Abstract
Objectives: This study aimed to investigate the magnetic resonance imaging (MRI) characteristics and clinical and MRI follow-up findings of patients with neurological complications of enterovirus 71-related hand, foot and mouth disease. Methods: Data were collected from 12 patients who developed neurological complications of enterovirus 71-related hand, foot, and mouth disease during an enterovirus-71 outbreak in Hainan Province, China, from May 2008 to October 2011. Patients were followed up for 2 years. Results: In the six patients with brainstem encephalitis, MRI showed posterior brainstem abnormalities with hyperintense areas on T2-weighted images and hypointense areas on T1-weighted images. In the four patients with acute flaccid paralysis but no brainstem encephalitis, sagittal MRI images showed linear hyperintense areas in the anterior spinal cord, transverse T2-weighted images showed hyperintense areas in the spinal cord, and contrast-enhanced axial T1-weighted images showed strong enhancement of the anterior horns or nerve roots. In the two patients with aseptic meningitis, MRI showed widening of the subarachnoid space and ventricles. The MRI and clinical signs of aseptic meningitis resolved within 4 weeks in both patients. Patients with isolated pontine abnormalities recovered faster than those with multiple brainstem abnormalities, patients with isolated brainstem encephalitis recovered faster than those with associated acute flaccid paralysis, patients with paralysis of one limb recovered faster than those with paralysis of multiple limbs, and patients with isolated thoracolumbar cord abnormalities recovered faster than those with cervical cord abnormalities. Conclusions: MRI is useful for assessment of the neurological complications of enterovirus 71-related hand, foot, and mouth disease. Patients who develop neurological complications characteristically have MRI abnormalities of the posterior brainstem or bilateral anterior horns of parts of the spinal cord. The MRI findings can help to predict prognosis.
Keywords: EV71 infection, hand, foot and mouth disease, MRI, neurological complications
Introduction
Hand, foot, and mouth disease (HFMD) is a common infectious disease caused by various enteroviruses, which was classified as a class C infectious disease by the Chinese Department of Health in May 2008. A high proportion of cases of severe HFMD are caused by enterovirus 71 (EV71) infection. Brainstem encephalitis, aseptic meningitis, acute flaccid paralysis (AFP), and various other neurological complications may occur, with rapid progression and high morbidity and mortality. A total of 1236 patients were treated for HFMD at our hospital from May 2008 to October 2011, of which 56 developed neurological complications. Thirty-eight of these 56 patients were diagnosed with EV71 infection by viral nucleic acid detection. The magnetic resonance imaging (MRI) findings of patients with EV71-related HFMD have been reported in previous studies, including studies by the present authors [1-7]. However, few studies have reported follow-up clinical and MRI findings in such patients. This study retrospectively analyzed MRI findings in 12 patients with neurological complications of EV71-related HFMD who underwent head and spinal cord MRI, and the follow-up findings over 2 years. Our findings may provide useful information regarding the pathological mechanisms underlying the neurological complications of EV71-related HFMD, and the MRI findings that can be used to predict prognosis.
Methods
Clinical data
HFMD was diagnosed according to the Chinese guidelines for the prevention and control of HFMD [8]. EV71 infection was diagnosed by the detection of EV71 nucleic acid in throat swab, stool, or cerebrospinal fluid samples tested by the Hainan Provincial Center for Disease Prevention and Control. The diagnostic criteria for HFMD-related encephalitis, AFP, and aseptic meningitis were described in our previous reports [1,2].
All 12 patients included in this study had a definitive diagnosis of HFMD and were treated in the Department of Pediatrics at our hospital from May 2008 to October 2011, during the HFMD outbreak in Hainan, China. Six (50%) of the 12 patients developed brainstem encephalitis (including three who also developed AFP), 4 (33%) developed AFP without brainstem encephalitis, and two (17%) developed aseptic meningitis.
The clinical details of the patients were recorded by doctors in the Departments of Pediatrics and Neurology. Routine blood testing, cerebrospinal fluid analysis, and testing for EV71 nucleic acid were performed after hospital admission. Surviving patients underwent clinical and MRI follow-up at 4 weeks and 2 years after the start of treatment.
