Abstract
Psoriatic arthritis (PsA) is an autoimmune disease with a complex interaction of gene and with a dysregulation of pro-inflammatory cytokine such as Macrophage migration Inhibitory Factor (MIF) and Tumor Necrosis Factor-alpha (TNFα). Two polymorphisms identified in the promoter region of the MIF gene have been described: the STR-794 CATT5-8 (rs5844572) and the SNP-173 G>C (rs755622), which are associated with increased MIF levels in circulation and with autoimmune diseases in several populations. In this case-control study we investigated whether commonly occurring functional MIF polymorphisms are associated with PsA susceptibility and clinical variables as well as with MIF and TNFα serum levels in a Mexican-Mestizo population. Genotyping of the -794 CATT5-8 and -173 G>C MIF polymorphisms was performed by PCR and PCR-RFLP respectively in 50 PsA patients and 100 healthy subjects (HS). MIF and TNFα serum levels were determined by ELISA. A significant increase of MIF (PsA: 7.8 vs. HS: 5.25 ng/mL; p < 0.001) and TNFα (PsA: 24.6 vs. HS: 9.9 pg/mL; p < 0.001) levels was found in PsA patients, a significant correlation was observed between MIF and TNFα (r = 0.41; p < 0.01). The 5,6 repeats genotype of the -794 CATT5-8 MIF was associated with protection to PsA (OR = 0.29; CI 0.77-0.98; p = 0.03), and the G/C genotype (OR = 7.5; CI 2.92-21.64; p < 0.001) and the -173*C allele (OR = 2.45; CI 1.43-4.20; p < 0.001) of the -173 G>C MIF were associated with susceptibility to PsA. In conclusion the -173*C allele is associated with susceptibility to PsA in Mexican-Mestizo population, whereas the correlation between MIF and TNFα soluble levels provided evidence that both cytokines are closely related in the pathophysiology of the PsA.
Keywords: Macrophage migration inhibitory factor, tumor necrosis factor alpha, polymorphism, psoriatic arthritis
Introduction
Psoriatic arthritis (PsA) is defined as an inflammatory arthritis associated with psoriasis, usually is seronegative for rheumatoid factor [1] and it is classified within of the seronegative Spondyloarthropathies [2]. PsA is characterized by paint joint, swelling joint, stiffness joint, arthritis, enthesitis, dactylitis, conjunctivitis and iridocyclitis with affect tendons, ligaments, articular capsule and bone [1]. The frequency of the disease varies from 7% to 40% of the patients who present psoriasis vulgaris [3].
PsA is an autoimmune disease with a complex interaction of gene, environment an immune mechanisms, its etiology is associated with a response of CD8+ T-cells and CD4+ T-cell with a dysregulation of pro-inflammatory cytokines [4-6].
The cytokine Macrophage migration Inhibitory Factor (MIF) is distinguished functionally by its ability to counter-regulate glucocorticoid immunosuppression and sustain pro-inflammatory activation by inhibiting activation-induced apoptosis [7]. MIF further co-stimulates T and B lymphocytes and upregulates the production of interleukin-6, interferon γ and Tumor Necrosis Factor alpha (TNFα) by a feed-forward, positive feedback loop [8-10]. Ten polymorphic sites have been described within the MIF gene [11]. Of these, only two polymorphisms identified in the promoter region relative to the star site of transcription appear to have functional importance: a) the short tandem repeat (STR) -794 CATT5-8 MIF (rs5844572) which is a microsatellite repetition of Cytosine-Adenine-Thymine-Thymine (CATT) at position -794 bp, in which the repeat length (5 to 8 repetitions) correlates with increased gene expression and with serum MIF circulation levels [12,13] and b) the another polymorphism is a single nucleotide polymorphism (SNP) -173 G>C MIF (rs755622) at position -173 of the MIF gene in which there is a change from Guanine (G) by Cytosine (C). The -173*C allele is associated with increased MIF levels in circulation in several populations [14,15], most likely by linkage disequilibrium with the -794 CATT7 high expression allele [12,14,16].
Previous studies in Mexican-Mestizo population have determined the frequency of the MIF promoter polymorphisms and their contribution for autoimmune/inflammatory pathologies such as rheumatoid arthritis (RA) and sy- stemic lupus erythematosus (SLE) [16-18]. Nevertheless, so far no studies have been conducted in Mexican-Mestizo PsA patients to determine the potential association of the MIF promoter polymorphisms.
Based on this knowledge we designed this study to investigate the association of -794 CATT5-8 and -173 G>C MIF polymorphisms with MIF and TNFα serum levels in PsA of Mexican-Mestizo population from western Mexico.
