Abstract
In this study, we observed synaptic connectivity among neurons in CA1 region of pilocarpine-induced chronic seizures in rats. Twenty healthy male Sprague-Dawley rats were divided randomly into an epilepsy group (n = 10) and a control group (n = 10). Approximately 60 days after status epilepticus (SE) , Fluorogold (FG) was injected into the CA1 area of the hippocampus in vivo. Somatostatin (SS) expression was observed using immunofluorescence. The distribution of FG-positive and FG/SS double-labeled neurons was observed using a confocal microscope. FG-labeled pyramidal cells could be seen remotely from the FG-injected site in the CA1 area and in the subiculum in the experimental group. FG/SS double-labeled interneurons were distributed remotely from the FG-injected site in the CA1 area in the epileptic rats. These changes suggest aberrant neuronal connectivity in CA1 region, which may lead to the formation of aberrant excitatory and inhibitory circuitry, and may play an important role in the generation or compensation for temporal lobe epilepsy.
Keywords: Temporal lobe epilepsy (TLE), fluorogold (FG), somatostatin (SS), neuronal connectivity, circuit rearrangement, interneuron, lithium chloride, pilocarpin
Introduction
Temporal lobe epilepsy (TLE) is a major type of intractable epilepsy that severely impairs physical and mental health. Currently, the cause of TLE and its spontaneous, recurrent seizures remains unknown. The potential mechanisms underlying self-compensation of seizure control are also unclear [1]. The occurrence of epilepsy is always associated with the following changes: neuronal loss, increased neuronal excitability, weak suppression, circuitry rearrangement and synaptic abnormalities. The rearrangement of excitatory and inhibitory circuitry in the hippocampus plays an important role in seizure onset in TLE [2,3]. Axonal sprouting in the hippocampus is a significant pathological feature in patients and animal models. Recent studies detected extensive axonal sprouting in hippocampal GABAergic inhibitory interneurons in a TLE model [4]. This evidence suggests that axonal sprouting is common in TLE and is an important structural basis for the rearrangement of excitatory and inhibitory circuitry [5]. Although there have been a number of reports on the rearrangement of excitatory circuitry [6-8], the changes in the synaptic connections of excitatory cells involved in TLE are unclear. In addition, there have been few studies on GABAergic inhibitory rearrangements [9]. Research on the synaptic connectivity that occurs between inhibitory interneurons during the chronic, spontaneous seizures of TLE is especially rare.
We studied the hippocampal somatostatin-positive (SS-positive) interneurons of pilocarpine-induced epileptic rats 30 days after status epilepticus (SE). We found that immunofluorescence for SS fibers increased visibly in the hippocampus stratum lacunosum-moleculare and in the outer molecular layer of the dentate gyrus. At 60 days after SE, many SS-positive fibers were found throughout the whole CA1 layer [10]. We speculate that the increased amount of SS-positive fibers was derived from an abnormal enhancement in axonal sprouting of SS interneurons from contiguous or distant regions. TLE is likely derived from aberrant hippocampal inhibitory circuits, and these SS-positive fibers may play an important self-repairing role following epileptic seizures. The continuous increase in the quantity of inhibitory fibers and the degeneration of a small number of neurons may contribute to spontaneous, chronic, recurrent seizures. Therefore, the increase in SS-positive fibers is worthy of further exploration.
To understand the changes in synaptic connections between excitatory and inhibitory rearrangements in TLE, we located and traced critical neurons and their dendrites. Fluorogold (FG) is a commonly used retrograde tracer, and when combined with immunofluorescence, FG retrograde tracing is a convenient, accurate and sensitive method to observe synaptic connections between the cell origin and dendrites using confocal microscopy [11-13]. The epileptic rat model induced by lithium chloride-pilocarpine is similar (behavior, EEG recordings, pathology and pharmacological properties) to human TLE and has been recognized as an ideal animal model for the study of TLE [14-17]. This study observed changes in synaptic connections among hippocampal CA1 pyramidal cells during spontaneous recurrent seizures using the lithium chloride-pilocarpine-induced epileptic rat model. By combining the FG retrograde tracer and immunofluorescent staining, we observed the origin of aberrant increased SS-positive fibers and their synaptic connectivity with inhibitory interneurons to reveal the “self-repairing” effect of excitatory and inhibitory circuits during TLE.
Methods
Animal model
In total, 20 adult male Sprague-Dawley (SD) rats (6-8 weeks old, weighing 250 ± 20 g) were used in this study. The SD rats were kept under stable environmental conditions (18°C-25°C, 50%-60% humidity, 12 h light/dark cycle, lights on at 06:00 am) with free access to standard laboratory chow and tap water. All experimental procedures were approved by The Animal Care and Use Ethics Committee of Xiangya Hospital (Changsha, China). The 20 SD rats were divided randomly into an epilepsy group (n = 10) or a control group (n = 10). The epilepsy model was developed based on the description of our previous study [10]. The rats in the control groups were intraperitoneally (i.p.) injected with 0.9% sodium chloride (125 mg/kg) at the corresponding spot times. All rats were observed for behavioral changes after injections.