MRI scanning
MRI scans were performed using a GE Sigma 1.5 T TwinSpeed/Excite II superconducting scanner with an 8-channel standard head coil and a 4-channel spinal cord coil (GE Medical Systems, Milwaukee, WI, USA). The following scan parameters were used: TR 1800 ms, TE 24 ms, and TI 750 ms for T1-weighted fluid-attenuated inversion recovery sequences; TR 3600 ms and TE 102 ms for T2-weighted fast spin-echo sequences; matrix 128 × 128, FOV 24 cm × 24 cm, single excitation, 6 mm transverse slice thickness, and 2 mm gap. Three patients received intravenous injection of gadolinium-DTPA complex (0.1 mmol/kg) for contrast-enhanced T1-weighted imaging.
Results
Clinical findings during the prodromal and peak stages
All 12 patients developed fever, with a maximum body temperature of 37.0-38.0°C in two patients, 38.1-39.0°C in six patients, and >39.0°C in four patients. The clinical course included development of a rash and/or vesicular mouth lesions, with a variable time course relative to the fever, in 12 (100%) of the patients; sore throat, runny nose, cough, other respiratory symptoms, and/or diarrhea during the prodromal phase in 2 (17%); headache and vomiting in 3 (25%); decreased level of consciousness in 2 (17%); oculomotor dysfunction and/or bulbar palsy in 3 (25%); single or multiple limb weakness in 8 (67%); limb tremor in 1 (8%); myoclonus in 1 (8%); ataxia in 1 (8%); facial paralysis in 2 (17%); loss of the swallowing reflex in 1 (8%); tendon areflexia and reduced muscle tone in 5 (42%); tendon hyperreflexia in 2 (17%); increased muscle tone in 1 (8%); positive Babinski’s sign in 1 (8%); and positive Kernig’s sign in 3 (25%) (Table 1).
Table 1.
Clinical and imaging characteristics of 12 patients with neurological complications of EV71-related HFMD
| Case No. | Sex | Age (months) | Clinical manifestations during the prodromal and peak stages | EV71 nucleic acid- positive sample | Brain MRI findings | Spinal cord MRI findings |
|---|---|---|---|---|---|---|
| Brainstem encephalitis | ||||||
| 1 | Female | 6 | Fever, rash, decreased level of consciousness, grade 0 muscle strength in all limbs, myoclonus, tendon hyporeflexia in all limbs, hypotonia | Stool | Abnormalities in the posterior brainstem, bilateral widening of the frontoparietal subarachnoid space and ventricles | Contrast enhancement of the anterior roots in the whole spinal cord |
| 2 | Male | 7 | Fever, rash, oculomotor dysfunction, bulbar palsy, grade 1 muscle strength in both upper limbs, grade 2 muscle strength in both lower limbs, tendon hyporeflexia in all limbs, hypotonia | Throat swab | Abnormalities in the medulla oblongata and posterior pons | Partial contrast enhancement of the anterior roots and anterior horns of the whole spinal cord |
