Abstract
Background: Acute myocardial infarction (AMI) was a type of disease with high mortality rate and high disability rate. And about 50% of the final area of myocardial infarction after AMI was led by ischemia/reperfusion (I/R) injury. The I/R injury was a kind of systemic inflammatory response, in which the main performance laid in the release of the large quantity of inflammatory cytokines. The basic experiments, clinical studies and the large scaled epidemiology investigations found that the low functions of vagus nerves had close relevance with the occurrence, development and prognosis of the cardiovascular diseases. This study investigate the effects of cholinergic anti-inflammatory pathway with with vagus never stimulation I/R injury in canine. Methods: 18 adult mongrel dogs were randomly divided into 3 groups (n = 6): sham operation group (sham Group), ischemia/reperfusion group (I/R group), right vagus nerve stimulation and ischemia/reperfusion group (STM group). The hemodynamic indexes were measured after reperfusion 120 min. Through internal jugular venous blood, serum acetylcholine (Ach), tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) concentrations were detected by ELISA. Alpha 7 subunit Ach acetylcholine receptor (α7nAchR) expression level was detected with immunohistochemical method. HE staining was used to observe the degree of neutrophil infiltration. Results: After ischemia/reperfusion 120 min, compared with sham group, TNF-α and IL-6 were significantly decreased, Ach content increased, the expression of α7nAchR protein was significantly reduced in I/R group (P < 0.05). Expression of α7nAchR protein, Ach content, TNF-α and IL-6 level had no significant difference in STM group (P < 0.05). Compared with I/R group, the expression of Ach and α7nAchR protein significantly increased the TNF- and IL-6 levels decreased in STM group (P < 0.05). Compared with the baseline, TNF-α and IL-6 levels significantly increased Ach content decreased in I/R group after ischemia /reperfusion 120 min (P < 0.05). Ach, TNF-α and IL-6 levels had no significant change in sham group and STM group of (P < 0.05). TNF-α and IL-6 were negatively correlated with Ach in I/R group (P < 0.05), and TNF-α, IL-6 were negatively correlated with Ach in group STM (P < 0.05). Massive infiltration of neutrophils were detected in myocardial tissue of I/R group, and a small number of neutrophils infiltration were detected in STM group. Conclusion: Right vagus nerve stimulation could activate anti-inflammatory pathway and inhibit the systemic and local inflammatory reaction to relieve myocardial I/R injury.
Keywords: Myocardial ischemia/reperfusion injury, cholinergic anti-inflammatory pathway, vagus nerve, acetylcholine
Introduction
The acute myocardial infarction (AMI) was a type of disease with high mortality rate and high disability rate. And about 50% of the final area of myocardial infarction after AMI was led by the Ischemia/reperfusion (I/R) damages [1]. Ridker putted forward that the ischemic heart disease belonged to the immune inflammatory reaction of organism [2]. And I/R damage were a kind of systemic inflammatory response, which the main performance laid in the release of the large quantity of inflammatory cytokines. The basic experiments, clinical studies and the large scaled epidemiology investigations found that the low functions of vagus nerves had close relevance with the occurrence, development and prognosis of the cardiovascular diseases [3-5]. The nervous system could significantly and rapidly inhibit the macrophage from releasing the cytokines such as tumor necrosis factor alpha-α (TMF-α) and white blood cells interleukin-6 (IL-6), etc, and reduce the systemic inflammatory responses. This physiological mechanism was called as cholinergic anti-inflammatory pathway [6]. The present study established I/R models, chose to implement the electrical stimulation on the right side vagus nerves cord before reperfusion after the occurrence of myocardial ischemia to realize the regulation over the inflammation and discussed the mechanism of stimulation on the unilateral vagus nerves cord to reduce the myocardial I/R damages of dogs was related to the activation of cholinergic anti-inflammatory pathway of the vagus nerves.
