Abstract
In order to find the possible mechanism of Dexamethasone (Dex) during curing fibrosis, the bleomycin (BLM)-induced mice model was used. After fibrosis were induced by BLM, histopathological evaluation and RT-PCR were employed to detect the expression of TGF-β1, Smad3 and STAT1. It was found that BLM promoted the development of inflammation, leading to severe pulmonary fibrosis with the increasing of TGF-β1, Smad3 and STAT1. After Dex treatment, the expression of TGF-β1, Smad3 and STAT1 showed a little higher with alleviation of the fibrosis. Thus it is concluded that there is a possible pathway of mouse pulmonary fibrosis model through TGF-β, Smad3 and JAK-STAT pathway.
Keywords: Bleomycin, dexamethasone, idiopathic pulmonary fibrosis, Smad3, JAK-STAT
Introduction
It is currently believed that idiopathic pulmonary fibrosis is an epithelial-fibroblastic disease. Risk factors associated with pulmonary fibrosis include smoking, environmental exposure, gastroesophageal reflux disease, genetic factors, diabetes mellitus, infectious agents, and commonly prescribed drugs, such as BLM [1]. Current therapeutic strategies are primarily aimed at controlling the inflammatory processes, often through the oral administration of glucocorticosteroids, which have been the first line of therapy for pulmonary fibrosis since the 1950s [2].
BLM plays an important role in the treatment of lymphoma, squamous cell carcinomas, germ cell tumors and malignant pleural effusion, where it is injected intrapleurally [3]. However, it is believed that BLM acts by causing single and double-strand DNA breaks in tumor cells. Pulmonary fibrosis develops in ~ 1% of patients receiving BLM [4]. BLM as an agent to induce experimental lung fibrosis was described in a lot of animals [5,6]. BLM induced animal models of experimental lung fibrosis often be employed to study the IPF. BLM causes inflammatory and fibrotic reactions within a short period of time, even more so after intratracheal instillation.
One study examining possibly safer therapies for pulmonary fibrosis revealed that low doses of DEX delivered constantly by autologous erythrocytes slowed the progression of lung disease [7].
In the early injury or inflammation, TGF-β promotes the development of inflammation, and increases activity at sites of inflammation, and induces the proliferation of the fibroblasts, leading to severe pulmonary fibrosis. The fulfilling of TGF-β function depends on the signal transduction and regulation of Smads proteins. Smad2/3 is mediated by TGF-β-induced liver fibrosis key elements [8].
In recent years, studies have shown the abnormal activation of STAT1 in BLM-induced mouse pulmonary fibrosis model [9], and STAT1 was involved in alveolitis and pulmonary fibrosis in the alveolar macrophages (AM). We also found that there is a pathway of pathogenesis in BLM-induced mouse model that involves the TGF-β1, IL-31 and JAKs/STATs pathway [10]. Finally, DEX affects the development of pulmonary fibrosis through TGF-β, Smad2/3, JAK-STAT. A BLM-induced mouse pulmonary fibrosis model was used to test the possible pathway of the DEX.
Materials and methods
Chemicals and reagents
BLM A5 hydrochloride and DEX were purchased from Zhejiang Haizheng (Hangzhou, China). The RT-PCR kit was obtained from Promega (WI, USA) and the PCR primers were synthesized by Bioasia Biologic Technology (Shanghai, China). The mouse antibodies of TGF-β1, Smad3 were purchased from Boster Bio-Engineering (Wuhan, China). All other chemicals were of the highest commercial grade available.
Animals
The studies described in this report were approved by the Animal Review Committee of Jiangsu University. CD-1 mice (Shanghai Laboratory Animal Center, Chinese Academy of Sciences, Shanghai, China) were maintained in a controlled environment and provided with water and standard rodent food.