| 3 | Male | 22 | Fever, rash, grade 2 muscle strength in both upper limbs, ataxia, tendon hyperreflexia in both lower limbs | Throat swab | Abnormalities in the medulla oblongata and posterior pons | Bilateral abnormalities at C1-C4 |
| 4 | Male | 9 | Vesicular lesions of the mouth, fever, headache, vomiting, facial paralysis, tremor, positive Kernig’s sign | Cerebrospinal fluid | Patchy areas of mildly hyperintense signals in the posterior pons on T2-weighted images | Normal |
| 5 | Female | 12 | Fever, rash, vomiting, decreased level of consciousness, bulbar palsy, absence of swallowing reflex | Throat swab | Abnormalities in the medulla oblongata and posterior pons | Normal |
| 6 | Male | 18 | Fever, rash, headache, vomiting, oculomotor dysfunction, bulbar palsy, facial paralysis, weakness of all limbs, hypertonia of all limbs, tendon hyperreflexia in all limbs, positive Babinski’s sign | Throat swab | Patchy abnormalities in the posterior brainstem | Normal |
| Aseptic meningitis | ||||||
| 7 | Female | 14 | Fever, rash, headache, vomiting, positive Kernig’s sign | Stool | Bilateral widening of the frontoparietal subarachnoid space and ventricles | Normal |
| 8 | Female | 37 | Fever, rash, sore throat, cough, headache, vomiting, positive Kernig’s sign | Throat swab | Bilateral widening of the frontoparietal subarachnoid space | Normal |
| Acute flaccid paralysis | ||||||
| 9 | Male | 12 | Fever, rash, vesicular lesions of the mouth, diarrhea, grade 2 muscle strength in both lower limbs, tendon hyporeflexia in both lower limbs, hypotonia | Cerebrospinal fluid | Normal | Bilateral abnormalities at T10-L1 |
| 10 | Male | 12 | Fever, vesicular lesions of the mouth, grade 1-2 muscle strength in both lower limbs, tendon hyporeflexia in both lower limbs, hypotonia | Stool | Normal | Bilateral abnormalities at T9-L1, bilateral contrast enhancement of the anterior roots |
| e11 | Male | 24 | Fever, rash, grade 0 muscle strength in the right upper limb, tendon areflexia in the right upper limb, hypotonia | Throat swab | Normal | Bilateral abnormalities at C4-C7 |
| 12 | Female | 19 | Fever, rash, grade 1 muscle strength in the left lower limb, tendon areflexia in the left lower limb, hypotonia | Cerebrospinal fluid | Normal | Left-sided abnormalities at T9-L1 |
