Abstract
Intestinal obstruction (IO) is an important risk factor for the development of bacteria translocation (BT), a serious condition associated with sepsis and potential mortality. Ankaferd is an herbal extract that is reported to exert anti-hemorrhagic, anti-oxidant, anti-microbial, and anti-inflammatory, effects in the intestine. In this study, we employed an animal model of intestinal obstruction to evaluate the effects of Ankaferd in the prevention of bacterial translocation and the suppression of the inflammatory response. Thirty male Wistar Albino rats were allocated randomly to three groups: Group 1 (sham) underwent ileal manipulation alone; Group 2 (intestinal obstruction, IO) underwent complete ileal ligation; Group 3 (intestinal obstruction + Ankaferd blood stopper, ABS) underwent complete ileal ligation and intraperitoneal Ankaferd injection. All rats were euthanized after 24 hours. Blood samples were collected for the measurement of serum oxidative stress parameters and cytokine expression. In addition, liver, mesenteric lymph node (MLN), spleen, and ileal specimens were obtained for microbiological culture to determine the rate of bacterial translocation. Liver and ileal tissues were collected for histopathological examination. A reduction in oxidative damage, inflammatory cytokine expression and bacterial translocation was observed in the ABS treatment group relative to the IO group (p<0.05). Furthermore, histopathological examination demonstrated a reduction in obstruction-induced mucosal injury in Ankaferd-treated rats. Data derived from this study provided the first evidence that Ankaferd treatment limits bacterial translocation and enhances intestinal barrier function in mice undergoing intestinal obstruction. Ankaferd may be useful in the prevention of BT associated with IO.
Keywords: Ankaferd, intestinal obstruction, bacterial translocation, cytokines, oxidative stress
Introduction
Intestinal obstruction (IO) is among the most common causes of acute abdominal problems. IO is associated with an elevated risk of bacterial translocation (BT), particularly among patients with multiorgan dysfunction syndrome (MODS) and septicemia, conditions which inflict high rates of mortality [1]. Improved diagnostic and therapeutic techniques are necessary in the treatment of intestinal obstruction. A better understanding of the pathophysiology of IO, and the application of antibiotic therapy, intestinal tube decompression, and intravenous fluid administration has reduced overall mortality among IO patients [2]. Despite recent advances, IO is associated with a mortality rate of 10% [3]. The human gut harbors approximately 500 unique commensal microbes, including anaerobic organisms [4]. Despite the exceedingly high microbial burden within the intestine, translocation across the intestinal mucosal barrier occurs relatively infrequently. Commensal microbes have significant physiological functions, acting as a barrier against pathogens and performing valuable metabolic activities [5].
In obstructed intestinal tissue, massive quantity of bacteria, their products, or both penetrates into extra-intestinal organs, which is termed BT [6]. Bacterial translocation may occur as a complication of many different conditions, including malnutrition, sepsis, trauma, and IO. Definitive diagnosis of BT involves the identification of gut-origin bacteria in the mesenteric lymph nodes (MLN), portal venous blood, or the systemic circulation [7].
Ankaferd (Ankaferd Blood Stopper®, ABS) is a unique traditional plant medicinal extract that has been used for centuries in Anatolia as an effective hemostatic agent. ABS forms an encapsulated protein network that provides critical attachment points for rapid erythrocyte aggregation without affecting the physiological coagulation systems [8]. In addition to hemostatic functions, anti-inflammatory [9], anti-infective, [10] antifungal, [11] and anti-oxidative [9] effects have been attributed to ABS.
Bacterial translocation secondary to bowel obstruction stimulates the release of reactive oxygen species and alters cytokine expression profiles [12]. Oxidative stress inflicts cellular damage as a result of the imbalance between antioxidants and reactive oxygen species [13]. Total oxidant activity (TOA), total antioxidant capacity (TAC) and the oxidative stress index (OSI) are useful markers of global changes in oxidation state [14]. Paraoxonase 1 (PON-1) protects lipoproteins against oxidative modification through the hydrolysis of hydrogen peroxide. Decreased PON-1 activity has been associated with increased systemic oxidative stress [15]. The measurement of oxidative balance may reflect changes in the inflammatory state subsequent to IO-induced BT.
Cytokines play a crucial role in the modulation of inflammation in the gastrointestinal tract. Pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) are necessary in the escalation of inflammatory processes [16]. A substantial number of experimental studies have demonstrated the hematological and biochemical safety profile of Ankaferd [17]. Cumulatively, the excellent safety profile and significant biological activity associated with Ankaferd suggest a variety of research applications. To our knowledge, this research is the first in vivo study investigating the effects of Ankaferd on BT and the inflammatory response in an experimental rat model of intestinal obstruction. The aim of the current study was to evaluate the therapeutic efficacy of Ankaferd in an experimental model of IO.
Materials and methods
Ankaferd blood stopper®
The standardized ABS (1 vial of 100 mL) used in these experiments was obtained from Trend Teknoloji Ilac AS, Istanbul, Turkey. The solution is a registered product of several plant extracts. The relative ratio of each plant extraction is shown in Table 1.