Materials and methods
Establishment of experimental animals and models
There were eighteen healthy adult mongrel dogs, with weight 14~22 Kg, male, which provided by the Animal center of Renmin Hospital of Wuhan University. The dogs were anaesthetized through forelimb injection of 30 m/kg pentobarbital sodium and then the injection would be added by 2 mg/kg per hour to maintain the state of anaesthetization. The trachea cannula was put and connected to the respirator. One lateral femoral artery and vein was separated and the 6F sheathing canal was implanted. The arterial channel linked to the pressure transducer to monitor the blood pressure and venous access for the dripping of saline infusion. After removing the hair, preparing the skin and sterilizing on the neck, the left jugular veins on the left clavicle was exposed horizontally for blood sampling. The indoor temperature was controlled during 25~30°C with air conditioner. In the course of the experiment, electric heating plate was placed under the dog to keep the normal body temperature of dogs. The next was to record the surface ECG (LEAD7000) continuously. First, the two sides of skins of the neck were exposed and then were cut nearby the trachea just below the jawbone to make blunt dissection of the right vagus nerves cord. And then a special silver-argentic chloride stimulation electrode was implanted into the nerve cord and connected to the pulser (SEN-7103, Nihon-Kohden, Tokyo, Japan). Prior stimulation of 3-4 times within 3 to 5 minutes to confirm that the heart rate declined by 10% remarkably and then the electrode was put well in place. The stimulation parameters were: frequency 10 HZ, pulse width 0.5 ms and stimulation strength 1.5-3 V. Afterwards, the chest was cut through the left fourth intercostals space to shear the cardiac vesicle and make the heart exposed. The needle and thread (2-0 non-invasive thread) went between the first diagonal branch and the second diagonal branch of the left anterior descending coronary artery together with a plastic tube with a small section of hard blunt head to complete the ligation, only to lead to ischemia and the changes in the electrocardiogram was recorded in time. The standard of successful ligation include that: the local cyanosis occurs in the myocardial ischemic area, the synchronous II lead ECG shows the ST section elevated significantly and ventricular arrhythmia takes place during the ischemia. 1 h after ligation, the ligation thread on the hard plastic tube was cut to recover the coronary blood flow for reperfusion for 6 h. The standard of successful reperfusion: some part of the ischemic area becomes red and the synchronous II lead ECG shows the ST section restores the level before ischemia and reperfusion ventricular arrhythmia occurs.
Grouping of experiment and intervention
The eighteen dogs were divided into three groups randomly (with six piece each group). 1. Sham operation group (Sham group): The right vagus cord was separated, chest was opened and the left coronary artery was separated without ligation. 2. Ischemia/Reperfusion (I/R group): the left anterior descending coronary artery was ligatured for 1 h and then reperfusion was made for 6 h. 3. Vagus nerves electrical stimulation group (STM group): the coronary artery was ligatured for 1 h ischemia. After ischemia for 15 min, the right vagus nerves were stimulated for 30 min and then reperfusion was made for 6 h.
Basic parameters of the experimental dogs in each group
10 min after the model establishment was completed and became steady, the heart rate (HR) and mean atrial pressure (MAP) were recorded, which were regarded as the baseline level. And then the HR and MAP at the time point of 120 min of reperfusion after ischemia of each group were recorded.
Detection of serum Ach, TNF-α and IL-6 levels
Blood was collected through the jugular veins of the experimental animals in the three groups. The blood specimen was injected with heparin sodium into the anticoagulant tube and then was shaken even. After that, it was put stay under room temperature for 10 min and then centrifugal movement at 3,000 r/min was made for 15 min. The supernatant was taken and placed in the EP tube. All the serum specimen were stored in the refrigerator under -80°C. The enzyme-linked immunosorbent assay (ELISA) kit for dogs was used to detect the levels of acetylcholine, TNF-α and IL-6 in the serum.
Observation of neutrophil infiltration with HE staining
After the experiment closed, the myocardial tissue were taken and were fixed for 24 h with 4% paraform in the surrounding area of the myocardial infarction in the I/R group and the STM group. Through the conventional dehydration, wax infiltration and embedding, the issues were made into paraffin section with thickness of 5mm to be stained with PE. And then the situation of neutrophil granulocyte infiltration was observed under optical microscope.