Material and methods
Subjects
A case-control study was conducted with two study groups; the case study group consisted of 50 PsA patients classified according to the 2006 Classification Criteria for Psoriatic Arthritis (CASPAR) [19] enrolled from the Psoriasis Clinic of the Instituto Dermatológico de Jalisco “Dr. José Barba Rubio”, Secretaría de Salud Jalisco, Mexico. All patients were examined according to a structured protocol that consisted of physical assessment, medical record and evaluation of 28 joints to determine the clinical activity according to the Disease Activity Score-28 (DAS-28) [20]. For the control study group, 100 Healthy Subjects (HS) identified by self-report and recruited from the general population in the same geographic area were matched for analysis. All subjects were from an unrelated Mexican-Mestizo population with a family history of ancestors, at least back to the third generation. The paternal ancestry estimated in western Mexican-Mestizos was mainly European (60-64%), Amerindian (21-25%) and African (15%) [21].
Ethical considerations
Informed written consent was obtained from all patients and subjects before enrollment to the study, according to the ethical guidelines of the 2008 Declaration of Helsinki and the investigation was approved by the ethical, investigation, and biosecurity committee of the Centro Universitario de Ciencias de la Salud, Universidad de Guadalajara (C.I. 084-2012).
Quantification of MIF and TNFα serum levels
Serum was obtained from all individuals at the time of inclusion, cytokine levels were quantified in a subset of 50 PsA patients that were not being treated with glucocorticoids and matched by age with 100 control subjects. The determination of MIF and TNFα serum levels was performed by commercial ELISA kits (RayBio® USA and Invitrogen™ USA, respectively) according to manufacturer’s instructions. The MIF assay sensitivity was 6 pg/mL and the TNFα assay sensitivity was 1.7 pg/mL.
Genotyping of -794 CATT5-8 and -173 G>C MIF polymorphisms
Total genomic DNA (gDNA) was isolated from peripheral blood leukocytes by the salting out method [22]. The -794 CATT5-8 MIF polymorphism was analyzed by conventional polymerase chain reaction (PCR) and polyacrylamide gel electrophoresis using the primers reported by Radstake et al. [12]. The -173 G>C MIF polymorphism was genotyped by the PCR-Restriction Fragment Length Polymorphism (RFLP) technique. Amplification of the polymorphic fragment was done using the primers reported by Makhija et al. [23]. The PCR protocols used in both polymorphisms were as reported in our previous studies [16,18].
To confirm the results, genotyping of both polymorphisms was done in duplicate in all cases and confirmed by automatized sequencing of a randomly selected subset of -794 CATT5-8 and -173 G>C MIF genotypes (Applied Biosystems, USA).
Statistical analysis
Statistical analysis was performed using the statistical software STATA v 9.2 and GraphPad Prism v 5.0. For the descriptive analysis, nominal variables were expressed as frequencies, continuous variables with nonparametric distribution were expressed as medians, percentile 5-95th and interquartile ranges 25-75th. We determined genotype and allele frequencies for the polymorphisms -794 CATT5-8 and -173 G>C MIF gene by direct counting and performed chi-square test to compare proportions between groups, to compare the genotype and allele frequencies and to evaluate the Hardy-Weinberg equilibrium. To compare nonparametric quantitative determinations we used the U Mann-Whitney test, Odds ratio (OR) and 95% confidence interval (95% CI) were used to analyze the risk for PsA associated with the MIF gene polymorphisms. For correlation analysis of continuous variables with nonparametric distribution we used the Spearman correlation test. Differences were considered significant at p < 0.05.
Results
Clinical and demographic characteristics
The clinical and demographic characteristics of the 50 PsA patients are shown in the Table 1. The median age of patients was 52 years; 52% were male and 48% female. The median of evolution time for psoriatic disease was 14 years, whereas for PsA was 4 years. The disease activity was evaluated by DAS28 score and we observed 40% with severe activity, 54% moderate activity, 2% low activity and 4% in remission.
Table 1.
Clinical features of PsA patients
| Variable | n = 50 |
|---|---|
| Demographics | |
| Age (years)a | 52 ± 13 |
| Gender b | |
| Male | 52 (26) |
| Female | 48 (24) |
| Disease status | |
| Disease evolution of psoriasis (years)c | 14 (2-32) |
| Disease evolution of PsA (years)c | 4 (1-31) |
| Clinical assessment | |
| DAS-28 scorea | 4.93 ± 1.27 |
| DAS-28 b | |
| Remission (< 2.6) | 4 (2) |
| Low activity (≥ 2.6 < 3.2) | 2 (1) |
| Moderate activity (≥ 3.2 < 5.1) | 54 (27) |
| High activity (≥ 5.1) | 40 (20) |
| Rheumatoid factor UI/mLc | 3.4 (0.7-14.1) |
| Rheumatoid factor b | |
| Negative (< 20 UI/mL) | 92 (46) |
| Positive (≥ 20 UI/mL) | 8 (4) |
| C reactive protein (mg/dL)c | 13.9 (0.7-162) |
| Type of psoriasis b | |
| Plaque | 66 (33) |
| Guttate | 6 (3) |
| Palmoplantar | 4 (2) |
| Scalp | 22 (11) |
| Inverse | 2 (1) |
| Nails change b | |
| Without change | 28 (14) |
| Dystrophy | 36 (18) |
| Hypertrophy | 8 (4) |
| Pitting | 28 (14) |
| Treatmentb | |
| Methotrexate (7.5-10 mg/week) | 18 (9) |
| Methotrexate (12.5-20 mg/week) | 14 (7) |
| Biologics (Etanercep) | 4 (2) |
| Non-steroidal topical treatment | 34 (17) |
| Without treatment | 30 (15) |
Data provided in mean ± DS;
Data provided in percentages and n;
Data provided in median (p5-p95).