Table 1.
Ingredients of Ankaferd®
| Form of the active substance | |
|---|---|
|
|
|
| Amount of the active substance | Ampule (mg) |
| Thymus vulgarisa | 0.10 |
| Glycyrrhiza glabrab | 0.18 |
| Vitis viniferab | 0.16 |
| Alpinia officinarumb | 0.14 |
| Urtica dioicac | 0.12 |
Dried grass extract;
Dried leaf extract;
Dried root extract.
Experimental animals
The study protocol was reviewed and approved by the Committee of Experimental Animals of Dicle University. All of the experimental protocols were performed according to the guidelines for the Care and Use of Laboratory Animals. Wistar albino rats were obtained from Dicle University Health Sciences Application and Research Center. This study included 30 male Wistar Albino rats aged 9-12 weeks with a mean weight of 300-350 g. Animals were housed under standardized humidity (45%-50%), lighting (12 hours of daylight/12 hours of dark), and temperature (21±2°C) conditions. Animals were fed with a consistent diet of laboratory grade pellets and fresh water each day. All animals were observed carefully during the experiment. A wire grate was placed in the bottom of the cage in order to prevent coprophagy. Rats of all groups were deprived of food for 24 h before surgery but were given free access to water. An effort was made to minimize animal suffering and the number of animals used.
The experimental groups
The 30 male Wistar Albino rats were allocated randomly to one of three equal groups (n=10 each), only one of which received ABS, as follows:
Group 1 (Sham): only ileal manipulation was performed and no drug was given.
Group 2 (IO): ileal manipulation and ligation; no drug was given.
Group 3 (IO + ABS): ileal manipulation and ligation, with 3 ml of ABS solution after 1:3 dilutions with saline delivered intraperitoneally at the end of the experimental study.
Animal preparation
Rats were anesthetized with 50 mg/kg ketamine hydrochloride (Ketalar®, Parke Davis, Eczacibasi, Istanbul, Turkey) and 10 mg kg xylazine (Rompun®; Bayer AG, Leverkusen, Germany) via intramuscular injection. Rats were then placed supinely on a pad. A 10% povidone iodine solution (Betadine®) was applied for shaved skin cleansing.
Surgical procedure
During laparotomy, a 2 cm midline incision was performed on all the rats under sterile conditions. The terminal ileum was isolated and ligated [18]. The distal ileum was ligated with a 3-0 silk suture at 1 cm proximal to the caecum, obstructing the passage without ligation of the mesenteric vessels. After the operation, 3 ml of the normal saline (NS) was injected into the peritoneum, and the incision was closed in a single layer. Twenty-four hours later, blood samples were collected from the anaesthetized rats by cardiac puncture under sterile conditions. Instantly after cardiac puncture, the rats were sacrificed and repeat laparotomies were performed. Before collecting blood and tissue specimens, a peritoneal swab was taken for culture from the peritoneal cavity with a sterile swab chopstick. For microbiological analyses, 1 ml blood samples were collected from the inferior vena cava, mesenteric lymph nodes (MLNs), liver, and spleen tissues. The latter allowed for the collection of liver tissues and ileal segments 2-3 cm in diameter proximal to the ligation for subsequent histopathological examinations. Serum was obtained following centrifugation of the blood and rapidly transferred to Eppendorf tubes for biochemical analyses, and stored at -80°C. Furthermore, the tissues were prepared for histopathological evaluation. Foreign tissue residues and blood were removed, washed with saline, and put into plastic containers containing 10% formaldehyde solution.
Microbiological evaluation
Blood samples were collected from the inferior vena cava and cultured aerobically and anaerobically utilizing BacTecTM Peds cruets (Bectone-Dickinson Diagnostic Inc., Sparks, MD, USA). Identification was completed using the BD-Phoenix 100 TM system. Peritoneal swabs and positive cultures were plated out on blood agar, chocolate agar, eosin methylene blue agar, or Sabouraud dextrose agar. Simultaneously, MLN, spleen, and liver tissue was removed and placed in sterile glass bottles containing sterile brain-heart infusion medium. The bottles were re-weighed and tissue homogenates were prepared in 2 ml brain-heart infusion medium using a sterile muller and pestle. A portion (0.1 ml) of each homogenate was cultured on blood agar, chocolate agar, eosin methylene blue agar, or Sabouraud-dextrose agar. All agar plates were analyzed after 24 and 48 h of incubation at 37°C. The incidence of bacterial translocation was calculated by determining the number of rats with positive bacterial culture divided by the total number of rats studied.
Biochemical analyses
PON-1, TAC, TOA, TNF-α, IL-1β, IL-6 and C-reactive protein (CRP) were measured in the serum samples. In addition, the OSI was calculated.
Measurement of the paraoxonase (PON-1)
Serum PON-1 activity was measured spectrophotometrically using a modified Eckerson method [19]. The PON-1 results were expressed in terms of U/L.