Detection of expression of subunit of nicotinic Ach receptor (α7nAchR)
After the experiment closed, the myocardial tissue in the surrounding area of the myocardial infarction of the dogs in the three groups was taken and the location for taking off the muscular tissue in the sham operation group conformed to that in I/R group. The myocardial tissue was fixed for 24 h with 4% paraform. Through paraffin embedding, they were cut into coronal slices with thickness of 4 μm and then the specimen were dewaxed and high pressure hot fix antigen was made. The α7nAchR was stained with SP immunohistochemical staining method. For the negative control, PBS took place of the primary antibody; the color expression of α7nAchR was expressed in that the endochylema was dyed into brownish yellow, indicating positive results. According to the degree, the colors were: colorless for negative, pale brownish yellow for positive and brown for strong positive. Five non-repetitive high performance fields were chose from the slices to calculate the number of positive cells respectively.
Statistical analysis
The statistical software SPSS16.0 was adopted to conduct the statistical analysis. The measurement data were expressed by the mean ± standard deviation (x̅±s). The comparison between every two groups was made with t detection of two separate fully randomly designed, the comparison among multiple groups was made with one-factor analysis of variance while comparison between each two groups among the multiple groups was detected with LSD method, and the difference was regarded to have significance when 0.05.
Results
Completion of I/R model preparation
The coronary artery was ligatured and myocardial ischemia may occur in the area between the first diagonal branch and the second diagonal branch with local cyanosis. The synchronous II lead was made and the ECG showed the remarkable elevation in the ST section. Meanwhile, ventricular arrhythmia took place during the ischemia. 1 h after ligation, reperfusion was made for 120 min. The color in the ischemic area became red locally. The synchronous II lead was made and the ECG showed that the ST section gradually restored to the level prior to ischemia and reperfusion ventricular arrhythmia came out, See Figure 1. During the period of preparing the I/R model and vagus stimulation I/R, two dogs died due to ventricular fibrillation and the others existed for the successful completion of the experiment. In the course of experiment, the animals were supplemented with the identical body quality and health state to the experiment according to the number of dead animals.
Figure 1.
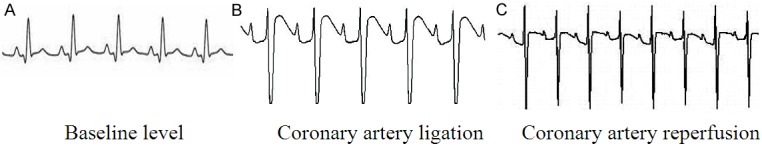
Features of surface II Lead ECG in the course of making I/R model.
Analysis on the results of hemodynamic of the experimental dogs in the three groups
Compared with the basic levels in these groups, the differences of HR and MAP had no statistical significance (P < 0.05). 120 min after reperfusion upon ischemia, compared with those in the sham group, both the HR and the MAP in the I/R group declined remarkably (P < 0.05) while the difference of HR and MAP in the STM group had no statistical significance (P > 0.05). Compared with those in the I/R group, both the HR and the MAP in the STM group increased remarkably (P < 0.05). Compared with those on the baseline levels, HR and MAP 120 min after reperfusion upon ischemia in the I/R group decreased remarkably (P < 0.05), while the HR and MAP in the sham group and STM group had no clear changes (P > 0.05), see Table 1.
Table 1.
Changes in the value of HR and MAP of the experimental dogs in the three groups (x̅±s)
| Group | case | Baseline level | 120 min after reperfusion/ischemia | ||
|---|---|---|---|---|---|
|
|
|
||||
| HR (Times/min) | MAP (mmHg) | HR (Times/min) | MAP (mmHg) | ||
| sham group | 6 | 134.17±3.12 | 138.50±8.77 | 135.24±4.23 | 136.27±4.25 |
| I/R group | 6 | 135.28±4.20 | 134.44±6.35 | 121.30±3.56a,b | 120.75±3.20a,b |
| STM group | 6 | 134.40±2.57 | 135.60±5.42 | 128.56±3.12c | 127.52±6.33c |
Note: compared with those on the baseline levels,
P < 0.05;
compared with those in the sham group,
P < 0.05 after 120 min through reperfusion upon ischemia;
compared with those in the I/R group,
P < 0.05.