Mouse model
60 mice were randomly divided into the following 3 groups (n=20 each): saline-water; BLM-water; BLM plus DEX. Mice in the saline group were injected intratracheally with 2 ml/kg saline; the others were injected intratracheally with BLM (5 mg/kg, 2 ml/kg in saline). Twenty-four hours after BLM treatment, mice were given by gavage 0.45 mg/kg/d DEX. The day of intratracheal injection with BLM or saline was designated day 0.
Histopathological evaluation
Mouse lung tissues were processed for routine paraffin embedding, and serial sections (5 μm) were stained with hematoxylin and eosin. The extent of alveolitis and fibrosis was blindly assessed using the previously described semi-quantitative criteria [11]. Tissues from all the mice were embedded in paraffin. Five μm-thick paraffin sections were collected by microscope slides. The sections were blocked and incubated with mouse anti-TGF-β1 and SMAD3 antibodies (Wuhan, China) and a biotinylated and streptomycin-labeled goat anti-mouse antibody (Wuhan, China). Cyclin D1 expression was detected by the reaction of peroxidase with 3, 3’-diaminobenzidine tetrahydrochloride (DAB).
RT-PCR analysis for cellular factors
Total RNA was extracted from 100-mg frozen lung tissue using the TRI Reagent (Sigma, MO, USA), and 2 μg of total RNA were reverse transcribed into cDNA at 42°C for 1 h with Moloney murine leukemia virus reverse transcriptase (Promega, WI, USA). The following primers were used for PCR: STAT1 (5’-ttgtgttgaatcccgaacct-3’; 5’-tcgaaccactgtgacatcct-3’), Actin (5’-gtccctcaccctcccaaaag-3’; 5’-gctgcctcaacacctcaaccc-3’). Amplifications were for 40 cycles of 60 s at 95°C, 45 s at 60°C, and 75 s at 72°C, and the PCR products were visualized on a 2% agarose gel using a Molecular Analyst Densitometer (Bio-Rad, Hercules, CA).
Statistical methods
Results are given as means ± SD. The differences were assessed by Student’s t-test, one-way or two-way ANOVA using Bonferroni posttests as appropriate. A P value of <0.05 was considered to indicate a significant difference.
Results
The mouse model of pulmonary fibrosis and effect of DEX treatment