Injection of FG
At 60 days after SE induction, the epilepsy group and control group were injected i.p. with sodium pentobarbital anesthesia (35 mg/kg), and were then placed in a stereotaxic apparatus (Narishige SN-3, Tokyo, Japan) with the fontanel in a horizontal plane. According to the brain stereotaxic atlas [18], a hole was drilled 2.0 mm from midline (bilaterally) and 3.1 mm posterior to the bregma. The needle of a 1 μl microsyringe (Hogon Scientific Instrument Co., Shanghai, China) was advanced 2.8-2.9 mm from the brain surface, and 0.5 μl of a FG solution (4% FG dissolved in 0.9% saline) (Fluorochrome Inc., Denver, CO, USA) was injected into the hippocampal CA1 area bilaterally.
Tissue fixation and section preparation
At 5-7 days after FG injection, rats were deeply anesthetized with sodium pentobarbital (35 mg/kg) and perfused through the ascending aorta with approximately 400 ml of 0.9% sodium chloride. The brains were removed, postfixed overnight with 500 ml of 4% paraformaldehyde and cryoprotected in 30% sucrose until they sank to the bottom. The freezing microtome (AO Company, Buffalo NY, USA) was adjusted to -18°C to -20°C, and the tissues were trimmed and fixed to the pedestal. The brain was serially sectioned in the coronal plane from the midbrain to the frontal lobes with a slice thickness of 35 μm. The hippocampal brain sections were collected and placed in 0.01 mol/l phosphate-buffered saline (PBS, pH 7.2-7.4). The sections were mounted onto polylysine-treated microscope slides and kept in a -20°C freezer. Direct sunlight was avoided during the sectioning and mounting to the slides.
SS immunofluorescent staining
The sections were dried and rinsed 3 times with 0.01 mol/l PBS. Each wash had duration of 10 min. The sections were then incubated with 0.03% TritonX-100 (DingGuo Biotech Co., Beijing, China) at 37°C for 25 min and rinsed 3 times with 0.01 mol/l PBS (10 min each). The sections were then blocked with 10% normal goat serum (DingGuo Biotech Co., Beijing, China) for 25 min at 37°C. The excess liquid was poured off without washing. The sections were then incubated at 4°C overnight (40-48 h) with 50 μl of the primary antibody (rabbit anti-rat antibody SS polyclonal antibody at a 1:1500 dilution in 2% normal goat serum (Sigma, St Louis, MO, USA). Following 3 PBS washes (1 min each), the sections were incubated with the secondary antibody (diluted with 2% blocking serum (Sigma, St Louis, MO, USA)) for 2 h at room temperature. The sections were rinsed twice with PBS (5 min each), dried and mounted with 60% glycerol.
Results and statistical analyses
We used a LSM510 confocal microscopy (Carl Zeiss AG, Oberkochen, Germany) to view the sections. In the epilepsy group, 5 sections from each rat were randomly selected. Each section contained the injection hole. Similar sections were randomly selected from the control group. Three non-overlapping images were photographed in the CA1 region in the FG injection site, the CA1 region distant from the injection site using a Motic microscope. The number of SS-positive cells and the number of FG- and SS-positive (FG/SS double-labeled) cells were counted. Statistical analyses were performed for the ratio of the number of FG/SS double-labeled interneurons to the total number of SS-labeled interneurons.
The experimental data were presented as the mean ± standard deviation. The differences between the groups were analyzed for statistical significance by a one-way analysis of variance (ANOVA) followed by paired comparisons. Student’s t-test was used to test for differences between the epilepsy group and the control group. Significance was defined as P < 0.05. The statistical analyses were performed using SPSS 17.0 software (SPSS, Inc., Chicago, USA).
Results
Behavioral changes
After the i.p. injection of lithium chloride, the rats in the experimental group behaved normally. The animals developed peripheral cholinergic reactions 5-30 minutes following injection of pilocarpine, such as piloerection, salivation, tremors and bloody tears. The following stereotypical behaviors appeared simultaneously or successively: gazing; chewing; sniffing; exploratory behavior; wet dog-like shakes; repeated looking up followed by blinking, facial spasms; nodding and repeated, bilateral forelimb clonus accompanied by upright posture, falling or turning over. Some animals had tonic, clonic seizures of the extremities. The seizures occurred infrequently at first, and the frequency increased over time. A total of 9 epileptic rats reached the Racine III-V level. A total of 2 rats died during convulsions, and the remaining 7 rats entered the resting phase after 24-72 h, most of the rats behaved normally during this interval. After 15-45 days, spontaneous seizures appeared in all of the surviving rats. These seizures were tonic, clonic seizures with short durations (lasting approximately 30 sec to 1 min). The seizure frequency ranged from several times per day to once every few days. No deaths were observed within the chronic phase. The rats behaved normally after injection of FG.