Results of detection on the levels of Ach, TNF-α and IL-6 in the serum
Compared with those on the baseline level in the groups, the differences of Ach, TNF-α and IL-6 had no statistical significance (P < 0.05). Compared with those through reperfusion for 6 h after ischemia, TNF-α and IL-6 in the I/R group remarkably decreased compared with those in the sham group, while the Ach content went up (P < 0.05), but the differences of Ach, TNF-α and IL-6 had no statistical significance (P > 0.05). Compared with those in the I/R group, Ach increased while TNF-α and IL-6 decreased (P < 0.05) in the STM group. Compared with those on the baseline level, TNF-α and IL-6 increased remarkably while the Ach content decreased (P < 0.05) in the I/R group through reperfusion for 120 min after ischemia, and the Ach, TNF-α and IL-6 in the sham group and STM group had no clear changes (P > 0.05) (Table 2).
Table 2.
Detection Results of the Ach, TNF-α and IL-6 in the Serum (x̅±s)
| Group | case | Baseline level | 6 h after reperfusion/ischemia | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| TNF-α (ng/ml) | IL-6 (pg/ml) | Ach (ng/ml) | TNF-α (ng/ml) | IL-6 (pg/ml) | Ach (ng/ml) | ||
| sham group | 6 | 0.51±0.03 | 83.32±2.35 | 56.38±3.20 | 0.60±0.02 | 89.35±2.70 | 58.45±2.30 |
| I/R group | 6 | 0.58±0.02 | 89.24±5.36 | 49.36±2.53 | 2.54±0.12a,b | 122.33±3.33a,b | 31.32±3.56a,b |
| STM group | 6 | 0.68±0.02 | 85.57±4.28 | 52.80±2.47 | 0.72±0.02c | 90.52±3.54c | 45.55±3.02c |
Note: compared with those on the baseline levels,
P < 0.05;
compared with those in the sham group,
P < 0.05 after 6 h through reperfusion upon ischemia;
compared with those in the I/R group,
P < 0.05.
Analysis on the correlation among the TNF-α, IL-6 and Ach in the serum in the I/R group and the STM group
The analysis on the correlations showed that, TNF-α and IL-6 present negative correlation with Ach in the I/R group (P < 0.05). Along with the decrease in the Ach content, the TNF-α and IL-6 increased remarkably. In the STM group, the TNF-α and IL-6 present negative correlation with Ach (P < 0.05). Along with the Ach content increased, TNF-α and IL-6 decreased obviously, see Table 3.
Table 3.
Correlation between the TNF-α, IL-6 and Ach in the Serum in the I/R group and STM group
| Variance | case | I/R group | STM group | ||
|---|---|---|---|---|---|
|
|
|
||||
| r | P | r | P | ||
| TNF-α | 6 | -0.905 | 0.015 | -0.582 | 0.026 |
| IL-6 | 6 | -0.535 | 0.036 | -0.367 | 0.042 |
Observation of neutrophil infiltration with HE staining
After the experiment closed, the myocardial tissue in the surrounding area of the myocardial infarction of the dogs in the I/R group and STM group were taken and the location for taking off the myocardial tissue in the sham operation group conform to that in the I/R group. The myocardial tissue was fixed for 24 h with 4% paraform. Through paraffin embedding, they were cut into slices with thickness of 5 mm and then the specimen was stained with HE. The development of neutrophil infiltration of the myocardial tissue was observed under the optical microscope, and it was seen that small amount of neutrophil filtration in the STM group, see Figure 2.
Figure 2.