C Reactive Protein (CRP) was found increased with a median of 13.9 mg/dL. The Rheumatoid Factor (RF) was negative in 92% and only 8% were positive in PsA patients.
The PsA patients showed the following psoriasis type: plaque (66%, 33/50), guttate (6%, 3/50), palmoplantar (4%, 2/50), scalp (22%, 11/50) and inverse (2%, 1/50). In addition, the nails change presented were dystrophy (36%, 18/50), hypertrophy (8%, 4/50) and pitting (28%, 14/50). The 28% (14/50) of PsA patients showed no changes. Regarding the therapy, PsA patients were treated with methotrexate (16/50, 7.5-20 mg/week), Etanercept (2/50), non-steroidal topical treatment (17/50) and (15/50) patients have not treatment at the moment of study. Any patients were treated with steroids.
MIF and TNFα serum levels
The MIF levels in serum were significantly higher in patients with PsA (7.8 ng/mL) versus HS (5.25 ng/mL) (p ≤ 0.001, Figure 1A) and similarly TNFα serum levels were higher in PsA (24.6 pg/mL) versus HS (9.9 pg/mL) (p ≤ 0.001, Figure 1B).
Figure 1.
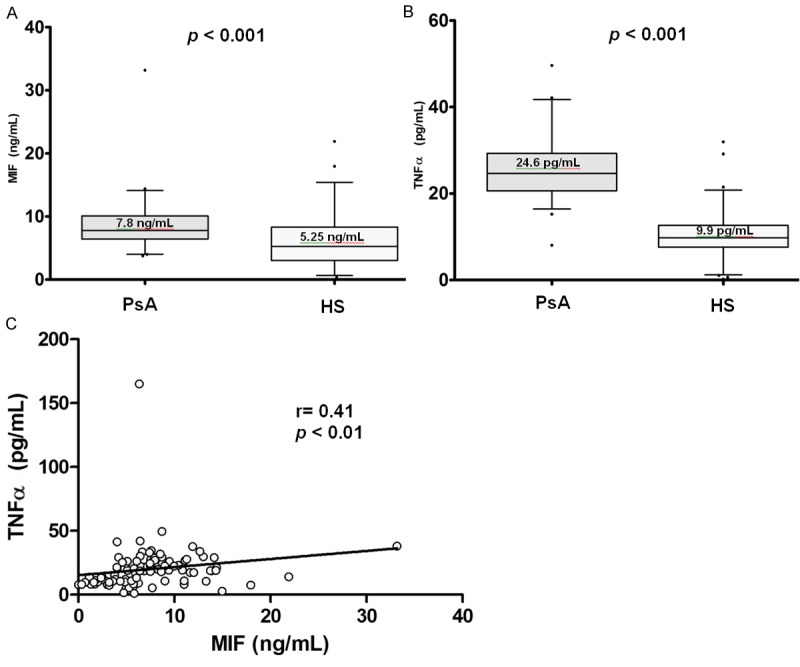
Serum levels by study groups and correlation of MIF and TNFα. A. Comparison of MIF serum levels by study groups; B. Comparison of TNFα serum levels by study groups. Data provided in median (p25-p75). U Mann-Whitney test. C. Positive correlation of serum levels of MIF and soluble TNFα. Spearman’s correlation test.
In previous studies, our research group has reported a correlation between MIF and TNFα in patients with RA and SLE [16,18]. Based on these findings, we investigated the correlation between MIF and TNFα serum levels in our study groups (PsA and HS). As depicted on Figure 1C, a positive correlation for MIF and TNFα was determined (r = 0.41, p ≤ 0.01) in both study groups.
Correlations between cytokines, acute phase reactants and disease evolution
A significant positive correlation was observed between MIF and CRP (r = 0.29, p < 0.01) as well as we observed a positive correlation between TNFα and CRP (r = 0.67, p < 0.001).
To assess MIF and TNFα relationship with the disease evolution of PsA, the patients were grouped according to years of evolution of the disease (from ≤ 1 year to > 5 years). Only a significant increase of MIF levels in PsA with ≤ 2 years of evolution (8.8 ng/mL) versus > 2 years (7.4 ng/mL) of evolution was found (p = 0.02). As interesting finding we observed a decreased of MIF levels in relation to the course of the disease. Nevertheless we did not observe a significant difference when analyzing the levels of TNFα in relation to PsA years of evolution (data not shown).