Pyramidal cells labeled with FG
The hippocampal CA1 neurons labeled with FG were primarily pyramidal cells and some non-chief cells. A yellow, granular material (Figure 1B) was observed in the cytoplasm of the labeled cells. FG-labeled cells in the control group were neatly and tightly arranged. Their axons and dendrites were orderly and arranged in parallel (longitudinally) (Figure 2B). FG-labeled pyramidal cells in the epilepsy group were shrunken and appeared disorderly. Their axons were not compact, and their dendrites were not well-visualized (Figure 2A). FG-labeled pyramidal cells were found in 5 of 7 rats in the epilepsy group in the CA1 region far from the injection site and close to the CA2 region (Figure 1C). No FG-labeled pyramidal cells were detected in any rats in the control group. Rather, FG-labeled cells were confined to the region adjacent to the injection site. FG-labeled pyramidal cells were also found in 2 of 7 rats in the epilepsy group in the hippocampal subiculum (Figure 1D). However, no FG-labeled pyramidal cells were found in the control group in this region. The fluorescent signal weakened with increasing distance from the injection site (Figure 1A, 1C and 1D).
Figure 1.
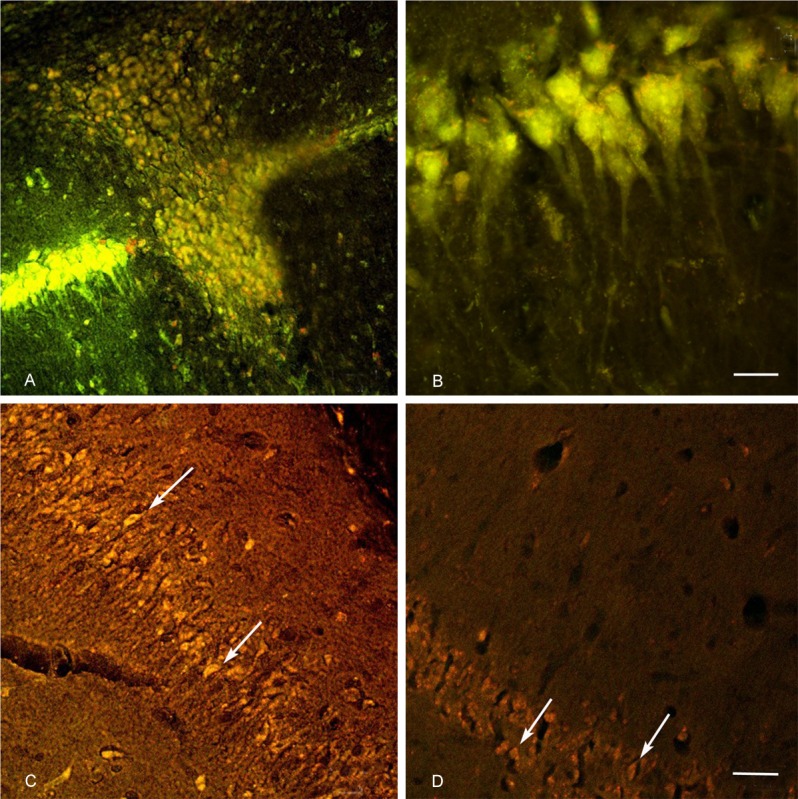
FG-labeled with immunofluorescence: A: The FG injection site in Hippocampal CA1 region (magnification, ×200; scale bars = 50 μm). B: FG-labeled pyramidal cells in CA1 region, a yellow, granular material was observed in the cytoplasm of the labeled cells (magnification, ×1000; scale bars = 10 μm). C: CA1 region, distant from the injection site in the epilepsy group, FG-labeled pyramidal cells were found (arrows) (magnification, ×200; scale bars = 50 μm). D: FG-labeled pyramidal cells were also found in the hippocampal subiculum of the epilepsy group (magnification, ×200; scale bars = 50 μm). A, C and D: The fluorescent signal weakened with increasing distance from the injection site.
Figure 2.
