosis is a potential contributor in the pathogenesis of TMD [19,20]. The PI3K/Akt pathway has been identified as a major regulator of cellular proliferation, differentiation and death in multiple cell types [21]. Specifically, normal expression of this pathway has an irreplaceable role in regulating the proliferation and differentiation of chondrocytes [8,11,22]. Maximal Akt activity is achieved through PI3K and subsequent phosphorylation at Ser473 [23]. Activated Akt can phosphorylate and inactivate Bad [24], thereby inhibiting their functions of promoting apoptosis [25]. In the current study, we found upregulation of Bad and Caspase3 expression and downregulation of Akt, p-Aktser473 and p-Badser136 expression in the SR group compared with the CC and LC groups. These results indicate that alterations in the expressions of Akt, p-Aktser473, Bad, p-Badser136 and Caspase3 concur with an increase of apoptotic activity in the condyle cartilage after sleep restriction.
Cell apoptosis is a complex process involving a variety of proteases. As this was a preliminary study, we chose to study only several major apoptosis-associated proteins. However, it remains uncertain whether Akt directly affects the changes of Bad and Caspase3 or whether it acts through other pathways to activate the cascade of reactions and thus influence these two apoptotic factors. The detailed molecular mechanism therefore needs further investigation.
In summary, our results demonstrate that chronic sleep restriction may lead to pathological changes in the condyle cartilage of rat, and alterations in Akt, Bad and Caspase3 may be associated with the potential mechanism by which sleep restriction influences the condylar cartilage. Our study also suggested that chronic sleep restriction may play a causal role in the occurrence and development of TMD, and the PI3K/Akt signalling pathway might be involved.
Acknowledgements
This study was supported by the Science and Technology Development Plan of Shandong Province (2012G0021833) and Natural Science Foundation of China (81400573; 61471384). The authors are deeply thankful to Dr. Haiying Chen of Liaocheng People’s Hospital of China (Liaocheng, China) for her earnest guidance.
Disclosure of conflict of interest
None.
References
- 1.Scrivani SJ, Keith DA, Kaban LB. Temporomandibular disorders. N Engl J Med. 2008;359:2693–705. doi: 10.1056/NEJMra0802472. [DOI] [PubMed] [Google Scholar]
- 2.De Leeuw R. J Orofac Pain. Chicago: Quintessence; 2008. Orofacial pain: guidelines for assessment, diagnosis, and management. [Google Scholar]
- 3.Smith MT, Wickwire EM, Grace EG, Edwards RR, Buenaver LF, Peterson S, Klick B, Haythornthwaite JA. Sleep disorders and their association with laboratory pain sensitivity in temporomandibular joint disorder. Sleep. 2009;32:779. doi: 10.1093/sleep/32.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auvenshine RC. Temporomandibular disorders: associated features. Dent Clin North Am. 2007;51:105–27. doi: 10.1016/j.cden.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Wickwire E, Bellinger K, Kronfli T, et al. Relations between objective sleep data, sleep disorders, and signs and symptoms of temporomandibular joint disorder (TMD) J Pain. 2008;9:14. [Google Scholar]
- 6.Michalak M, Paulo M, Bożyk A, Zadrożny Ł, Wysokińska-Miszczuk J, Michalak I, Borowicz J. Incidence of abnormalities in temporomandibular joints in a population of 1,100 urban and rural patients lacking teeth and other parafunctions in 2003-2008. An international problem. Ann Agr Env Med. 2012;20:86–90. [PubMed] [Google Scholar]
- 7.Kim HA, Suh DI, Song YW. Relationship between chondrocyte apoptosis and matrix depletion in human articular cartilage. J Rheumatol. 2001;28:2038–45. [PubMed] [Google Scholar]
- 8.Malemud CJ. Protein kinases in chondrocyte signaling and osteoarthritis. Clin Orthop Relat Res. 2004;427:S145–S51. doi: 10.1097/01.blo.0000143802.41885.50. [DOI] [PubMed] [Google Scholar]
- 9.Oh CD, Chun JS. Signaling mechanisms leading to the regulation of differentiation and apoptosis of articular chondrocytes by insulin-like growth factor-1. J Biol Chem. 2003;278:36563–71. doi: 10.1074/jbc.M304857200. [DOI] [PubMed] [Google Scholar]