Genotype and allele associations of -794 CATT5-8 and -173 G>C MIF polymorphisms in PsA patients and HS
Both MIF promoter polymorphisms analyzed were in Hardy-Weinberg equilibrium in the control group (-794 CATT5-8 p = 0.15 and -173 G>C p = 0.029). The distribution of -794 CATT5-8 and -173 G>C MIF polymorphisms in PsA patients and HS are shown in Table 2.
Table 2.
Genotype and allele frequencies of -794 CATT5-8 and -173 G>C MIF polymorphisms in PsA and healthy subjects
| Polymorphism | PsA % (n = 50) | HS % (n = 100) | p * value | OR (CI 95%) | p * value |
|---|---|---|---|---|---|
| -794 CATT5-8 MIF | |||||
| Genotype | 0.04 | ||||
| 5,5 | 4 (2) | 1 (1) | 3.65 (0.17-221.9) | 0.28 | |
| 5,6 | 10 (5) | 31 (31) | 0.29 (0.77-0.98) | 0.03 | |
| 5,7 | 10 (5) | 9 (9) | 1.01 (0.23-4.05) | 0.98 | |
| 6,6§ | 34 (17) | 31 (31) | 1 | - | |
| 6,7 | 32 (16) | 25 (25) | 1.16 (0.45-3.02) | 0.72 | |
| 7,7 | 10 (5) | 3 (3) | 3.03 (0.51-21.6) | 0.14 | |
| Allele | 0.06 | ||||
| 5 | 14 (14) | 21 (42) | 0.71 (0.33-1.47) | 0.33 | |
| 6§ | 55 (55) | 59 (118) | 1 | - | |
| 7 | 31 (31) | 20 (40) | 1.66 (0.90-3.04) | 0.08 | |
| Genetic model | |||||
| Do | 0.07 | ||||
| -,-§ | 48 (24) | 63 (63) | 1 | - | |
| -,7 + 7,7 | 52 (26) | 37 (37) | 1.84 (0.88-3.88) | 0.07 | |
| -173 G>C MIF | |||||
| Genotype | < 0.001 | ||||
| G/G§ | 14 (7) | 54 (54) | 1 | - | |
| G/C | 82 (41) | 42 (42) | 7.5 (2.92-21.64) | < 0.001 | |
| C/C | 4 (2) | 4 (4) | 3.85 (0.28-32.45) | 0.13 | |
| Allele | < 0.001 | ||||
| G§ | 55 (55) | 75 (150) | 1 | - | |
| C | 45 (45) | 25 (50) | 2.45 (1.43-4.20) | < 0.001 | |
| Genetic model | |||||
| Do | < 0.001 | ||||
| G/G§ | 14 (7) | 54 (54) | 1 | - | |
| G/C + C/C | 86 (43) | 46 (46) | 7.21 (2.82-20.6) | < 0.001 |
Chi square test χ2;
OR: odds ratio; CI: confidence interval; Do: dominant inheritance genetic model (-,- = genotypes without risk allele; -,7 = heterozygous genotypes with allele risk);
reference category.
We identified a significant association in the genotype distribution of the -794 CATT5-8 MIF polymorphism in both studied groups (p = 0.04) with an OR of 0.29 (CI 0.77-0.98, p = 0.03) for the 5,6 repeats heterozygote genotype; no significant differences in the comparison of allele frequency was found. Applying a genetic model of dominant inheritance where the genotypes without risk allele (-,-) and heterozygous + homozygous genotypes with allele risk (-,7 + 7,7) where grouped, a similar pattern without significant differences, was observed.
For the -173 G>C MIF polymorphism, we observed a higher frequency of the G/C heterozygote genotype with an OR of 7.5 (CI 2.92-21.64, p < 0.001) for this genotype and the -173*C allele showed an OR of 2.45 (CI 1.43-4.20, p < 0.001). Applying a genetic model of dominant inheritance where the genotypes that contain the -173*C risk allele (G/C+C/C) were grouped, we found a significant association with an OR of 7.21 (CI 2.82-20.6, p < 0.001). This suggests that subjects carrying the -173*C allele has 7.21-folds more susceptibility to present PsA compared with subjects who are G/G genotype carriers (Table 2).
Association between -794 CATT5-8 and -173 G>C MIF polymorphisms with serum levels of MIF and TNFα
To determine whether the MIF promoter polymorphisms are associated with soluble protein levels, we quantified MIF and TNFα levels in the serum of PsA patients and HS. Regarding the -794 CATT5-8 polymorphism, this polymorphisms did not show significant differences with MIF levels, but we observed higher MIF levels in 6,6 repeats carriers with PsA in comparison with HS [7.52 ng/mL (PsA) vs. 5.75 ng/mL (HS)] a similar pattern was observed in the 6,7 repeats carriers [8.29 ng/mL (PsA) vs. 3.85 ng/mL (HS)] (Figure 2A, p < 0.05). Following a genetic model proposed for this polymorphism, we identified a significant increase of MIF in the PsA patients without risk allele genotypes [7.46 ng/mL (PsA) vs. 5.09 ng/mL (HS)] (Figure 2B, p < 0.05). For the TNFα soluble levels, only found a significant increase of this cytokine in the PsA patients carriers of 6,7 repeats genotype in comparison with HS [16.78 pg/mL (PsA) vs. 9.67 pg/mL (HS)] (Figure 2C, p < 0.05). Subsequently, we observed that TNFα serum levels was higher in all PsA patients according to the genetic model proposed for the -794 CATT5-8 MIF polymorphism (Figure 2D, p < 0.05) in comparison with HS.