Measurement of the total antioxidant capacity (TAC)
TAC was determined in the supernatant fractions using a novel automated measurement method developed by Erel [20]. Here, the antioxidative effect of the sample against the potent free radicals, as initiated by hydroxyl radical production, is measured. The results are expressed as mmol Trolox equiv./L.
Measurement of total oxidant activity (TOA)
The TOA of supernatant fractions was determined using a novel automated measurement method developed by Erel [14]. The assay is calibrated with hydrogen peroxide and the results are expressed in terms of µmol H2O2 equiv./L.
Oxidative stress index (OSI)
OSI is a parameter indicating the degree of oxidative stress as follows: OSI (arbitrary units)=[TOA/TAC] ×10014.
Measurement of TNF-α, IL-1β, IL-6, and CRP
Circulating TNF-α, IL-1β, and IL-6 (Diasource, Nivelles, Belgium) cytokines were measured using an enzyme amplified sensitivity immunoassay method. The serum CRP levels (DRG, NJ, USA) were determined using an enzyme-linked immunosorbent assay method.
Histopathological analysis
The liver tissues and ileal segment were fixed in 10% formalin in phosphate buffer for 48 hours and embedded in paraffin blocks. Then paraffin blocks were placed in a microtome (EM UC7, Leica, Germany) and tissue sections were cut into 5 µm slices. The tissue sections were deparaffinized, hydrated using a series of xylenes and graded alcohol, and stained with hematoxylin-eosin (H&E). After the staining process was completed, the sections were examined. The H&E stained sections of liver and ileal segments were examined for graded inflammatory cell infiltration and the degree of ileal mucosal injury. Histological slides were examined under a light microscope (Nikon ECLIPSE 80i). All histomorphological analyses described below were performed by an experienced and blinded pathologist (Dr.G.T.) without knowledge of the animal treatment groups. The degree of tissue injury was classified as (0) normal-no damage, (1) mild damage, (2) moderate damage and (3) severe damage [21,22]. Ten microscopic fields were analyzed on each slide. In addition, all tissue sections were stained with Giemsa and examined under a light microscope for evaluation of bacterial translocation.
Statistical analysis
All biochemical findings are presented as mean ± standard deviation (SD) and the histopathological scores are presented as median values. A one-sample Kolmogorov-Smirnov test was performed in order to evaluate the data distribution relative to a normal distribution. Inter-group comparisons were performed using the Kruskal-Wallis and Mann-Whitney U-test. The Chi-square test was used for the comparison of categorical variables between groups. A P value of less than 0.05 was considered statistically significant. All data were processed using the statistical package SPSS 18.0 for Windows (IBM Corporation, Armonk, NY).
Results
No mortality observed within the 24 h following the experimental procedures.
Comparison of oxidative and antioxidative parameters
According to the biochemical analyses of oxidative and antioxidative serum parameters there were significant differences in PON-1, TAC, TOA and OSI levels in the IO group relative to the to the sham group (p<0.001 for all differences in oxidative parameters). The IO group exhibited significantly lower PON-1 and TAC levels, and higher TOA and OSI levels compared relative to the sham group (p<0.001, for both) (Table 2). When Ankaferd was administered to rats with experimental IO (intestinal obstruction plus Ankaferd group) a significant increase in PON-1 and TAC (p<0.001 and p=0.019, respectively) and significant decreases in TOA and OSI levels were observed relative to the group with untreated IO (p=0.009 and p=0.002, respectively) (Table 2). No significant differences in PON-1, TAC, or TOA values between the Sham and Intestinal obstruction + Ankaferd groups were found (p>0.05) (Table 2).
Table 2.
Levels of paraoxonase, total antioxidant capacity, total oxidant activity, and oxidative stress index in serum samples of rat (mean ± Standard deviation)
| Shama (n=10) | Intestinal obstructionb (n=10) | Intestinal obstruction + Ankaferdc (n=10) | p | |
|---|---|---|---|---|
| PON-1 | 38.39±7.15 | 18.09±4.22 | 53.30±18.20 | a-b<0.001, a-c0.089, b-c<0.001, a-b-c<0.001 |
| TAC | 0.87±0.08 | 0.65±0.09 | 0.79±0.10 | a-b<0.001, a-c0.089, b-c0.019, a-b-c<0.001 |
| TOA | 12.23±1.41 | 33.29±10.51 | 18.27±10.73 | a-b<0.001, a-c0.218, b-c0.009, a-b-c<0.001 |
| OSI | 1423±238 | 5275±2204 | 2370±1464 | a-b<0.001, a-c0.029, b-c0.002, a-b-c<0.001 |
Group 1;
Group 2;
Group 3.
PON-1 (U/L)=paraoxanase-1, TAC (mmol Trolox Equiv./L)=total antioxidant capacity, TOA (mmol H2O2 Equiv./L)=total oxidant activity, OSI (Arbitrary Unite)=oxidative stress index.