Among the seven patients with AFP (including three who also had brainstem encephalitis), the right arm, left arm, right leg, and left leg were affected with similar frequency. Patients with AFP developed muscle weakness and tendon hyporeflexia or areflexia but no sensory dysfunction. Patients with brainstem encephalitis were severely ill, resulting in some cases in decreased level of consciousness, cranial nerve dysfunction, and other neurological abnormalities.
Laboratory findings
Five patients had a normal initial white blood cell count, of which three had an increased lymphocyte ratio. Seven patients had an increased white blood cell count (range 10.0-19.5 × 10<9/L), of which three had an increased granulocyte ratio (0.75-0.85) and four had an increased lymphocyte ratio. Cerebrospinal fluid analysis showed a cell count ranging from 10 × 106/L to 500 × 106/L with increased numbers of monocytes in all patients, abnormal protein levels in three patients, and normal glucose and chloride levels.
MRI findings
MRI showed posterior brainstem abnormalities in all six patients with brainstem encephalitis, with unilateral or bilateral patchy hypointense areas on T1-weighted images and slightly hyperintense areas on T2-weighted images. Typical cases are shown in Figures 1 and 2. These six patients all had MRI abnormalities in the pons, five had abnormalities in the medulla oblongata, and two had abnormalities in the midbrain. One of these patients also had bilateral widening of the frontoparietal subarachnoid space and ventricles (Table 1).
Figure 1.
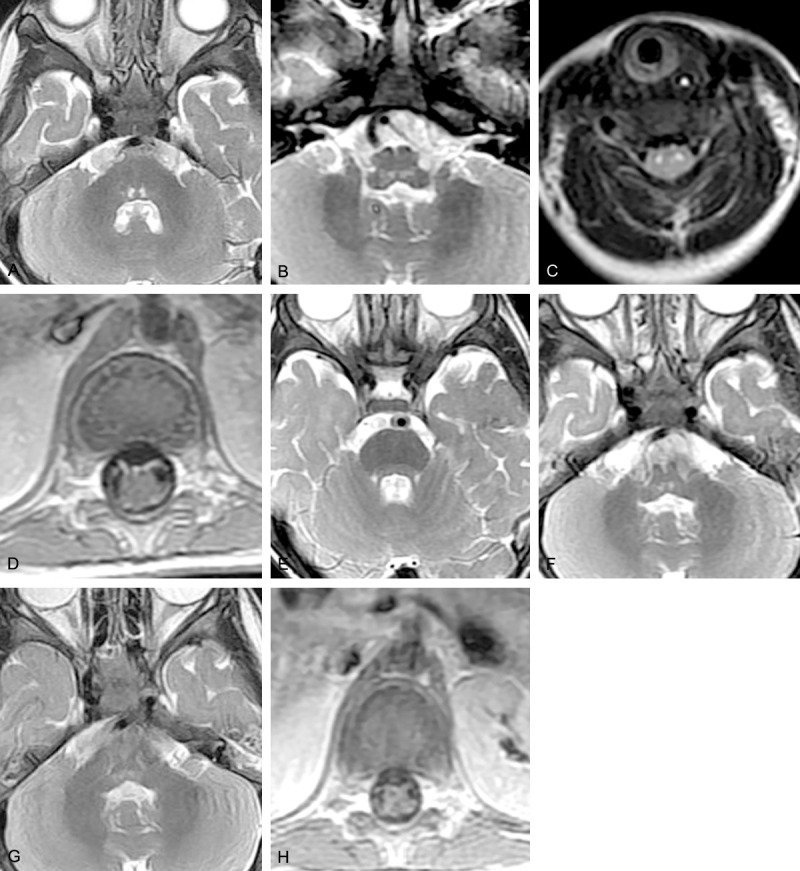
Brain MRI of Case 2. Before treatment: (A) T2-weighted image showing bilateral hyperintense signals in the posterior pons, (B) T2-weighted image showing bilateral hyperintense signals in the posterior medulla oblongata, (C) T2-weighted image showing bilateral hyperintense signals in the anterior roots of the spinal cord, (D) contrast-enhanced image showing enhancement of the bilateral anterior nerve roots. Repeat MRI after 4 weeks: (E) T2-weighted image showing absence of hyperintense signals in the posterior pons, (F) decreased size of the lesion in the medulla oblongata. Repeat MRI after 1 year: (G) partial resolution of the lesion in the medulla oblongata, (H) contrast-enhanced image showing absence of nerve root enhancement.
Figure 2.
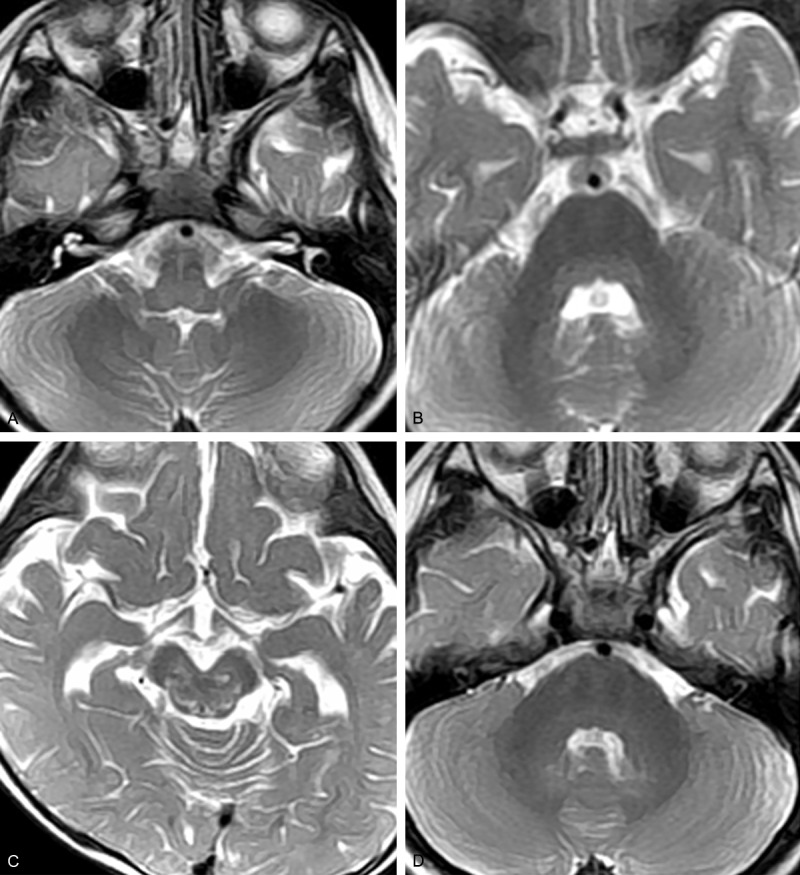
Brain MRI of Case 6. Before treatment: (A) T2-weighted image showing bilateral hyperintense signals in the posterior medulla oblongata, (B) T2-weighted image showing mildly hyperintense signals in the posterior pons, (C) T2-weighted image showing patchy hyperintense signals in the red nucleus, substantia nigra, oculomotor region, and trochlear nerve nucleus. (D) Repeat MRI after 4 weeks showing disappearance of the pontine lesion.