BLM administration resulted in the development of extensive fibrosis areas in the lung on day 28 after treatment. Dex administration attenuated pulmonary fibrosis. Histopathology showed that mice in the control group had a normal structure (Figure 1A). On day 3 post treatment, BLM treated mice and Dex treated mice showed mild fibrosis in the alveoli and interstitium (Figure 1B, 1D). On day 28, BLM treated mice showed marked histopathological changes, such as large fibrous areas and collapsed alveolar spaces (Figure 1C). However, Dex treated mice only showed mild fibrosis at day 28 (Figure 1E).
Figure 1.
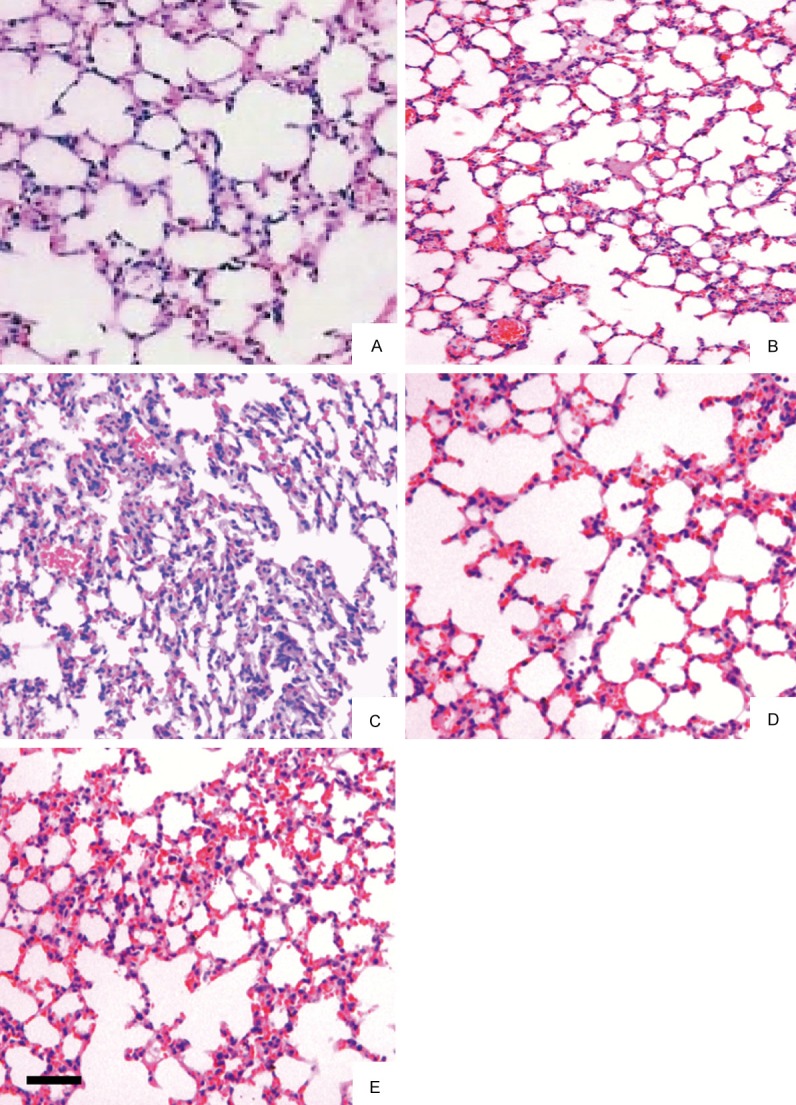
Histological section of lung fields (A-E). The lung sections were stained with HE (A: The control group, B: The bleomycin (BLM) model group 3 d, C: The BLM model group 28 d, D: The BLM model plus dexamethasone (DEX) group 3 d, E: The BLM model plus Dex group 28 d). The results of the control group were normal (A); the pulmonary tissues in the BLM group had apparent fibrotic changes (B, C); and it shows a mild fibrosis in the alveoli and interstitium by Dex treatment (D, E). Bar: 100 μm.
The expression of TGF-β1 detected by immunohistochemical staining
The sections at different times were stained by anti TGF-β1 antibodies. This marker expressed weakly in control lung tissues. At 7 and 14 days after BLM treatment, the signal of TGF-β1 was significantly stronger than that of the control group. At 28 days after treatment, the TGF-β1 signal became a little weaker. At 7 and 14 days of BLM plus Dex group, the signal of TGF-β1 was also stronger than that of the control group. However, at 28 days, the TGF-β1 signal became weaker and was a little stronger than the level of control group. All the results were given by comparison of the average IOD value (Table 1).
Table 1.
TGF-β1 immunohistochemical staining by comparison of the average IOD values
| Group | 7 d | 14 d | 28 d |
|---|---|---|---|
| Control | 13.34±1.12 | 13.23±0.99 | 13.16±0.87 |
| BLM | 32.93±1.77** | 29.09±1.56** | 25.79±1.55* |
| Dex | 26.37±2.38**,† | 23.23±2.14**,† | 18.22±1.36**,† |
Data are mean ± SEM, n=15,
p<0.05;
p<0.01;
p<0.05.
The expression of Smad3 of mice lung tissue
Immunohistochemical staining was performed to detect the expression of Smad3 in the alveolar macrophages and alveolar epithelial cells. We found the expression of Smad3 increased significantly after treatment from 3 d to 28 d (Figure 2B). However, after treated with Dex, the expression level became weaker (Figure 2C).
Figure 2.