Comparison of inflammatory cytokines and C-reactive protein
Following biochemical analyses of the inflammatory cytokines, the IO group exhibited higher TNF-α, IL-1β and IL-6 expression levels compared with the sham group (p<0.001 for all cytokines). In animals treated with Ankaferd (IO + Ankaferd group), TNF-α, IL-1β, and IL-6 was significantly decreased relative to the IO group (p<0.001, p=0.002 and p<0.001, respectively). Significantly higher CRP levels were found in the IO group compared with the sham or IO + Ankaferd groups (p<0.001). However, CRP levels in ABS group were significantly lower than in the IO group (p<0.001 for each) as indicated in Table 3.
Table 3.
The serum levels of TNF-α, IL-1β, IL-6 and C-reactive protein in rats untreated and treated with Ankaferd (mean ± standard deviation)
| Shama (n=10) | Intestinal obstructionb (n=10) | Intestinal obstruction + Ankaferdc (n=10) | p | |
|---|---|---|---|---|
| TNF-α (pg/mL) | 1.85±0.88 | 7.94±1.71 | 2.87±1.14 | a-b<0.001, a-c0.075, b-c<0.001, a-b-c<0.001 |
| IL-1β (pg/mL) | 0.43±0.10 | 1.72±0.54 | 0.53±0.84 | a-b<0.001, a-c=0.043, b-c=0.002, a-b-c<0.001 |
| IL-6 (pg/mL) | 30.48±8.52 | 65.82±20.44 | 36.97±7.34 | a-b< 0.001, a-c0.105, b-c< 0.001, a-b-c<0.001 |
| CRP (mg/mL) | 29.29±4.91 | 165.26±41.05 | 67.19±11.71 | a-b< 0.001, a-c< 0.001, b-c< 0.001, a-b-c<0.001 |
Group 1;
Group 2;
Group 3.
TNF-α=tumor necrosis factor-α, IL-1β=interleukin-1β, IL-6=interleukin-6, CRP=C reactive protein.
Comparison of histomorphological findings
Significant inflammatory pathology was observed in the intestinal obstruction group. In contrast to the sham rats (Figure 1A), moderate to severe inflammation, swelling of the intestinal mucosa and intense edema of the lamina propria, and vacuolization of the epithelial cells were observed in the small intestine of IO group rats (Figure 1B). In addition, the intestinal villi were dramatically blunted and shortened. The animals treated with the Ankaferd exhibited significant preservation of the intestinal tissue structure (Figure 1C). In H&E staining of the liver tissue sections, the sham group exhibited minimal vascular congestion and no inflammatory activity (Figure 2A). Moderate portal inflammation and vascular congestion in the liver tissue were observed in the IO group (Figure 2B). Hepatic histomorphologic findings were consistent with suppressed inflammatory activity in the IO + ABS group relative to the IO group (Figure 2C).
Figure 1.
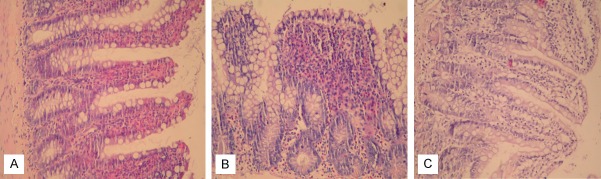
Histological images of the small intestine of rats. A. Sham group. Mild mucosal inflamation (HE stain, ×200); B. Intestinal obstruction group. Severe inflammation and edema in the mucosa with subtotal villous atrophy (HE stain, ×200); C. Intestinal obstruction + ankaferd group. Mild to moderate inflammation and edema in mucosa of the villi, some of which show slightly blunted ends (HE stain, ×200).
Figure 2.
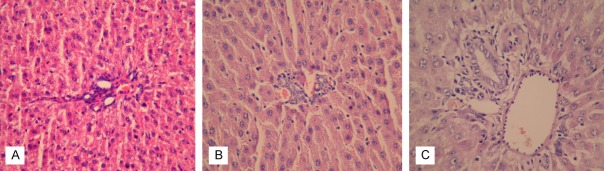
Histopathological changes in liver tissue (A) Minimal vascular congestion without inflammation in the liver parenchyma was shown in sham group (HE stain, ×200); (B) Moderate portal inflammation and vascular congestion were shown in intestinal obstruction group (HE stain, ×200); (C) In intestinal obstruction + ankaferd group liver tissue seemingly normal other than to minimal portal inflammation (HE stain, ×200).
The histopathological grading of the liver and ileum are summarized in Table 4. According to the histopathological evaluation, ileum and liver tissue inflammatory scores were significantly higher in the IO group compared with the sham of IO + ABS groups (p<0.001 and p<0.001, respectively). The ileal mucosal histological damage scores for animals in the IO group were also significantly higher than in the sham group (p<0.001). There was no difference in liver inflammatory scores between the IO + ABS and sham groups, (p=0.481). However, the liver and ileum inflammation scores in the IO + ABS group were significantly lower than in the IO group (p=0.001 and p<0.001, respectively) (Table 4).