- 10.Priore R, Dailey L, Basilico C. Downregulation of Akt activity contributes to the growth arrest induced by FGF in chondrocytes. J Cell Physiol. 2006;207:800–08. doi: 10.1002/jcp.20620. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi R, Ryo A, Komitsu N, Mikuni-Takagaki Y, Fukui A, Takagi Y, Shiraishi T, Morishita S, Yamazaki Y, Kumagai K, Aoki I, Saito T. Low-intensity pulsed ultrasound activates the phosphatidylinositol 3 Kinase/Akt pathway and stimulates the growth of chondrocytes in three-dimensional cultures: a basic science study. Arthritis Res Ther. 2008;10:R77. doi: 10.1186/ar2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nuttall ME, Nadeau DP, Fisher PW, Wang F, Keller PM, DeWolf WE Jr, Goldring MB, Badger AM, Lee D, Levy MA, Gowen M, Lark MW. Inhibition of caspase-3-like activity prevents apoptosis while retaining functionality of human chondrocytes in vitro. J Orthop Res. 2000;18:356–63. doi: 10.1002/jor.1100180306. [DOI] [PubMed] [Google Scholar]
- 13.Machado RB, Hipólide DC, Benedito-Silva AA, Tufik S. Sleep deprivation induced by the modified multiple platform technique: quantification of sleep loss and recovery. Brain Res. 2004;1004:45–51. doi: 10.1016/j.brainres.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Buescher JJ. Temporomandibular joint disorders. Am Fam Physician. 2007;76:1477–82. [PubMed] [Google Scholar]
- 15.Selaimen CM, Jeronymo JC, Brilhante DP, Grossi ML. Sleep and depression as risk indicators for temporomandibular disorders in a cross-cultural perspective: a case-control study. Int J Prosthodont. 2005;19:154–61. [PubMed] [Google Scholar]
- 16.Quartana PJ, Wickwire EM, Klick B, Grace E, Smith MT. Naturalistic changes in insomnia symptoms and pain in temporomandibular joint disorder: a cross-lagged panel analysis. Pain. 2010;149:325–31. doi: 10.1016/j.pain.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Wu G, Zhu G, Wang P, Chen H, Zhao H. Influence of sleep deprivation on expression of MKK4 and c-fos in the mandibular condylar cartilage of rats. Br J Oral Maxillofac Surg. 2013;51:e250–e55. doi: 10.1016/j.bjoms.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Kim Y, Chen L, McCarley RW, Strecker RE. Sleep allostasis in chronic sleep restriction: the role of the norepinephrine system. Brain Res. 2013;1531:9–16. doi: 10.1016/j.brainres.2013.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang XD, Kou XX, He DQ, Zeng MM, Meng Z, Bi RY, Liu Y, Zhang JN, Gan YH, Zhou YH. Progression of cartilage degradation, bone resorption and pain in rat temporomandibular joint osteoarthritis induced by injection of iodoacetate. PLoS One. 2012;7:e45036. doi: 10.1371/journal.pone.0045036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loreto C, Almeida LE, Trevilatto P, Leonardi R. Apoptosis in displaced temporomandibular joint disc with and without reduction: an immunohistochemical study. J Oral Pathol Med. 2011;40:103–10. doi: 10.1111/j.1600-0714.2010.00920.x. [DOI] [PubMed] [Google Scholar]
- 21.Beier F, Loeser RF. Biology and pathology of Rho Gtpase, Pi-3 Kinase-Akt, and MAP kinase signaling pathways in chondrocytes. J Cell Biochem. 2010;110:573–80. doi: 10.1002/jcb.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ulici V, Hoenselaar KD, Gillespie JR, Beier F. The PI3K pathway regulates endochondral bone growth through control of hypertrophic chondrocyte differentiation. BMC Dev Biol. 2008;8:40. doi: 10.1186/1471-213X-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mistry D, Oue Y, Chambers MG, Kayser MV, Mason RM. Chondrocyte death during murine osteoarthritis. Osteoarthritis Cartilage. 2004;12:131–41. doi: 10.1016/j.joca.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106:2973–79. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- 25.Murriel CL, Churchill E, Inagaki K, Szweda LI, Mochly-Rosen D. Protein kinase Cδ activation induces apoptosis in response to cardiac ischemia and reperfusion damage a mechanism involving Bad and the mitochondria. J Biol Chem. 2004;279:47985–91. doi: 10.1074/jbc.M405071200. [DOI] [PubMed] [Google Scholar]