Figure 2.
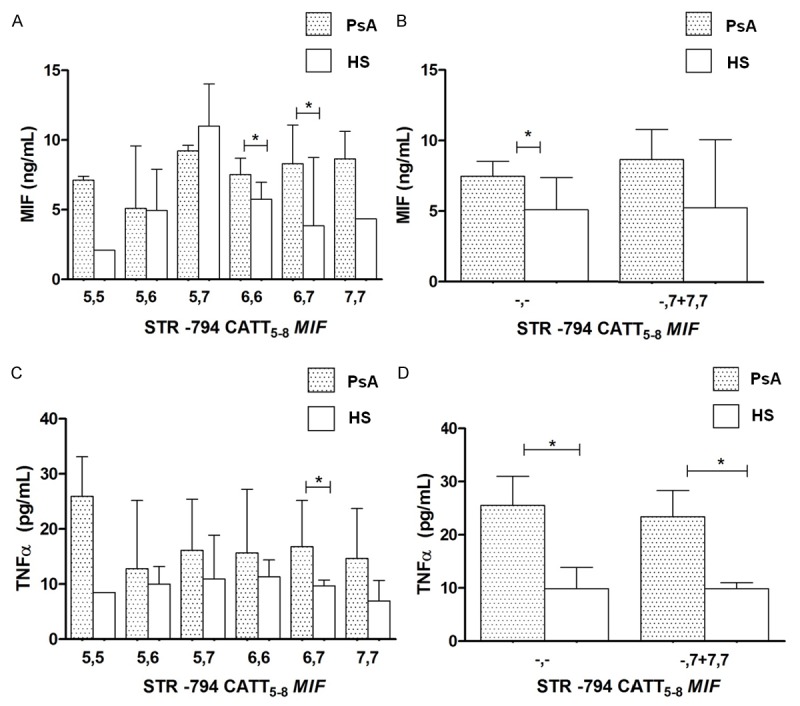
MIF and TNFα concentrations by -794 CATT5-8 MIF genotypes and a dominant genetic model in PsA and healthy subjects. Data provided in median (p25 -p75). *U Mann-Whitney test, p < 0.05.
When we examine the relationship between -173 G>C MIF polymorphism with soluble MIF, no significant differences were found by genotypes and applying the dominant model proposed for this polymorphism (Figure 3A, 3B), only we observed significant differences with higher levels of TNFα in patients with PsA vs. HS in the dominant model proposed (Figure 3C, 3D, p < 0.05).
Figure 3.
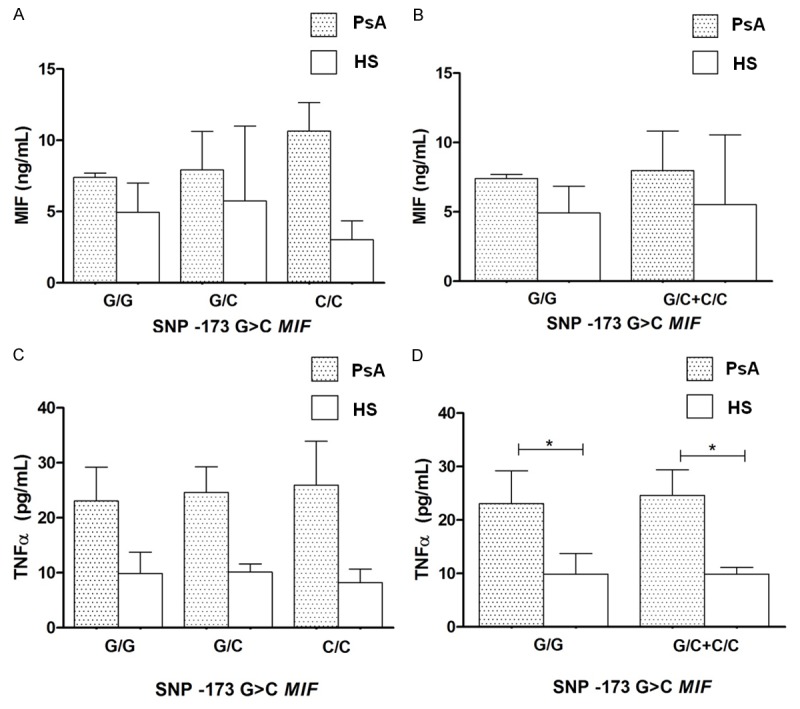
MIF and TNFα concentrations by -173 G>C MIF genotypes and a dominant genetic model in PsA and healthy subjects. Data provided in median (p25 - p75). *U Mann-Whitney test, p < 0.05.