Table 4.
The histopathologic grading of liver and ileum in rats according to groups [Values are median (minimum-maximum)]
| Shama (n=10) | Intestinal obstructionb (n=10) | Intestinal obstruction + Ankaferdc (n=10) | p | |
|---|---|---|---|---|
| Liver inflammation score | 0.00 (0.0-1.0) | 1.50 (1.0-2.0) | 0.00 (0.0-1.0) | a-b<0.001, a-c0.481, b-c<0.001, a-b-c<0.001 |
| Ileum inflammation score | 1.0 (0.0-1.0) | 3.0 (1.0-3.0) | 1.0 (1.0-2.0) | a-b<0.001, a-c0.190, b-c0.001, a-b-c<0.001 |
| Ileal injury score | 0.00 (0.0-0.0) | 1.50 (1.0-3.0) | 1.0 (0.0-2.0) | a-b<0.001, a-c0.002, b-c0.052, a-b-c<0.001 |
Group 1;
Group 2;
Group 3.
Comparison of microbiological findings
The culture results, expressed as the number of rats with positive bacterial culture divided by the total number of rats, are presented in Table 5. No significant difference in peritoneal culture results was observed among the three treatment groups. Comparison of the IO group with the sham and IO + Ankaferd groups revealed a significantly higher proportion of positive culture results in the blood (p=0.004), liver (p<0.001), spleen (p=0.018), and MLN (p=0.002) cultures of the IO group (Table 5). Ankaferd treatment significantly decreased the proportion of positive MLN and liver cultures (p=0.007 and p=0.007, respectively). However, no significant differences in the proportion of positive peritoneal, spleen, or blood cultures in the IO + ABS group relative to the IO group (p=0.074 for each).
Table 5.
Positive microbiological culture results according to groups
| Sham (n=10) | Intestinal obstruction (n=10) | Intestinal obstruction + Ankaferd (n=10) | p | |
|---|---|---|---|---|
| Peritoneal culture | 2/10 | 7/10 | 3/10 | 0.054 |
| MLN culture | 1/10 | 8/10 | 2/10 | 0.002 |
| Spleen culture | 1/10 | 7/10 | 3/10 | 0.018 |
| Liver culture | 0/10 | 8/10 | 2/10 | <0.001 |
| Blood culture | 0/10 | 7/10 | 3/10 | 0.004 |
MLN: Mesenteric lymph node.
Discussion
Intestinal obstruction of the small bowel is relatively common, despite advances in diagnosis and the treatment. Bowel obstruction remains a significant cause of morbidity and mortality, particularly among critically ill patients [2]. Bacterial translocation at the intestinal barrier and invasion of the systemic circulation has been proposed as one of the primary pathogenetic mechanisms of sepsis and multi-organ dysfunction among critically ill patients with IO [7]. In spite of the well-known anti-oxidative, anti-inflammatory, and anti-microbial effects associated with Ankaferd, this is the first report to demonstrate that Ankaferd ameliorates the detrimental effects of experimental IO. Our results demonstrate that Ankaferd suppresses BT and oxidative damage caused by IO.
Obstruction of the intestine without interruption of blood flow results in important modifications of intestinal physiology, characterized primarily by bowel distension. Obstruction-induced intestine distension is correlated with the accumulation of fluid and gas in the intestinal lumen due to swallowing and bacterial fermentation, resulting in part from disrupted absorptive function and augmented bacterial growth in the obstructed intestine [3]. In addition, IO may injure intestinal mucosa and increase intestinal permeability to bacteria and endotoxins, resulting in BT and sepsis [23]. As a result of disrupted intestinal flow during IO, we observed bacterial overgrowth following IO in agreement with previous reports by El-Awady et al. and Çıtak et al. [24,25].
The agar gel diffusion test is widely used as an in-vitro method for evaluation of antimicrobial activity of various chemicals [26]. This may suggest an alternate method of evaluating antimicrobial activity associated with Ankaferd. During periods of increased intestinal permeability, microorganisms may invade the systemic circulation, MLN and liver. In the present study, BT was demonstrated in cultures of blood, spleen, MLN, and liver samples obtained from animals with IO. However, no evidence of BT was observed in animals in the sham group. This difference in BT rates between the experimental and sham groups was statistically significant. Furthermore, our results showed that Ankaferd significantly reduced BT in MLN and liver cultures when compared with the IO group. The reduction in BT associated with Ankaferd may result from the preservation of the intestinal barrier and maintenance of intestinal impermeability. The mechanism through which Ankaferd protects against BT is unclear; further studies are required to clarify these mechanisms and open the way for the use of Ankaferd in co-therapeutic treatments.