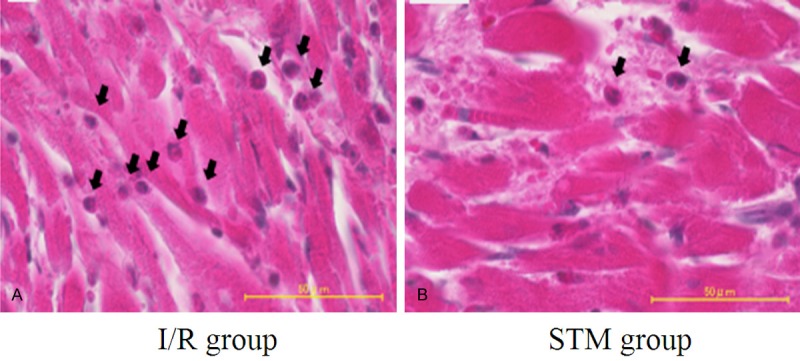
Neutrophil filtration of myocardial tissue in I/R group and STM group (HE staining, ×400). The black arrow stands for neutrophil granulocyte.
Detection results of α7nAchR protein expression
The results of protein expression of the myocardial tissue of the dogs in the groups showed that, brown positive granules were seen in the sham group and STM group, small amount of brownish positive granules was seen in the I/R group. Compared with those in the sham group, the α7nAchR protein expression decreased obviously and the differences had statistical significance (P < 0.05); there was no clear increase in the α7nAchR protein expression. Compared with those in the I/R group, the α7nAchR protein expression in the STM group increased remarkably (P < 0.05). See Figure 3 and Table 4.
Figure 3.
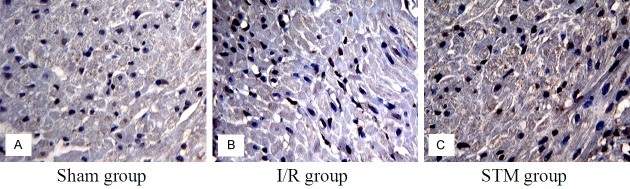
Positive protein expression of α7nAchR in the myocardial tissue of dogs in the three groups.
Table 4.
Number of positive cells with α7nAchR after 6 h perfusion upon ischemia (positive number/visual fields, x̅±s)
| Designation of groups | Numbers of positive cells expression |
|---|---|
| sham group | 60.35±3.54 |
| I/R group | 30.65±2.35a |
| STM group | 62.54±5.10b |
Note: compared with those in the sham group,
P < 0.05;
compared with those in the I/R group,
P < 0.05.
Discussions
At present, some small amount of randomized controlled clinical studies proved that the vagus stimulation could protect the ischemic myocardial muscle. However, it was still necessary to make large scaled preclinical researches, evaluation and optimization of the paths and actions of vagus stimulation protecting the ischemic myocardial diseases. In the present study, the results of hemodynamic showed that 120 min after reperfusion upon ligation of coronary artery of the dogs in the I/R group, the values of HR and MAP declined clearly than the basic values. Combined with the elevation on ligation as well as restoration to the baseline level after reperfusion of lead II ST section shown on the surface electrocardiogram, it hinted that the I/R model in the dogs of the experiment succeed. In addition, no obvious changes were seen in the values of HR and MAP 120 min after ischemia reperfusion in the STM group under the electric vagus stimulation. These results pointed that the invention measures of vagus stimulation to protect the I/R damages succeeded under the circumstances without affecting the hemodynamic. Such results in the experiment conform to those described by Uemura et al. and Kawada et al. [7,8].
I/R damage were a type of clinical syndrome featured with excessive systematic inflammatory responses. On I/R damage, the monocyte/marcrophase, leukomonocyte and neutrophil granulocyte, etc would start the cellular inflammatory responses to produce a great number of a variety of inflammatory cytokines [9]. In 2002, Tracey putted forward the concept of “Inflammatory Reflex”, that was the whole course of regulating the inflammatory responses through vagus nerves [10]. Vagus nerves were an essential part consisting of the cholinergic anti-inflammatory pathway and played an important role in the bidirectional communications between the center nerves and the peripheral immune system. Ach was the main transmitter released by vagus nerves and could combine with the receptors on the immune cells to inhibit the release of inflammatory factors. It was reported in some researches that the main receptor of the cholinergic transmitter was α7nAchR. Therefore, α7nAchR would be regarded as the main unit to regulate the cytokine synthesis in the cholinergic anti-inflammatory pathway and exert the crucial actions [11-13]. The present study showed that a large number of deep brown α7nAchR granules were seen and Ach content increases greatly in the STM group. This hints that the number of α7nAchR and the content of Ach decrease upon I/R. After electrical stimulation on vagus, the activity α7nAchR of the myocardial cell membranes increased or the density increased and the Ach content went up the research results suggested that, α7nAchR and Ach took part in the protection roles in vagus electric stimulation on the I/R damages and α7nAchR, the electric vagus stimulation exerts anti-inflammatory actions cholinergic anti-inflammatory pathway through the effector of cholinergic anti-inflammatory pathway, α7nAchR.