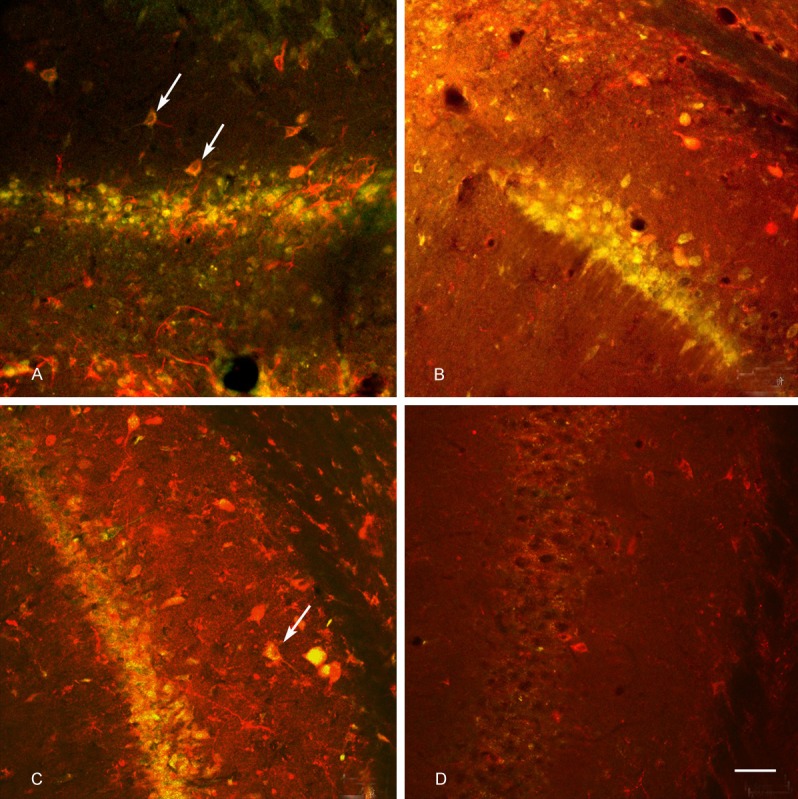
Double-labeling of FG and SS (magnification, ×200; scale bars = 50 μm): A: The FG injection site in the CA1 region of the epilepsy rat, FG-labeled pyramidal cells were shrunken and appeared disorderly, their axons were not compact, and their dendrites were not well-visualized. FG/SS double-labeled neurons had red cell bodies, axons and dendrites covered with scattered, dark yellow fluorescence (arrows). B: In the control rat, FG-labeled cells were neatly and tightly arranged, their axons and dendrites were orderly and arranged in parallel (longitudinally), FG/SS double-labeled neurons were detected. C: CA1 region, distant from the FG injection site, near the CA2 region in the epilepsy rat, FG/SS double-labeled neurons were found (arrows). D: CA1 region, distant from the FG injection site, near the CA2 region in the control rat, FG/SS double-labeled were not found.
FG-labeled SS interneurons
FG-labeled slices immunolabeled for SS were observed by confocal microscopy. There was a drastic increase in the number of SS-positive fibers in 5 rats in the epilepsy group. The other 2 rats presented little increase. FG/SS double-labeled neurons had red cell bodies, axons and dendrites covered with scattered, dark yellow fluorescence. FG-negative cells presented extremely bright yellow fluorescence [13]. At the FG injection sites, many FG/SS double-labeled neurons were found in the epilepsy group (Figure 2A) (accounted for 60% to 81% of the total number of neurons). FG/SS double-labeled axons and dendrites were also detected. FG/SS double-labeled neurons were also found in the control group (Figure 2B) (accounted for approximately 52% to 80% to the total number of neurons). There was no significant difference between the experimental group and the control group (P>0.05, Table 1). FG/SS double-labeled neurons were identified in the CA1 region distant from the FG injection site near the CA2 region in the epilepsy group (Figure 2C) in 5 rats (accounting for 35% to 55% of the total number of neurons). FG/SS double-labeled neurons were not found in the control group (Figure 2D). There was a statistically significant difference between the epilepsy and control groups (P<0.01, Table 1).
Table 1.
Ratio of FG/SS double-labeled neurons to total SS-positive neurons in different parts of the CA1 region (% ± SD)
| Group | Injection site | Distant from the injection site and near the CA2 region |
|---|---|---|
| Control group | 0.66 ± 0.096 | 0 |
| Epilepsy group | 0.70 ± 0.090# | 0.43 ± 0.083* |
Comparison of the same site between two groups, P > 0.05;
Comparison of the epilepsy group between two sites, P < 0.01.
Abnormal labeling and the incidence of spontaneous seizures
During the chronic stage of SE, spontaneous seizures occurred 1 to 5 times every 40 hours (5 days per week). We divided the incidence of spontaneous seizure into the following 3 levels: low frequency: ≤ 0.2 times per day (n = 2 rats); moderate frequency: between 0.4 and 0.6 times per day (n = 3 rats); and high frequency: ≥ 0.8 times per day (n = 2 rats). There was no significant increase of SS-positive fibers in the CA1 region of the 2 rats with a low incidence of seizures. Aberrant connections among SS-positive neurons and pyramidal cells were most obvious in the CA1 region of rats with a moderate frequency of seizures, pyramidal cells also demonstrated obvious morphological abnormalities in these rats. In addition, aberrant distribution of FG-labeled pyramidal cells was observed in the subiculum of 2 rats with a high frequency of seizures.