Ankaferd includes a standardized preparation of the plants Thymus vulgaris, Glycyrrhiza glabra, Vitis vinifera, Alpinia officinarum, and Urtica dioica [9]. Each of these herbs has known effects on the endothelium, blood cells, angiogenesis, cell proliferation, and other physiologic mediators [8]. Thymus vulgaris has antimicrobial activity and anti-oxidative properties, including the inhibition of lipid peroxidation [27]. Recent studies have demonstrated that Glycyrrhiza glabra has antifungal, antimicrobial, antioxidant, and powerful anti-inflammatory effects [28]. Vitis vinifera seed extract is associated with an extensive spectrum of pharmacological effects including antioxidative, anti-inflammatory, as well as an-timicrobial effects [29]. Alpinia officinarum also has antimicrobial and antioxidant activity [30]. However, Urtica dioica has been reported as an antimicrobial agent in pharmaceutical and food industry [31].
The most commonly isolated bacteria in cases of BT is Escherichia coli. The antimicrobial activity of Ankaferd has been tested against many pathogens [26]. The in vitro antibacterial activity of Ankaferd has been studies using the agar well diffusion test and is highly effective against several gram-positive and gram-negative bacteria including common food borne pathogens [32]. Ankaferd inhibits growth of several common sources of nosocomial infection, including vancomycin-resistant enterococci (VRE), imipenem-resistant Acinetobacter, and methicillin-resistant Staphylococcus aureus (MRSA) isolates [10]. Another recent study demonstrated in vitro antibacterial activity of Ankaferd against other multi-antibiotic resistant bacteria, such as E. coli, Enterococcus spp., Pseudomonas spp., and Klebsiella spp., as well as fungi including Candida albicans, Mucor spp., and Aspergillus spp. [33]. E. coli was found to be the most commonly translocated bacteria in our study. The proportion of positive cultures in the MLN, spleen, and liver were significantly lower in the Ankaferd treatment group than in the IO group. These results suggest that Ankaferd inhibits the in vivo growth of gram-positive and gram-negative bacteria. Exposure to Ankaferd may promote enhanced oxygenation through erythrocyte aggregation [26,33,34].
TAC, TOA and OSI are important biochemical of oxidative status. In this study, TOA and OSI were higher in the serum of experimental IO rats than among the control group. These data suggest that during IO the production of oxygen free radicals is elevated. Therefore, administration of antioxidants may limit the harmful effects of oxygen free radical production in IO.
PON-1 is a high-density lipoprotein (HDL) associated enzyme with antioxidant properties. PON-1 levels are an indicator of the antioxidant defense system [35]. In the present study, PON-1 and TAC levels were decreased in IO rats relative to the control and ABS-treated groups. These results suggest that the antioxidant properties of Ankaferd may limit oxidative damage following IO.
TNF-α is an inflammatory cytokine known to induce increased epithelial permeability [16]. The level of TNF-α was elevated following IO, which may contribute to the induction of intestinal barrier damage. IL-1β and IL-6 are potent inflammatory cytokines up-regulated during bowel obstruction. In the present study, TNF-α, IL-1β and IL-6 were elevated in the IO group relative to the control group. However, administration of Ankaferd decreased expression of pro-inflammatory cytokines. In an experimental model of IO, CRP was elevated relative to control animals [36]. Similarly, the present data demonstrate a significant increase in CRP levels in the IO group. We also found that Ankaferd treatment was associated with significantly decreased CRP expression. Taken together, the results are evidence of the anti-inflammatory function of Ankaferd in an experimental bowel obstruction model.
The histological results of the current study demonstrate that bowel obstruction by mechanical ligation caused mucosal injury and gut barrier damage. Damage to the ileal mucosa seen in the IO group may be attributed to increased intra-luminal hydrostatic pressure and compression of the intestinal mucosal villi as a consequence of obstruction [37]. The histological damage in our IO rats was similar to other models of mechanical obstruction, with pronounced swelling and edematous villous structure [38,39]. In the Ankaferd treatment group, the histopathological changes resulting from the bowel obstruction damage were notably less severe relative to the IO group. The mechanism of anti-inflammatory activity following Ankaferd administration remains unclear.
Conclusion
The data from this study clearly demonstrate that oxidative stress parameters are elevated in experimental IO relative to sham treatment. Ankaferd was found to be protective against the oxidative damage in IO. Furthermore, Ankaferd inhibited production of pro-inflammatory cytokines. Interestingly, Ankaferd limited histological damage to the ileum and liver during IO. Taken together, our results demonstrated the potential protective effects of Ankaferd as an antioxidant, antimicrobial, and anti-inflammatory agent. This herbal mixture may improve prognosis in patients who are at potential risk of BT following IO. The mechanisms by which Ankaferd prevents BT should be investigated by future studies.
Disclosure of conflict of interest
None.