Discussion
In this study, our interest was to evaluate the association of -794 CATT5-8 and -173 G>C MIF promoter polymorphisms with serum MIF and TNFα levels in PsA Mexican-Mestizo patients from western Mexico.
To our knowledge this is the first case-control study that reports the genotypic and allelic frequencies of -794 CATT5-8 and -173 G>C MIF polymorphisms in PsA. Previously, only one study has reported a frequency of -173 G>C MIF polymorphism in PsA patients with a frequency of the 27.7% for the 173*G allele and 72.3% for the -173*C allele in Caucasian population [24]. However, in the present study we identify a higher frequency of the -173*G allele (55%) and a minor frequency for the -173*C allele (45%), this differences could be attributed to the sample size and the criteria for inclusion in each study, and this can also be influenced by the racial differences between populations [21].
The genotype frequency of the -794 CATT5-8 MIF polymorphism is similar with the two previous reports from our research group in Mexican-Mestizo from western Mexico population [16,18]. However, similarly to those reported in our previous study in SLE we did not identify the -794 CATT8 high expression allele reported at a frequency of 1% in RA patients. When we realized the comparison of the genotypic and allelic frequencies for this polymorphism, we detected a borderline significant association for the 5,6 repeats genotype with PsA protection (OR 0.29). However, this data must interpret with caution, due to the confidence interval is close to 1; besides when we analyzing the allelic frequencies we could not establish this association.
In the case of the -173 G>C MIF polymorphism a significant difference in the allele frequency distribution, was found. An important finding was that the G/C genotype carriers present 7.5 fold more susceptibility to PsA. By applying of the genetic model of dominant inheritance proposed we highlighted differences by study group, finding an OR of 7.2 for the -173*C risk allele carriers to present PsA, which is a high OR in polymorphism of low penetrance.
However, several studies previously reported support the association of -794 CATT5-8 and -173 G>C MIF polymorphisms in autoimmune diseases such as RA and SLE where has been associated the high expression alleles (-794 CATT7 and -173*C) with early onset and clinical activity in RA and with susceptibility to SLE, as well as with increase TNFα serum levels. In addition, both MIF polymorphisms presented a strong linkage disequilibrium in our population (LD = 0.87, p < 0.01) [16,18].
Other diseases closely related with PsA such as the chronic plaque psoriasis have been associated with both MIF polymorphisms. Donn et al., associated the CATT7/-173*C haplotype with susceptibility to psoriasis in Caucasian patients [25], whereas that Wu et al., in Han population in northeastern China described no significant differences in the distribution of alleles, genotypes and haplotypes for both MIF polymorphisms. Nevertheless, when the patients were divided according to gender and age of onset, they identified an association with -173*C allele in male with late onset of psoriasis [26].
MIF is a relevant cytokine by its upstream action on the immune cells and to the possible feedback loop between MIF and TNFα [27]. Herein, MIF (7.8 ng/mL) and TNFα (24.6 ng/mL) were significantly increased in the PsA patients and a positive correlation between MIF and TNFα serum levels was observed (r = 0.41), this finding is consistent with previous studies in RA and SLE Mexican-Mestizo patients from western Mexico [17,18,28].
In the present study increased MIF serum levels were detected in early stages of disease (≤2 years of PsA evolution), this finding suggests that MIF may play a key role in PsA onset with a similar pattern with the reported in our previous study in RA, where the MIF levels were raised on early stages of RA with a tend to decrease according to years of evolution. According with this findings, we hypothesized that MIF soluble levels in PsA are increased when the inflammatory process is beginning and then, once that the inflammation is well established other mediators such as TNFα may act to maintain the inflammatory state, which could occurs similarly to that reported in RA [17], which is supported by previous studies were reported by immunostaining an increase of TNFα in the lining layer and to perivascular macrophages in the PsA synovium. In addition, also has been demonstrated the higher mRNA TNFα expression in the synovial tissue in PsA patients [4]. We also analyzed the association of MIF levels with clinical markers of disease, a positive correlation was found between MIF and CRP in PsA patients. This finding is consistent with the reported by Wheelhouse et al., who described that MIF can directly stimulate hepatocytes to secrete acute phase reactants such as CRP [17,29].
To evaluate the association of MIF polymorphisms with MIF and TNFα levels, we stratified our data of both study groups according to the dominant inheritance genetic models proposed for both MIF polymorphisms. Similarly with our previous studies [16,18,28] we were not able to replicate the association of MIF polymorphisms with MIF serum levels that have been reported in other populations [27-30], possibly due to differences in the genetic structure of our population which could be influenced by the MIF gene locus. However, subject carriers of genotypes of both polymorphisms showed higher levels of MIF with a slight tendency to increase according to high expression alleles were present in comparison with control subjects. These findings highlight the contribution of the MIF polymorphisms in the susceptibility to diseases with a strong autoimmune background such as PsA in Mexican-Mestizo population from western Mexico.