In the four patients with AFP but no brainstem encephalitis, sagittal spinal cord images showed hypointense areas on T1-weighted images and hyperintense areas on T2-weighted images in various parts of the cervical cord and conus medullaris, and cross-sectional images showed hypointense areas on T1-weighted images and hyperintense areas on T2-weighted images in the unilateral or bilateral anterior horns, mostly in the cervical cord and from T9 to L1. Two of the three patients with both brainstem encephalitis and AFP had abnormalities of the entire length of the spinal cord, and the other one had bilateral cervical cord abnormalities only. In one patient, contrast-enhanced MRI showed significant enhancement of the involved anterior roots (Figure 1D).
In the two patients with aseptic meningitis, MRI showed bilateral frontoparietal widening of the subarachnoid space, and one of these patients also had widening of the ventricles.
Follow-up
One patient with both brainstem encephalitis and AFP (Case 1) died from severe neurogenic pulmonary edema and multiple organ failure. The remaining 11 patients were followed up 4 weeks after the time of admission. The two patients with aseptic meningitis (Cases 7 and 8) had normal clinical and MRI findings at this time. The patients with brainstem encephalitis still had varying degrees of neurological dysfunction, including oculomotor palsy and facial paralysis. The MRI findings had improved in these patients (Figures 1 and 2). The patients with AFP still had varying degrees of muscle weakness (Table 2).
Table 2.
Clinical and MRI follow-up findings of 12 patients with neurological complications of EV71-associated HFMD
| Case no. | Follow-up after 4 months | Follow-up after 2 years | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Clinical findings | Brain MRI | Spinal cord MRI | Clinical findings | Brain MRI | Spinal cord MRI | |
| Brainstem encephalitis | ||||||
| 1 | N/A (dead) | N/A | N/A | N/A | N/A | N/A |
| 2 | Mild bulbar palsy, grade 3 muscle strength in both upper limbs, grade 4 muscle strength in both lower limbs, tendon hyperreflexia, hypertonia | Mildly abnormal signal in the medulla oblongata and posterior pons | Mildly abnormal signal in the whole length of the spinal cord | Mild right-sided facial weakness, slightly high-arched feet | Remaining medullary abnormalities | Mild abnormalities in the thoracolumbar cord |
| 3 | Grade 3 muscle strength in both upper limbs, tendon hyperreflexia in both lower limbs | The abnormal signals in the medulla oblongata and posterior pons had almost disappeared | Bilateral hypointense signal at C1-C4 | Mild limb weakness | Normal | Normal |
| 4 | Mild facial paralysis | The patchy mildly hyperintense signals on T2-weighted images in the posterior pons had almost disappeared | N/A | Normal | Normal | N/A |
| 5 | Mild bulbar palsy | The abnormal signals in the medulla oblongata and posterior pons had decreased | N/A | Normal | Normal | N/A |
| 6 | Mild oculomotor dysfunction, normal muscle strength in all limbs, hypertonia in all limbs, tendon hyperreflexia | The patchy signal in the posterior brainstem had almost disappeared | N/A | Normal | Normal | N/A |
| Aseptic meningitis | ||||||
| 7 | Normal | Normal | N/A | N/A | N/A | N/A |
| 8 | Normal | Normal | N/A | N/A | N/A | N/A |
| Acute flaccid paralysis | ||||||
| 9 | Grade 4 muscle strength in both lower limbs, normal tendon reflexes, hypertonia | N/A | Bilateral hypointense signal at T10-L1 | Normal | N/A | Normal |
| 10 | Grade 4 muscle strength in both lower limbs, tendon hyporeflexia in both lower limbs, hypertonia | N/A | Bilateral hypointense signal at T9-L1, the bilateral enhancement of the anterior roots had disappeared | Normal | N/A | Normal |
| 11 | Grade 2 muscle strength in the right upper limb, tendon hyporeflexia in the right upper limb, hypertonia | N/A | Bilateral mildly abnormal signal at C4-C7 | Mild upper limb weakness | N/A | Mild abnormalities in the cervical cord |
| 12 | Grade 4 muscle strength in the left lower limb | N/A | Left-sided mildly abnormal signal at T9-L1 | Normal | N/A | Normal |
At the 2-year follow-up, the three surviving patients with brainstem encephalitis without AFP (Cases 4, 5, and 6) had normal clinical and MRI findings. One of the two patients with both brainstem encephalitis and AFP (Case 2) had mild right-sided facial paralysis and slightly high-arched feet, and MRI showed remaining medullary abnormalities; and the other patient (Case 3) had mild limb weakness. One of the four patients with AFP without brainstem encephalitis (Case 11) had mild upper limb weakness, and MRI showed mild abnormalities of the cervical cord; and the other three patients (Cases 9, 10, and 12) had normal clinical and MRI findings (Table 2).