References
- 1.Cappell MS, Batke M. Mechanical obstruction of the small bowel and colon. Med Clin North Am. 2008;92:575–597. doi: 10.1016/j.mcna.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Diaz JJ Jr, Bokhari F, Mowery NT, Acosta JA, Block EF, Bromberg WJ, Collier BR, Cullinane DC, Dwyer KM, Griffen MM, Mayberry JC. Guidelines for management of small bowel obstruction. J Trauma. 2008;64:1651–64. doi: 10.1097/TA.0b013e31816f709e. [DOI] [PubMed] [Google Scholar]
- 3.Chang T, Lu R, Tsai L. Glutamine ameliorates mechanical obstruction-induced intestinal injury. J Surg Res. 2001;95:133–40. doi: 10.1006/jsre.2000.5983. [DOI] [PubMed] [Google Scholar]
- 4.Diniz SOF, Barbosa AJA, Araújo ID. Assessment of bacterial translocation in obstructive jaundice using Tc-99m Escherichia coli. Braz Arch Biol Technol. 2005;48:45–9. [Google Scholar]
- 5.Farthing MJ. Bugs and the gut: an unstable marriage. Best Pract Res Clin Gastroenterol. 2004;18:233–239. doi: 10.1016/j.bpg.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Shiomi H, Shimizu T, Endo Y, Murata S, Kurumi Y, Uji Y, Tani T. Relations among circulating monocytes, dendritic cells, and bacterial translocation in patients with intestinal obstruction. World J Surg. 2007;31:1806–1812. doi: 10.1007/s00268-007-9110-7. [DOI] [PubMed] [Google Scholar]
- 7.Papoff P, Ceccarelli G, d’Ettorre G, Cerasaro C, Caresta E, Midulla F, Moretti C. Gut microbial translocation in critically ill children and effects of supplementation with pre- and pro biotics. Int J Microbiol. 2012;2012:151393. doi: 10.1155/2012/151393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beyazit Y, Kurt M, Kekilli M, Goker H, Haznedaroglu IC. Evaluation of hemostatic effects of Ankaferd as an alternative medicine. Altern Med Rev. 2010;15:329–36. [PubMed] [Google Scholar]
- 9.Koçak E, Akbal E, Taş A, Köklü S, Karaca G, Can M, Kösem B, Üstün H. Anti-inflammatory efficiency of Ankaferd blood stopper in experimental distal colitis model. Saudi J Gastroenterol. 2013;19:126–30. doi: 10.4103/1319-3767.111955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saribas Z, Sener B, Haznedaroglu IC. Antimicrobial activity of Ankaferd Blood Stopper® against nosocomial bacterial pathogens. Central Eur J Med. 2010;5:198–202. [Google Scholar]
- 11.Ciftci S, Keskin F, Keceli Ozcan S, Erdem MA, Cankaya B, Bingol R, Kasapoglu C. In vitro antifungal activity of Ankaferd Blood Stopper against Candida albicans. Curr Ther Res. 2011;72:120–6. doi: 10.1016/j.curtheres.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choudhry MA, Fazal N, Goto M, Gamelli RL, Sayeed MM. Gut-associated lymphoid T cell suppression enhances bacterial translocation in alcohol and burn injury. Am J Physiol Gastrointest Liver Physiol. 2002;282:G937–47. doi: 10.1152/ajpgi.00235.2001. [DOI] [PubMed] [Google Scholar]
- 13.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37:112–119. doi: 10.1016/j.clinbiochem.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Gbandjaba NY, Ghalim N, Hassar M, Berrougui H, Labrazi H, Taki H, Saile R, Khalil A. Paraoxonase activity in healthy, diabetic, and hemodialysis patients. Clin Biochem. 2012;45:470–4. doi: 10.1016/j.clinbiochem.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Al-Sadi R, Boivin M, Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci (Landmark Ed) 2009;14:2765–78. doi: 10.2741/3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bilgili H, Captug O, Kosar A, Kurt M, Kekilli M, Shorbagi A, Kurt OK, Ozdemir O, Goker H, Haznedaroglu IC. Oral systemic administration of ankaferd blood stopper has no short-term toxicity in an “in vivo” rabbit experimental model. Clin Appl Thromb Hemost. 2010;16:533–536. doi: 10.1177/1076029609335912. [DOI] [PubMed] [Google Scholar]
- 18.dos Santos Rd, Viana ML, Generoso SV, Arantes RE, Davisson Correia M, Cardoso VN. Glutamine supplementation decreases intestinal permeability and preserves gut mucosa integrity in an experimental mouse model. JPEN J Parenter Enteral Nutr. 2010;34:408–13. doi: 10.1177/0148607110362530. [DOI] [PubMed] [Google Scholar]
- 19.Eckerson HW, Wyte CM, La Du BN. The human serum paraoxonase/arylesterase polymorphism. Am J Hum Genet. 1983;35:1126–38. [PMC free article] [PubMed] [Google Scholar]
- 20.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–11. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Akçay MN, Capan MY, Gündogdu C, Polat M, Oren D. Bacterial translocation in experimental intestinal obstruction. J Int Med Res. 1996;24:17–26. doi: 10.1177/030006059602400103. [DOI] [PubMed] [Google Scholar]