Myocardial ischemia could not only rapidly lead to clear biochemical and morphological changes in the myocardial tissue, but also induced the acute inflammatory responses. The proinflammatory cytokines of TNF-α, IL-6 and IL-18, etc, released through induction of I/R play an extremely important role in the pathogenesis mechanism of I/R [16]. Clinical studies suggested that the damages on the “vagus tensions” exist in the patients with ischemic heart disease and thus the improvement of activity of vagus verves can provide protective effects on the series pathological and physiological changes after myocardial ischemia [16]. It was reported by studies that the TNF-α in the plasma will rise immediately after the death of myocardial infarction. Another experiments founded that the correlations exist between TNF-α and myocardial reperfusion [17]. TNF-α was a type of important inflammatory cytokines, the level of which was comparatively low in the myocardial tissues in general conditions. However, the damaged cells would release a large number of TNF-α after myocardial ischemia reperfusion damages occur. Studies proved that TNF-α released first at the initial stage of ischemia reperfusion and its content was related to the severity as well as the prognosis of the disease [18]. The past studies on animals showed that it could reduce the number of circulating neutrophils and monocytes as well as their filtration to the damaged myocardial muscles and then to induce the clear protection action on the myocardial muscles through lifting the levels of TNF-α expression in the ischemic area prior to I/R [19]. Some other studies found the myocardial I/R led to the obvious increase of the IL-6 level in serum [20]. Sawa et al. [21], through in vitro experiments, observed that Ach has stronger actions on inhibiting TNF-α, in which Ach could suppress the release of inflammatory cytokines produced from the stimulation of endotoxin on the macrophasges. Results of the present study showed that, the levels of TNF-α and IL-6 increased remarkably in the I/R group, and simultaneously, Ach content decreased and a large amount of neutrophils filtration occurred; the levels of TNF-α and IL-6 in the STM group declined and simultaneously, Ach content increased and the neutrophils in filtration decreased clearly. The results of the study suggested, in the I/R model of dogs, the inflammatory cytokines of TNF-α and IL-6 took part in the pathological and physiological courses of I/R, and the decrease of Ach content hinted that the functions of vagus nerves were damaged. After the vagus nerves were given electric stimulation, the decreased levels of TNF-α and IL-6 reduced the damage of inflammatory cytokines on the myocardial muscles. The raising content of Ach hinted that the vagus nerves exerted the functions in the anti-inflammation mechanism in the I/R model. In the present study, the stimulation gave off the vagus nerves, Ach and N-acetylcholine receptor suppressed the inflammation responses and this may exert some certain protective action in the course of I/R damages of the heart. The study further hinted that cholinergic anti-inflammatory pathway can be used as an alternative or potential therapy in intervening some pathological courses or prevent some inflammation diseases which are still lack of effective treatment measures at present.
In most of the experiments related to the influences of vagus stimulation on the myocardial I/R damages at abroad, the unilateral [23,24] or bilateral [25,26] vagus nerves were cut and then the proximal part of the vagus was stimulated. This could cause clear low blood pressure and negative chronotropic effect to happen to the experimental animals. Therefore, the invasive cardiac protective intervention measures had defects of opportunity choice clinically, option of adaptability as well as complication at high risks and became hard to apply to the clinical treatment in a wide manner.
In conclusion, the present study adopted the protective action on I/R damages of electric stimulation to the complete vagus nerves cord. This could be transferred to be the verification of the protective action of vagus stimulation on the treatment of myocardial diseases, and embody its accurate clinical application value and prosperity. The right vagus nerve stimulation could activate anti-inflammatory pathway and inhibit the systemic and local inflammatory reaction to relieve myocardial I/R injury.