Discussion
Under pathological conditions, hippocampal granule cells spread mossy fibers that activate the dendrites of GABAergic interneurons. The activated interneurons sprout mossy fibers, which effectively resist the excitation of the granule cells. Therefore, Froscher et al. proposed that hippocampal mossy fiber-interneuron inhibitory synapses may be an important constituent of an inhibitory circuit rearrangement [19]. Aberrant hippocampal excitatory and inhibitory rearrangements may be closely related to the generation of TLE and its self-repairing function. Our study found rearrangement of excitatory and inhibitory circuitry in the hippocampal CA1 region of rats with pilocarpine-induced, chronic TLE. First, aberrant interactions were observed among pyramidal cells in the CA1 region and between the hippocampal subiculum and the CA1 region. Second, aberrant connectivity existed among interneurons in the CA1 region.
This study found that FG-labeled pyramidal cells existed in the CA1 region remotely from the injection site and in the subiculum in rats with chronic TLE. This finding was not observed in the control group, which suggests the presence of aberrant excitability in the neural network of rats with chronic TLE.
Aberrant connections among pyramidal cells in CA1
Local excitatory connections exist mostly in the CA3 region rather than the CA1 region [20,21]. Using an intracellular recording method and biotinylated probes, Deuchars et al. [22] determined that the pyramidal cell connectivity ratio was 1:16 in the CA3 region and only 1:100 in the CA1 region. Therefore, under normal circumstances, connections between pyramidal cells in the CA1 region are rare, which is consistent with the results of this study. In the chronic stage of TLE, we detected an enhancement of connections between pyramidal cells in the hippocampal CA1 region through FG labeling. Because FG is transported to the cell body mainly by retrograde axonal transport [11], this method of tracing may enhance the visualization of collateral connections between pyramidal cells that may result from aberrant axonal sprouting. This phenomenon also exists in kainic acid-induced seizures [8,23] and in human TLE with hippocampal sclerosis (moderate neuronal loss and gliosis) [24]. This evidence indicates that enhancement of collateral connections between pyramidal cells caused by axonal sprouting of pyramidal cells in the CA1 region is an important constituent of hippocampal excitatory circuit rearrangement. This mechanism could also compensate for the reduction in the number of pyramidal cells and the morphological changes in TLE.
Aberrant connections between subiculum and CA1
In this study, in addition to aberrant connections between pyramidal cells in the CA1 region, there were 2 rats in the epilepsy group with aberrant connections between the hippocampal subiculum and the CA1 region. Subiculum-hippocampal fibers have been reported previously and are believed to spread to the CA1 region through the stratum lacunosum-moleculare or stratum oriens [25]. Another study found that the subiculum delivered fibers to all levels of the plane above (including the pyramidal layer) [26], which suggests that cells in the CA1 region are controlled by subicular cells. In this study, FG-labeled, subicular pyramidal cells appeared in rats with chronic TLE, which is consistent with the results of Lehmann et al. [27], who used fluorescent dextran amine to observe an enhancement of subiculum-hippocampal projections. However, in the present study, this phenomenon was only observed in the 2 rats with a high frequency of spontaneous seizures. Therefore, the enhancement of excitatory synaptic connections between the subiculum and hippocampus could be associated with the frequency of spontaneous seizures. This finding needs to be confirmed with a larger sample size and electrophysiological studies.
Aberrant synaptic connections between excitatory pyramidal cells may promote the formation of aberrant excitatory circuits and enhance excitatory positive feedback from the hippocampus. These aberrant excitatory circuits may result in the synchronization of electrical discharges and play an important role in the generation of epilepsy.
Hippocampal inhibitory rearrangements in TLE
Previous research has shown that only a few hippocampal inhibitory interneurons are labeled by retrograde or anterograde tracers [28]. Zappone et al. [13] found sensitive FG fluorescence could be used to detect approximately 96% of SS-positive neurons in the hilar region of the hippocampus in normal SD rats. Therefore, we used this method to inject FG into the hippocampal CA1 region and performed SS-immunofluorescent staining. Confocal microscopy showed that FG/SS double-labeled interneurons were present in the CA1 region remotely from the FG injection site near the CA2 region in rats with chronic TLE. This finding was not obtained in rats in the control group, which suggests the presence of aberrant inhibitory neural networks between interneurons in the CA1 region in rats with chronic TLE.