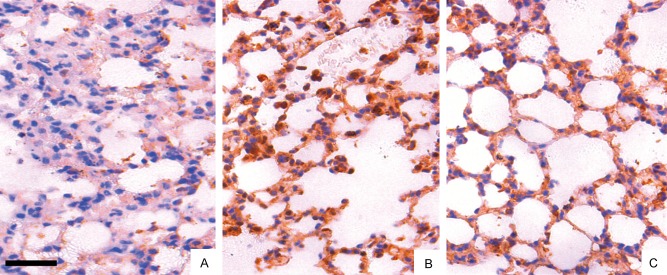
Immunohistological staining for Smad3 in the lung fields (A: The control group, B: The BLM model group, C: The BLM plus Dex group). Fewer positively stained cells were seen in the BLM plus Dex group (C) than in the BLM group (B); Smad3 expression was significantly weak in the control group (A). Bar: 100 μm.
The expression of STAT1 mRNA of mice lung tissues
The lung tissues of mice after BLM treatment at different days were collected and extracted mRNA. Then, RT-PCR technology was employed to get the expression of STAT1 gene. We found that the STAT1 gene was upregulated in mice lung after treated by BLM, even at 7 days after treatment (Figure 3). STAT1 gene was detected at a more high level in the model group. It expressed at the most high level at 14 d and becomes lower at 21 d and 28 d. Whenever, after treated with Dex, the expression level became lower than the model group.
Figure 3.
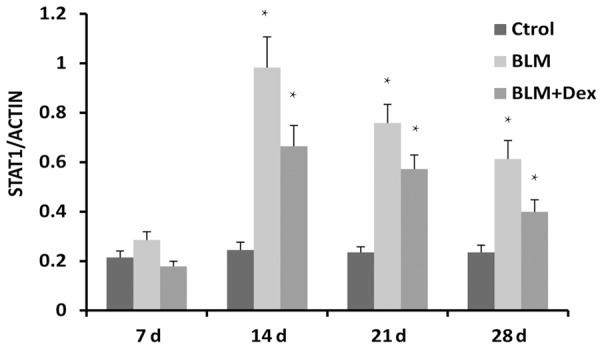
RT-PCR for STAT1 mRNA in different groups. STAT1 mRNA bands were measured by densitometric analysis and normalized with GAPDH. For each group n=15. Data are expressed as the mean ± SEM. *P<0.05. **P<0.01.
Discussion
In the present study, DEX was used in BLM-induced fibrosis mouse model, and as previous studies, DEX attenuated pulmonary fibrosis induced by BLM. The molecules TGF-β1, Smad3 and JAK-STAT increased after BLM treatment and showed decreasing trend. Because of the expressions of these molecules are in a similar mode whether in the BLM-induced model or BLM plus Dex group. Therefore, we speculate that they may constitute a possible pathway of mouse pulmonary fibrosis.
In a previous clinical report, short-term treatment with the corticosteroid DEX started 12-48 h after birth in infants with neonatal respiratory distress syndrome, increased survival without causing bronchopulmonary dysplasia [12]. This suggests that DEX treatment after injury is able to prevent lung fibrosis. Furthermore, one study revealed that low doses of DEX delivered constantly by autologous erythrocytes slowed the progression of lung disease such as pulmonary fibrosis. And in our result, we also saw the attenuating function of DEX.
TGF-β1 is a key molecule in the development and progression of pulmonary fibrosis. It promotes the transcription of collagen type I and fibronectin in fibroblasts through an intricate signaling cascade. In this study, TGF-β1 expression became significantly higher after BLM treatment and was a little lower if DEX was added.
A family of transcription factors, called Smad proteins [13,14] is intracellular signaling pathway downstream to the TGF-β1 receptors. Activation of the type I receptor results in phosphorylation of the pathway-restricted Smad2 and Smad3, which then form a heteromeric complex with Smad4 [13,14]. We found the expression of Smad3 is similar with TGF-β1. Therefore, we suspect that Smad3 is a downstream of TGF-β1 in the BLM-induced mice model.