Among patients with brainstem encephalitis, those with isolated pontine abnormalities recovered faster than those with multiple brainstem abnormalities, and those with isolated brainstem encephalitis recovered faster than those with both brainstem encephalitis and AFP. Among patients with AFP (with or without brainstem encephalitis), those with paralysis of one limb recovered faster than those with paralysis of multiple limbs, and those with isolated thoracolumbar cord abnormalities recovered faster than those with cervical cord abnormalities.
Discussion
The mechanisms underlying the neurological complications of EV71-related HFMD are not fully understood. Oral EV71 infection can cause persistent viremia with increased permeability of the blood-brain barrier. However, the viral load is lower in brain tissue than in other parts of the body, suggesting that hematogenous spread is not the main cause of central nervous system damage [9,10]. Seven-day-old rats that were infected with EV71 and developed hindlimb paralysis subsequently died after 5-9 days [11]. Histopathological examination of the rat specimens showed damage to various parts of the central nervous system including the spinal cord, brainstem, and thalamus. Degenerate neurons were observed in the anterior horns of the spinal cord, with neuronal loss and apoptosis, neuronophagia, vascular congestion, and dense perivascular infiltration of mononuclear leucocytes and neutrophils. Nissl’s staining showed a 20-30% reduction of neuronal cell bodies. Terminal dUTP nick end labeling (TUNEL) positive cells were observed in the gray matter of the spinal cord, and cavernous transformation was observed in the white matter adjacent to the involved gray matter. These results suggest that EV71 can cause direct damage to central nervous system cells [12].
The patients in this study had typical MRI findings of AFP, brainstem encephalitis, and aseptic meningitis. The anterior horns of the affected parts of the spinal cord had hypointense areas on T1-weighted images and hyperintense areas on T2-weighted images. The spinal cord abnormalities were mainly in the cervical region and from T9 to the conus medullaris. Contrast-enhanced MRI showed partial enhancement of the anterior roots and anterior horns. Our findings differ from previously reported findings in that two patients (Cases 1 and 2) with both brainstem encephalitis and AFP had MRI abnormalities of the whole length of the spinal cord [13]. These patients developed paralysis of all four limbs. There were no cases of posterior root or posterior horn MRI abnormalities, or of sensory impairment, suggesting infection with an EV71 variant with affinity for specific neural cell types.
In patients with brainstem encephalitis, MRI showed abnormalities in the posterior brainstem with unilateral or bilateral hypointense areas on T1-weighted images and hyperintense areas on T2-weighted images [14]. These areas are mostly composed of gray nuclei, but it is unclear why these parts of the brain were the most affected by EV71 infection. The findings differed from the MRI findings in other types of infection-related encephalopathy, and to our knowledge such changes have not previously been reported. We attributed the variable MRI findings to different stages or severity of disease, but sequential scans would be needed to confirm this. Aseptic meningitis is not associated with specific MRI abnormalities, but meningeal enhancement, widening of the subarachnoid space, and hydrocephalus may be observed.
In our patients with neurological complications of HFMD, the MRI findings were consistent with the clinical manifestations. Limb paralysis was always associated with abnormalities of the corresponding anterior horns or anterior roots of the spinal cord. All seven patients with AFP had abnormalities of the corresponding areas of the spinal cord, except for one who had bilateral abnormalities at C4-C7. This exception suggests a relationship between the strength of the abnormal signal and the clinical manifestations.