Swanson et al. [29] found that FG labeled pyramidal cells in the normal hippocampus in the CA1 and CA3 region, but the stratum radiatum and stratum oriens of the CA1 and CA3 region were rarely labeled. This lack of labeling may have occurred because the interneurons of the non-injected sites of the same sub-region had no ability to transport FG, which means that few synaptic connections exist between normal hippocampal CA regions. However, upon FG injection in the dendritic region, FG-labeled interneurons were observed in the molecular layer, stratum radiatum and stratum oriens [13]. These previous results are consistent with our findings in the control group.
Figure 1B shows that the soma of the neuron contain many yellow granules, which are called FG-positive cytoplasmic granules. These granules are formed by lysosomal inclusions after fluorogold is ingested into the soma. The distribution of the particles reflects the relative density of neurons projecting to the axon at the injection site [30]. This study found that SS-positive interneurons in the hippocampal CA1 region remotely from the injection site were all labeled with FG, and several FG-positive cytoplasmic granules were observed in the cytoplasm of the soma. However, with increasing distance from the injection site, the number of cytoplasmic particles decreased, which indicates that with increasing distance, fewer axons project to the injection site. Another explanation may be that interneuronal sprouting neurites were too short to arrive at the injection site and thus did not sufficiently absorb FG, resulting in fewer granules transported to the soma. In addition, with increasing distance from the injection site, there were fewer double-labeled interneurons, which also suggests that with increasing distance, the uptake ability of neurons is decreased. However, regardless of sprouting or the ingestion of FG, the findings described above confirm that synaptic connections between interneurons in the CA1 region are enhanced in the chronic stage of TLE compared to controls. Only a few SS-positive fibers were labeled with FG, which is consistent with the study by Deller et al. [31], most likely because of masking of FG by strong red fluorescence of SS. Nonetheless, our results show that the increased amount of SS-positive fibers in the CA1 region partially originated from enhanced axonal sprouting of SS interneurons distant from the CA1 region.
In a model of pilocarpine-induced seizures, Houser et al. [32] found that GAD immunoreactivity of the stratum radiatum and stratum lacunosum-moleculare was enhanced and proposed that GABAergic circuit rearrangement likely occurred within the hippocampal CA1 region. However, no evidence has associated a seizure model with synaptic plasticity between GABAergic interneurons in the CA1 region. Only Wittneret al. [33] observed enhancements in synaptic connections between calbindin-positive interneurons in the CA1 region in human TLE. In this study, using FG retrograde tracing and immunofluorescence techniques, we confirmed that synaptic connections between SS-positive interneurons in the CA1 region were enhanced in the chronic stage of TLE in animal models. Aberrant inhibitory GABAergic circuit rearrangement existed in the CA1 region.
This type of rearrangement may lead to 2 functional outcomes. First, inhibitory synaptic connections are enhanced between interneurons so that the interneurons maybe disinhibited. SS-positive interneurons are classified as a dendritic type of GABAergic interneuron, and their main function is to control the input of pyramidal cells in the CA1 region [34,35]. Therefore, disinhibition of SS-positive interneurons may weaken their inhibitory control of pyramidal cells and enhance the excitability of the pyramidal cells [36,37]. Second, the increase in axonal sprouting of interneurons directly inhibits the excitability of pyramidal cells. Either functional outcome or both could play an important role in chronic spontaneous seizures.
Conclusion
Therefore, in the pathological state of TLE, inhibitory GABAergic interneurons are not just simply dead or alive. Their axonal sprouting and changes in synaptic connections may be important factors for inhibitory circuit rearrangement and may play an important role in the generation of epilepsy and self-repairing [5]. This circuit rearrangement is closely related to axonal sprouting, making the complex neural network system even more complicated. It is still unclear whether circuit rearrangement is the cause or result of chronic, spontaneous seizures. Further electrophysiological studies are needed.
Acknowledgements
This study was supported by grants from The National Natural Science Foundation of China (Grant No. 81201001) and The Project of Free Exploration for Young Teachers in Central South University (Grant No. 201012200171).
Disclosure of conflict of interest
None.