Another study found that STAT-1 was abnormally activated in alveolar macrophages (AMs) of rats with BLM-induced interstitial pulmonary fibrosis (IPF) [9]. The abnormal STAT-1 activation may play a role in the pathogenesis of acute alveolitis and pulmonary fibrosis. Other previous studies found JAKs/STATs pathway is important in lung disease.
The data from this study show that it is a possible pathway of pulmonary fibrosis. And the rescue function of DEX further confirmed this pathway. It begins at TGF-β1. TGF-β1 induced Smad2 phosphorylation finally stimulates the JAK-STAT.
Acknowledgements
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. This work was funded by Jiangsu University Clinical Science and Technology Development Fund (JLY2012063), and Natural Science Foundation of Shanghai (10ZR1422600).
References
- 1.Zisman DA, Keane MP, Belperio JA, Strieter RM, Lynch JP 3rd. Pulmonary fibrosis. Methods Mol Med. 2005;117:3–44. doi: 10.1385/1-59259-940-0:003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamman L, Rich AR. Acute diffuse fibrosis of the lungs. Bull Johns Hopkins Hosp. 1944;74:177–212. [Google Scholar]
- 3.Kundu S, Misra S, Haldar R, et al. Severe Paraneoplastic Peripheral Blood Eosinophilia and Eosinophilic Malignant Pleural Effusion as Rare Manifestations of Squamous Cell Carcinoma Lung. Int Med J. 2013;20:34. [Google Scholar]
- 4.Blenoxane®. Canadian Pharmacists Association: 2006. Compendiumof Pharmaceuticals and Specialties. [Google Scholar]
- 5.Fleischman RW, Baker JR, Thompson GR, Schaeppi UH, Illievski VR, Cooney DA, Davis RD. Bleomycin-induced interstitial pneumonia in dogs. Thorax. 1971;26:675–682. doi: 10.1136/thx.26.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adamson IY, Bowden DH. The pathogenesis of bleomycin-induced pulmonary fibrosis in mice. Am J Pathol. 1974;77:185–197. [PMC free article] [PubMed] [Google Scholar]
- 7.Rossi L, Castro M, D’Orio F, Damonte G, Serafini S, Bigi L, Panzani I, Novelli G, Dallapiccola B, Panunzi S, Di Carlo P, Bella S, Magnani M. Low doses of dexamethasone constantly delivered by autologous erythrocytes slow the pro gression of lung disease in cystic fibrosis patients. Blood Cells Mol Dis. 2004;33:57–63. doi: 10.1016/j.bcmd.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Bartram U, Speer CP. The role of transforming growth factor beta in lung development and disease. Chest. 2004;125:754–765. doi: 10.1378/chest.125.2.754. [DOI] [PubMed] [Google Scholar]
- 9.Fan XM, Wang ZL, Li ZH. STAT1 activation and STAT1-dependent immune-response gene ICAM-1 expression in alveolar macrophages of rats suffered from interstitial pulmonary fibrosis. Chin J Cell Mol Immunol. 2003;19:3–6. [PubMed] [Google Scholar]
- 10.Shi K, Jiang J, Ma T, Xie J, Duan L, Chen R, Song P, Yu Z, Liu C, Zhu Q, Zheng J. Pathogenesis pathways of idiopathic pulmonary fibrosis in bleomycin-induced lung injury model in mice. Respir Physiol Neurobiol. 2014;190:113–117. doi: 10.1016/j.resp.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988;41:467–470. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garland JS, Alex CP, Pauly TH, Whitehead VL, Brand J, Winston JF, Samuels DP, McAuliffe TL. A three-day course of dexamethasone therapy to prevent chronic lung disease inventilated neonates: a randomized trial. Pediatrics. 1999;104:91–99. doi: 10.1542/peds.104.1.91. [DOI] [PubMed] [Google Scholar]
- 13.Massaous J, Hata A. TGF-beta signalling through the Smad pathway. Trends Cell Biol. 1997;7:187–192. doi: 10.1016/S0962-8924(97)01036-2. [DOI] [PubMed] [Google Scholar]
- 14.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]