Acknowledgements
We thank Drs. Lilei Yu and Hong Jiang (Department of Cardiology, People’s Hospital of Wuhan University) for technical assistance in the experiment. This work was supported by grant 2013911119 from Science and Technology Supporting Project of Xinjiang Uygur Autonomous Region.
Disclosure of conflict of interest
None.
References
- 1.Ridker PM. Inflammation, infection, and cardiovascular risk: how good is the clinical evidence? Circulation. 1998;97:1671–1674. doi: 10.1161/01.cir.97.17.1671. [DOI] [PubMed] [Google Scholar]
- 2.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 3.Coquet I, Mousson C, Rifle G, Laurent G, Moreau D, Cottin Y, Zeller M, Touzery C, Wolf JE. Influence of ischemia on heart-rate variability in chronic hemodialysis patients. Ren Fail. 2005;27:7–12. [PubMed] [Google Scholar]
- 4.Brook RD, Julius S. Autonomic imbalance, hypertension, andcardiovascular risk. Am J Hypertens. 2000;13:112S–122S. doi: 10.1016/s0895-7061(00)00228-4. [DOI] [PubMed] [Google Scholar]
- 5.Camm AJ, Pratt CM, Schwartz PJ, Al-Khalidi HR, Spyt MJ, Holroyde MJ, Karam R, Sonnenblick EH, Brum JM AzimiLide post Infarct surVival Evaluation (ALIVE) Investigators. Mortality in patients after a recent myocardial infarction: arandomized, placebo-controlled trial of azimilide using heart rate variability for risk stratification. Circulation. 2004;109:990–996. doi: 10.1161/01.CIR.0000117090.01718.2A. [DOI] [PubMed] [Google Scholar]
- 6.Czura CJ, Traeey KJ. Autonomic neural regulation of immunity. J Intern Med. 2005;257:156–166. doi: 10.1111/j.1365-2796.2004.01442.x. [DOI] [PubMed] [Google Scholar]
- 7.Kawada T, Yamazaki T, Akiyama T, Kitagawa H, Shimizu S, Mizuno M, Li M, Sugimachi M. Vagal stimulation suppresses ischemia-induced myocardial interstitial myoglobin release. Life Sci. 2008;83:490–495. doi: 10.1016/j.lfs.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Uemura K, Zheng C, Li M, Kawada T, Sugimachi M. Early short-term vagal nerve stimulation attenuates cardiac remodeling after reperfused myocardial infarction. J Card Fail. 2010;16:689–699. doi: 10.1016/j.cardfail.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Doyle SL, O’Neill LA. Toll-like receptors: from the discovery of NFkappaB to new insights into transcriptional regulations in innate immunity. Biochem Pharmacol. 2006;72:1102–1113. doi: 10.1016/j.bcp.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetycholine receptor alpha7 subunit is an essential regulator of in flammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 12.Francis J, Zhang ZH, Weiss RM, Felder RB. Neural regulation of the proinflammatory cytokine response to acute myocardial infarction. Am J Physiol Heart Circ Physiol. 2005;288:977–978. doi: 10.1152/ajpheart.00099.2004. [DOI] [PubMed] [Google Scholar]
- 13.van Westerloo DJ, Giebelen IA, Florquin S, Daalhuisen J, Bruno MJ, de Vos AF, Tracey KJ, van der Poll T. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. J Infect Dis. 2005;191:2138–2148. doi: 10.1086/430323. [DOI] [PubMed] [Google Scholar]
- 14.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Susarla S, Czura CJ, Tracey KJ. Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J Vasc Surg. 2002;36:1231–1236. doi: 10.1067/mva.2002.129643. [DOI] [PubMed] [Google Scholar]