- 22.Demirkan A, Aksoy M, Kuzu MA. The effects of indomethacine on intestinal permeability and bacterial translocation in intestinal obstruction. J of Ankara University Faculty of Medicine. 2006;59:119–27. [Google Scholar]
- 23.Batista MA, Nicoli JR, Martins Fdos S, Machado JA, Arantes RM, Quirino IE, Correia MI, Cardoso VN. Pretreatment with citrulline improves gut barrier after intestinal obstruction in mice. JPEN J Parenter Enteral Nutr. 2012;36:69–76. doi: 10.1177/0148607111414024. [DOI] [PubMed] [Google Scholar]
- 24.El-Awady SI, El-Nagar M, El-Dakar M, Ragab M, Elnady G. C-reactive protein reliability? Acta Cir Bras. 2009;24:98–106. doi: 10.1590/s0102-86502009000200005. [DOI] [PubMed] [Google Scholar]
- 25.Çıtak A, Yilmaz O, Pekçetin Ç, Ozbal S, Lambrecht FY. Influence of uracil on bacterial translocation in an intestinal obstruction model in rats. Int J Surg. 2013;11:27–30. doi: 10.1016/j.ijsu.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Tasdelen Fisgin N, Tanriverdi Cayci Y, Coban AY, Ozatli D, Tanyel E, Durupinar B, Tulek N. Antimicrobial activity of plant extract Ankaferd Blood Stopper. Fitoterapia. 2009;80:48–50. doi: 10.1016/j.fitote.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Lee SJ, Umano K, Shibamoto T. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymusvulgaris L.) and their antioxidant properties. Food Chem. 2005;91:131–7. [Google Scholar]
- 28.Dirican E, Turkez H. In vitro studies on protective effect of Glycyrrhiza glabra root extracts against cadmium-induced genetic and oxidative damage in human lymphocytes. Cytotechnology. 2014;66:9–16. doi: 10.1007/s10616-012-9531-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nassiri-Asl M, Hosseinzadeh H. Review of the pharmacological effects of Vitis vinifera (grape) and its bioactive compounds. Phytother Res. 2009;23:1197–1204. doi: 10.1002/ptr.2761. [DOI] [PubMed] [Google Scholar]
- 30.Srividya AR, Dhanabal SP, Misra VK, Suja G. Antioxidant and Antimicrobial Activity of Alpinia officinarum. Indian J Pharm Sci. 2010;72:145–148. doi: 10.4103/0250-474X.62233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Modarresi-Chahardehi A, Ibrahim D, Fariza-Sulaiman S, Mousavi L. Screening antimicrobial activity of various extracts of Urtica dioica. Rev Biol Trop. 2012;60:1567–76. doi: 10.15517/rbt.v60i4.2074. [DOI] [PubMed] [Google Scholar]
- 32.Akkoc N, Akcelik M, Haznedaroglu I. In vitro anti-bacterial activities of ankaferd blood stopper. Int J of Lab Hem. 2008;30:95–9. [Google Scholar]
- 33.Akkoc N, Akcelik M, Haznedaroglu IC. In vitro anti-bacterial activities of Ankaferd medicinal plant extract. Turk J Med Sci. 2009;29:410–415. [Google Scholar]
- 34.Haznedaroglu BZ, Beyazit Y, Walker SL, Haznedaroglu IC. Pleiotropic cellular, hemostatic, and biological actions of Ankaferd hemostat. Crit Rev Oncol Hematol. 2012;83:21–34. doi: 10.1016/j.critrevonc.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Topsakal C, Kilic N, Ozveren F. Effects of prostaglandin E1, melatonin, and oxytetracycline on lipid peroxidation, antioxidant defense system, paraoxonase (PON1) activities, and homocysteine levels in an animal model of spinal cord injury. Spine. 2003;28:1643–52. doi: 10.1097/01.BRS.0000083163.03910.B1. [DOI] [PubMed] [Google Scholar]
- 36.Cevikel MH, Ozgün H, Boylu S, Demirkiran AE, Aydin N, Sari C, Erkus M. C-reactive protein may be a marker of bacterial translocation in experimental intestinal obstruction. ANZ J Surg. 2004;74:900–4. doi: 10.1111/j.1445-1433.2003.02681.x. [DOI] [PubMed] [Google Scholar]
- 37.Hayanga AJ, Bass-Wilkins K, Bulkley GB. Current management of small-bowel obstruction. Adv Surg. 2005;39:1–33. doi: 10.1016/j.yasu.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Kabaroudis A, Papaziogas B, Koutelidakis I, Kyparissi-Kanellaki M, Kouzi-Koliakou K, Papaziogas T. Disruption of the small intestine mucosal barrier after intestinal occlusion: a study with light and electron microscopy. J Invest Surg. 2003;16:23–28. [PubMed] [Google Scholar]
- 39.Wu CC, Lu YZ, Wu LL, Yu LC. Role of myosin light chain kinase in intestinal epithelial barrier defects in a rat model of bowel obstruction. BMC Gastroenterol. 2010;10:39. doi: 10.1186/1471-230X-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]