References
- 1.Sinha S, Siddiqui KA. Definition of intractable epilepsy. Neurosciences (Riyadh) 2011;16:3–9. [PubMed] [Google Scholar]
- 2.Kaila K, Ruusuvuori E, Seja P, Voipio J, Puskarjov M. GABA actions and ionic plasticity in epilepsy. Curr Opin Neurobiol. 2014;26C:34–41. doi: 10.1016/j.conb.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Jinde S, Zsiros V, Nakazawa K. Hilar mossy cell circuitry controlling dentate granule cell excitability. Front Neural Circuits. 2013;7:14. doi: 10.3389/fncir.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bausch SB. Axonal sprouting of GABAergic interneurons in temporal lobe epilepsy. Epilepsy Behav. 2005;7:390–400. doi: 10.1016/j.yebeh.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 5.Maglóczky Z, Freund TF. Impaired and repaired inhibitory circuits in the epileptic human hippocampus. Trends Neurosci. 2005;28:334–40. doi: 10.1016/j.tins.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Cross DJ, Cavazos JE. Synaptic reorganization in subiculum and CA3 after early-life status epilepticus in the kainic acid rat model. Epilepsy Res. 2007;73:156–65. doi: 10.1016/j.eplepsyres.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavazos JE, Jones SM, Cross DJ. Sprouting and synaptic reorganization in the subiculum and CA1 region of the hippocampus in acute and chronic models of partial-onset epilepsy. Neuroscience. 2004;126:677–88. doi: 10.1016/j.neuroscience.2004.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maglóczky Z. Sprouting in human temporal lobe epilepsy: excitatory pathways and axons of interneurons. Epilepsy Res. 2010;89:52–9. doi: 10.1016/j.eplepsyres.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Zappone CA, Sloviter RS. Translamellar disinhibition in the rat hippocampal dentate gyrus after seizure-induced degeneration of vulnerable hilar neurons. J Neurosci. 2004;24:853–64. doi: 10.1523/JNEUROSCI.1619-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long L, Xiao B, Feng L, Yi F, Li G, Li S, Mutasem MA, Chen S, Bi F, Li Y. Selective loss and axonal sprouting of GABAergic interneurons in the sclerotic hippocampus induced by LiCl-pilocarpine. Int J Neurosci. 2011;121:69–85. doi: 10.3109/00207454.2010.530007. [DOI] [PubMed] [Google Scholar]
- 11.Köbbert C, Apps R, Bechmann I, Lanciego JL, Mey J, Thanos S. Current concepts in neuroanatomical tracing. Prog Neurobiol. 2000;62:327–51. doi: 10.1016/s0301-0082(00)00019-8. [DOI] [PubMed] [Google Scholar]
- 12.Shozo J, Toshio K. Immunocytochemical characterization of hippocamposeptal projecting GABAergic nonprincipal neurons in the mouse brain: a retrograde labeling study. Brain Res. 2002;945:219–31. doi: 10.1016/s0006-8993(02)02804-4. [DOI] [PubMed] [Google Scholar]
- 13.Zappone C, Sloviter RS. Commissurally projecting inhibitory interneurons of the rat hippo campal dentate gyrus: a colocalization study of neuronal markers and the retrograde tracer fluoro-gold. J Comp Neurol. 2001;441:324–44. doi: 10.1002/cne.1415. [DOI] [PubMed] [Google Scholar]
- 14.Klitgaard H, Matagne A, Vanneste-Goemaere J, Margineanu DG. Pilocarpine-induced epileptogenesis in the rat: impact of initial duration of status epilepticus on electrophysiological and neuropathological alterations. Epilepsy Res. 2002;51:93–107. doi: 10.1016/s0920-1211(02)00099-2. [DOI] [PubMed] [Google Scholar]
- 15.Proper EA, Jansen GH, van Veelen CW, van Rijen PC, Gispen WH, de Graan PN. A grading system for hippocampal sclerosis based on the degree of hippocampal mossy fiber sprouting. Acta Neuropathol. 2001;101:405–9. doi: 10.1007/s004010000301. [DOI] [PubMed] [Google Scholar]
- 16.Loscher W. Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Res. 2002;50:105–23. doi: 10.1016/s0920-1211(02)00073-6. [DOI] [PubMed] [Google Scholar]
- 17.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–94. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 18.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. Amsterdam: Elsevier/Academic Press; 2007. [Google Scholar]
- 19.Frotscher M, Jonas P, Sloviter RS. Synapses formed by normal and abnormal hippocampal mossy fibers. Cell Tissue Res. 2006;326:361–7. doi: 10.1007/s00441-006-0269-2. [DOI] [PubMed] [Google Scholar]
- 20.Christian EP, Dudek FE. Characteristics of local excitatory circuits studied with glutamate microapplication in the CA3 area of rat hippocampal slices. J Neurophysiol. 1988;59:90–109. doi: 10.1152/jn.1988.59.1.90. [DOI] [PubMed] [Google Scholar]