In conclusion, this study showed that the -173*C allele is associated with susceptibility to PsA in Mexican-Mestizo population from western Mexico, whereas the correlation between MIF and TNFα soluble levels provided evidence that both cytokines are closely related in the pathophysiology of the PsA.
Acknowledgements
This study was supported by Grant No. 180663 (JFMV) from the National Council of Science and Technology (CONACYT Ciencia Básica-Universidad de Guadalajara).
Disclosure of conflict of interest
None.
References
- 1.Cantini F, Niccoli L, Nannini C, Kaloudi O, Bertoni M, Cassarà E. Psoriatic arthritis: a systematic review. Int J Rheum Dis. 2010;13:300–17. doi: 10.1111/j.1756-185X.2010.01540.x. [DOI] [PubMed] [Google Scholar]
- 2.Moll JMH, Wright V. Psoriatic arthritis. Semin Arthritis Rheum. 1973;3:55–78. doi: 10.1016/0049-0172(73)90035-8. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd P, Ryan C, Menter A. Psoriatic arthritis: an update. Arthritis. 2012;2012:176298. doi: 10.1155/2012/176298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veale DJ. Immunopathology of psoriasis and psoriatic arthritis. Ann Rheum Dis. 2005;1:II26–II29. doi: 10.1136/ard.2004.031740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nograles KE, Brasington RD, Bowcock AM. New insights into the pathogenesis and genetics of psoriatic arthritis. Nat Clin Pract Rheumatol. 2009;5:83–91. doi: 10.1038/ncprheum0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuchacovich R, Perez-Alamino R, Garcia-Valladares I, Espinoza LR. Steps in the management of psoriatic arthritis: a guide for clinicians. Ther Adv Chronic Dis. 2012;3:259–69. doi: 10.1177/2040622312459673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aeberli D, Leech M, Morand EF. Macrophage migration inhibitory factor and glucocorticoid sensitivity. Rheumatology (Oxford) 2006;45:937–43. doi: 10.1093/rheumatology/kel142. [DOI] [PubMed] [Google Scholar]
- 9.Bacher M, Metz CN, Calandra T, Mayer K, Chesney J, Lohoff M, Gemsa D, Donnelly T, Bucala R. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc Natl Acad Sci U S A. 1996;93:7849–54. doi: 10.1073/pnas.93.15.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denkinger CM, Metz C, Fingerle-Rowson G, Denkinger MD, Forsthuber T. Macrophage migration inhibitory factor and its role in autoimmune diseases. Arch Immunol Ther Exp (Warsz) 2004;52:389–400. [PubMed] [Google Scholar]
- 11.Yende S, Angus DC, Kong L, Kellum JA, Weissfeld L, Ferrell R, Finegold D, Carter M, Leng L, Peng ZY, Bucala R. The influence of macrophage migration inhibitory factor gene polymorphisms on outcome from community-acquired pneumonia. FASEB J. 2009;23:2403–11. doi: 10.1096/fj.09-129445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radstake TR, Sweep FC, Welsing P, Franke B, Vermeulen SH, Geurts-Moespot A, Calandra T, Donn R, van Riel PL. Correlation of rheumatoid arthritis severity with the genetic functional variants and circulating levels of macrophage migration inhibitory factor. Arthritis Rheum. 2005;52:3020–9. doi: 10.1002/art.21285. [DOI] [PubMed] [Google Scholar]
- 13.Renner P, Roger T, Bochud PY, Sprong T, Sweep FC, Bochud M, Faust SN, Haralambous E, Betts H, Chanson AL, Reymond MK, Mermel E, Erard V, van Deuren M, Read RC, Levin M, Calandra T. A functional microsatellite of the macrophage migration inhibitory factor gene associated with meningococcal disease. FASEB J. 2012;26:907–16. doi: 10.1096/fj.11-195065. [DOI] [PubMed] [Google Scholar]
- 14.Donn R, Alourfi Z, Zeggini E, Lamb R, Jury F, Lunt M, Meazza C, De Benedetti F, Thomson W, Ray D British Paediatric Rheumatology Study Group. A functional promoter haplotype of macrophage migration inhibitory factor is linked and associated with juvenile idiopathic arthritis. Arthritis Rheum. 2004;50:1604–10. doi: 10.1002/art.20178. [DOI] [PubMed] [Google Scholar]
- 15.Donn R, Alourfi Z, De Benedetti F, Meazza C, Zeggini E, Lunt M, Stevens A, Shelley E, Lamb R, Ollier WE, Thomson W, Ray D British Paediatric Rheumatology Study Group. Mutation screening of the macrophage migration inhibitory factor gene: positive association of a functional polymorphism of macrophage migration inhibitory factor with juvenile idiopathic arthritis. Arthritis Rheum. 2002;46:2402–9. doi: 10.1002/art.10492. [DOI] [PubMed] [Google Scholar]