- 15.Shahani R, Marshall JG, Rubin BB, Li RK, Walker PM, Lindsay TF. Role of TNF-alpha in myocardial dysfunction after hemorrhagic shock and lower-torso ischemia. Am J Physiol Heart Circ Physiol. 2000;278:H942–H950. doi: 10.1152/ajpheart.2000.278.3.H942. [DOI] [PubMed] [Google Scholar]
- 16.Wu W, Lu Z. Loss of anti-arrhythmic effect of vagal nerve stimulation on ischemia-induced ventricular tachyarrhythmia in aged rats. Tohoku J Exp Med. 2011;223:27–33. doi: 10.1620/tjem.223.27. [DOI] [PubMed] [Google Scholar]
- 17.Ott I, Neumann FJ, Kenngott S, Gawaz M, Schömig A. Procoagulant inflammatory responses of monocytes after direct balloon angioplasty in acute myocardial infarction. Am J Cardiol. 1998;82:938–42. doi: 10.1016/s0002-9149(98)00509-8. [DOI] [PubMed] [Google Scholar]
- 18.Neumann FJ, Ott I, Gawaz M, Richardt G, Holzapfel H, Jochum M, Schömig A. Cardiac release of cytokines and inflammatory responses in acute myocardial infarction. Circulation. 1995;92:748–55. doi: 10.1161/01.cir.92.4.748. [DOI] [PubMed] [Google Scholar]
- 19.Strande JL, Routhu KV, Hsu A, Nicolosi AC, Baker JE. Gadolinium decreases inflammation related to myocardial ischemia and reperfusion injury. J Inflamm (Lond) 2009;6:34. doi: 10.1186/1476-9255-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akiyama D, Hara T, Yoshitomi O, Maekawa T, Cho S, Sumikawa K. Postischemic infusion of sivelestat sodium hydrate, a selective neutrophil elastase inhibitor, protects against myocardial stunning in swine. J Anesth. 2010;24:575–581. doi: 10.1007/s00540-010-0948-8. [DOI] [PubMed] [Google Scholar]
- 21.Sawa Y, Ichikawa H, Kagisaki K, Ohata T, Matsuda H. Interleukin-6 derived from hypoxic myocytes promotes neutrophil-mediated reperfusion injury in myocardium. J Thorac Cardiovasc Surg. 1998;116:511–517. doi: 10.1016/S0022-5223(98)70018-2. [DOI] [PubMed] [Google Scholar]
- 22.Lyudmila V, Borovikova , Svetlana lvanova, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW Borovikova. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–461. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 23.Katare RG, Ando M, Kakinuma Y, Arikawa M, Handa T, Yamasaki F, Sato T. Vagal nerve stimulation prevents reperfusion injury through inhibition of opening of mitochondrial permeability transition pore independent of the bradycardiac effect. J Thorac Cardiovasc Surg. 2009;137:223–231. doi: 10.1016/j.jtcvs.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 24.Kakinuma Y, Ando M, Kuwabara M, Katare RG, Okudela K, Kobayashi M, Sato T. Acetylcholine from vagal stimulation protects cardiomyocytes against ischemia and hypoxia involving additive non-hypoxic induction of HIF-alpha. FEBS Lett. 2005;579:2111–2118. doi: 10.1016/j.febslet.2005.02.065. [DOI] [PubMed] [Google Scholar]
- 25.Uemura K, Li M, Tsutsumi T, Yamazaki T, Kawada T, Kamiya A, Inagaki M, Sunagawa K, Sugimachi M. Efferent vagal nerve stimulation induces tissue inhibitor of metalloproteinase-1 in myocardial ischemia-reperfusion injury in rabbit. Am J Physiol Heart Circ Physiol. 2007;293:H2254–2261. doi: 10.1152/ajpheart.00490.2007. [DOI] [PubMed] [Google Scholar]
- 26.Mioni C, Bazzani C, Giuliani D, Altavilla D, Leone S, Ferrari A, Minutoli L, Bitto A, Marini H, Zaffe D, Botticelli AR, Iannone A, Tomasi A, Bigiani A, Bertolini A, Squadrito F, Guarini S. Activation of an efferent cholinergic pathway produces strong protection against myocardial ischemialreperfusion injury in rats. Crit Care Med. 2005;33:2621–2628. doi: 10.1097/01.ccm.0000186762.05301.13. [DOI] [PubMed] [Google Scholar]