- 21.Christian EP, Dudek FE. Electrophysiological evidence from glutamate microapplications for local excitatory circuits in the CA1 area of rat hippocampal slices. J Neurophysiol. 1988;59:110–23. doi: 10.1152/jn.1988.59.1.110. [DOI] [PubMed] [Google Scholar]
- 22.Deuchars J, Thomson AM. CA1 pyramid-pyramid connections in rat hippocampus in vitro: dual intracellular recordings with biocytin filling. Neuroscience. 1996;74:1009–18. doi: 10.1016/0306-4522(96)00251-5. [DOI] [PubMed] [Google Scholar]
- 23.Wheal HV, Chen Y, Mitchell J, Schachner M, Maerz W, Wieland H, Van Rossum D, Kirsch J. Molecular mechanisms that underliestructural and functional changes at the postsynaptic membrane during synaptic plasticity. Prog Neurobiol. 1998;55:611–40. doi: 10.1016/s0301-0082(98)00026-4. [DOI] [PubMed] [Google Scholar]
- 24.Lehmann TN, Gabriel S, Kovacs R, Eilers A, Kivi A, Schulze K, Lanksch WR, Meencke HJ, Heinemann U. Alterations of neuronal connectivity in area CA1 of hippocampal slices from temporal lobe epilepsy patients and from pilocarpine-treated epileptic rats. Epilepsia. 2000;41:S190–4. doi: 10.1111/j.1528-1157.2000.tb01580.x. [DOI] [PubMed] [Google Scholar]
- 25.Berger TW, Swanson GW, Milner TA, Lynch GS, Thompson RF. Reciprocal anatomical connections between hippocampus and subiculum in the rabbit evidence for subicular innervation of regio superior. Brain Res. 1980;183:265–76. doi: 10.1016/0006-8993(80)90463-1. [DOI] [PubMed] [Google Scholar]
- 26.Köhler C. Intrinsic projections of the retrohippocampal region in the rat brain. I. The subicular complex. J Comp Neurol. 1985;236:504–22. doi: 10.1002/cne.902360407. [DOI] [PubMed] [Google Scholar]
- 27.Lehmann TN, Gabriel S, Eilers A, Njunting M, Kovacs R, Schulze K, Lanksch WR, Heinemann U. Fluorescent tracer in pilocarpine-treated rats shows widespread aberrant hippocampal neuronal connectivity. Eur J Neurosci. 2001;14:83–95. doi: 10.1046/j.0953-816x.2001.01632.x. [DOI] [PubMed] [Google Scholar]
- 28.Patton PE, McNaughton B. Connection matrix of the hippocampal formation. I. The dentate gyrus. Hippocampus. 1995;5:245–86. doi: 10.1002/hipo.450050402. [DOI] [PubMed] [Google Scholar]
- 29.Swanson LW, Sawchenko PE, Cowan WM. Evidence for collateral projections by neurons in Ammon’s horn, the dentate gyrus, and the subiculum: a multiple retrograde labeling study in the rat. J Neurosci. 1981;1:548–59. doi: 10.1523/JNEUROSCI.01-05-00548.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmued LC, Kyriakidis K, Fallon JH, Ribak CE. Neurons containing retrogradely transported Fluoro-Gold exhibit a variety of lysosomal profiles: a combined brightfield, fluorescence, and electron microscopic study. J Neurocytol. 1989;18:333–43. doi: 10.1007/BF01190836. [DOI] [PubMed] [Google Scholar]
- 31.Deller T, Nitsch R, Frotscher M. Heterogeneity of the commissural projection to the rat dentate gyrus: a Phaseolus vulgaris leucoagglutinin tracing study. Neuroscience. 1996;75:111–21. doi: 10.1016/0306-4522(96)00255-2. [DOI] [PubMed] [Google Scholar]
- 32.Houser CR, Esclapez M. Vulnerability and plasticity of the GABA system in the pilocarpine model of spontaneous recurrent seizures. Epilepsy Res. 1996;26:207–18. doi: 10.1016/s0920-1211(96)00054-x. [DOI] [PubMed] [Google Scholar]
- 33.Wittner L, Eross L, Szabó Z, Tóth S, Czirják S, Halász P, Freund TF, Maglóczky ZS. Synaptic reorganization of calbindin-positive neurons in the human hippocampal CA1 region in temporal lobe epilepsy. Neuroscience. 2002;115:961–78. doi: 10.1016/s0306-4522(02)00264-6. [DOI] [PubMed] [Google Scholar]
- 34.Cossart R, Dinocourt C, Hirsch JC, Merchan-Perez A, De Felipe J, Ben-Ari Y, Esclapez M, Bernard C. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat Neurosci. 2001;4:52–62. doi: 10.1038/82900. [DOI] [PubMed] [Google Scholar]
- 35.Miles R, Tóth K, Gulyás AI, Hájos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16:815–23. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- 36.Traub RD, Jefferys JG, Whittington MA. Functionally relevant and functionally disruptive (epileptic) synchronized oscillations in brain slices. Adv Neurol. 1999;79:709–24. [PubMed] [Google Scholar]
- 37.Mann EO, Paulsen O. Role of GABAergic inhibition in hippocampal network oscillations. Trends Neurosci. 2007;30:343–9. doi: 10.1016/j.tins.2007.05.003. [DOI] [PubMed] [Google Scholar]