- 16.Llamas-Covarrubias MA, Valle Y, Bucala R, Navarro-Hernández RE, Palafox-Sánchez CA, Padilla-Gutiérrez JR, Parra-Rojas I, Bernard-Medina AG, Reyes-Castillo Z, Muñoz-Valle JF. Macrophage migration inhibitory factor (MIF): genetic evidence for participation in early onset and early stage rheumatoid arthritis. Cytokine. 2013;61:759–65. doi: 10.1016/j.cyto.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Llamas-Covarrubias MA, Valle Y, Navarro-Hernández RE, Guzmán-Guzmán IP, Ramírez-Dueñas MG, Rangel-Villalobos H, Estrada-Chávez C, Muñoz-Valle JF. Serum levels of macrophage migration inhibitory factor are associated with rheumatoid arthritis course. Rheumatol Int. 2012;32:2307–11. doi: 10.1007/s00296-011-1951-6. [DOI] [PubMed] [Google Scholar]
- 18.De la Cruz-Mosso U, Bucala R, Palafox-Sánchez CA, Parra-Rojas I, Padilla-Gutiérrez JR, Pereira-Suárez AL, Rangel-Villalobos H, Vázquez-Villamar M, Angel-Chávez LI, Muñoz-Valle JF. Macrophage migration inhibitory factor: association of -794 CATT5-8 and -173 G>C polymorphisms with TNF-α in systemic lupus erythematosus. Hum Immunol. 2014;75:433–9. doi: 10.1016/j.humimm.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H CASPAR Study Group. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;5:2665–73. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 20.Prevoo ML, van Gestel AM, van T Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL. Remission in a prospective study of patients with rheumatoid arthritis. American Rheumatism Association preliminary remission criteria in relation to the disease activity score. Br J Rheumatol. 1996;35:1101–5. doi: 10.1093/rheumatology/35.11.1101. [DOI] [PubMed] [Google Scholar]
- 21.Rangel-Villalobos H, Muñoz-Valle JF, González-Martín A, Gorostiza A, Magaña MT, Páez-Riberos LA. Genetic admixture, relatedness, and structure patterns among Mexican populations revealed by the Y-chromosome. Am J Phys Anthropol. 2008;135:448–61. doi: 10.1002/ajpa.20765. [DOI] [PubMed] [Google Scholar]
- 22.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makhija R, Kingsnorth A, Demaine A. Gene polymorphisms of the macrophage migration inhibitory factor and acute pancreatitis. JOP. 2007;8:289–95. [PubMed] [Google Scholar]
- 24.Eder L, Chandran V, Ueng J, Bhella S, Lee KA, Rahman P, Pope A, Cook RJ, Gladman DD. Predictors of response to intra-articular steroid injection in psoriatic arthritis. Rheumatology (Oxford) 2010;49:1367–73. doi: 10.1093/rheumatology/keq102. [DOI] [PubMed] [Google Scholar]
- 25.Donn RP, Plant D, Jury F, Richards HL, Worthington J, Ray DW, Griffiths CE. Macrophage migration inhibitory factor gene polymorphism is associated with psoriasis. J Invest Dermatol. 2004;123:484–7. doi: 10.1111/j.0022-202X.2004.23314.x. [DOI] [PubMed] [Google Scholar]
- 26.Wu J, Chen F, Zhang X, Li Y, Ma H, Zhou Y, Jin Y, Wang H, Bai J, Zhang G, Fu S. Association of MIF promoter polymorphisms with psoriasis in a Han population in northeastern China. J Dermatol Sci. 2009 Mar;53:212–5. doi: 10.1016/j.jdermsci.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Pons-Estel GJ, Alarcón GS, Scofield L, Reinlib L, Cooper GS. Understanding the Epidemiology and Progression of Systemic Lupus Erythematosus. Semin Arthritis Rheum. 2010;39:257–68. doi: 10.1016/j.semarthrit.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sreih A, Ezzeddine R, Leng L, LaChance A, Yu G, Mizue Y, Subrahmanyan L, Pons-Estel BA, Abelson AK, Gunnarsson I, Svenungsson E, Cavett J, Glenn S, Zhang L, Montgomery R, Perl A, Salmon J, Alarcón-Riquelme ME, Harley JB, Bucala R. Dual effect of the macrophage migration inhibitory factor gene on the development and severity of human systemic lupus erythematosus. Arthritis Rheum. 2011;63:3942–51. doi: 10.1002/art.30624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wheelhouse NM, Dowidar N, Dejong CH, Garden OJ, Powell JJ, Barber MD, Sangster K, Maingay JP, Ross JA. The effects of macrophage migratory inhibitory factor on acute-phase protein production in primary human hepatocytes. Int J Mol Med. 2006;18:957. [PubMed] [Google Scholar]
- 30.Donn RP, Ray DW. Macrophage migration inhibitory factor: molecular, cellular and genetic aspects of a key neuroendocrine molecule. J Endocrinol. 2004;182:1–9. doi: 10.1677/joe.0.1820001. [DOI] [PubMed] [Google Scholar]