When patients with HFMD develop symptoms suggestive of lesions in the region of the posterior cerebral circulation, such as tremor, myoclonus, and cranial nerve dysfunction (particularly of the abducens and facial nerves), brainstem lesions should be considered. Huang et al. considered that the pontine tegmentum is the area most frequently involved in patients with neurological complications of HFMD [4]. The initial symptoms of neurological involvement include myoclonus, tremor, and ataxia. If the abnormalities extend to the head and tail of the hippocampus, the symptoms may be more severe. All six patients in this study with brainstem encephalitis had pontine abnormalities on MRI, which is consistent with the pons being more sensitive to EV71 infection than other parts of the brain. However, only three patients (Cases 1, 3, and 4) developed myoclonus, tremor, or ataxia, in contrast to the findings reported by Huang et al [4].
One of the patients in this study with brainstem encephalitis died, and the other patients recovered, indicating that EV71-related AFP has a better prognosis than poliomyelitis. Review of the MRI findings showed that patients with unilateral thoracolumbar cord abnormalities on MRI recovered faster than those with cervical cord abnormalities or abnormalities of the whole length of the spinal cord, and those patients with abnormalities of the spinal cord only recovered faster than those with both brainstem and spinal cord abnormalities.
Follow-up of the patients with brainstem encephalitis showed that those with isolated pontine abnormalities recovered faster than those with multiple brainstem abnormalities. Patients with brainstem encephalitis without AFP recovered faster than those with both brainstem encephalitis and AFP.
The results of this study show that MRI is a sensitive method of evaluating EV71-related central nervous system damage, and can help to evaluate the extent of pathological changes. MRI also plays a very important role in analyzing symptoms and predicting the prognosis.
Acknowledgements
This study was supported by a grant from the Hainan Natural Science Foundation 812149 & 813201, Hainan Health Institution Project 2012PT-06, Hainan Social Development Fundation SF201312, the National key clinical specialist construction Programs of China.
References
- 1.Chen F, Li J, Liu T, Wang L, Li Y. MRI characteristics of brainstem encephalitis in hand-foot-mouth disease induced by enterovirus type 71--will different MRI manifestations be helpful for prognosis? Eur J Paediatr Neurol. 2013;17:486–91. doi: 10.1016/j.ejpn.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Chen F, Liu T, Wang L. MRI findings of neurological complications in hand-foot-mouth disease by enterovirus 71 infection. Int J Neurosci. 2012;122:338–44. doi: 10.3109/00207454.2012.657379. [DOI] [PubMed] [Google Scholar]
- 3.Shen WC, Chiu HH, Chow KC, Tsai CH. MR imaging findings of enteroviral encephaloymelitis: an outbreak in Taiwan. AJNR Am J Neuroradiol. 1999;20:1889–95. [PMC free article] [PubMed] [Google Scholar]
- 4.Huang CC, Liu CC, Chang YC, Chen CY, Wang ST, Yeh TF. Neurologic complications in children with enterovirus 71 infection. New Engl J Med. 1999;341:936–42. doi: 10.1056/NEJM199909233411302. [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Peng Y, Duan XM, Wang Xu, Zeng JJ, Sun GQ. MRI findings of spine: acute flaccid paralysis associated with enterovirus 71 infected hand-foot-mouth disease. Zhonghua Fang She Xue Za Zhi. 2008;42:1237–40. [Google Scholar]
- 6.Liu K, Ma YX, Zhang CB, Chen YP, Ye XJ, Bai GH, Yu ZK, Yan ZH. Neurologic complications in children with enterovirus 71-infected hand-foot-mouth disease: clinical features, MRI findings and follow-up study. Zhonghua Yi Xue Za Zhi. 2012;92:1742–6. [PubMed] [Google Scholar]
- 7.Jang S, Suh SI, Ha SM, Byeon JH, Eun BL, Lee YH, Seo HS, Eun SH, Seol HY. Enterovirus 71-related encephalomyelitis: usual and unusual magnetic resonance imaging findings. Neuroradiology. 2012;54:239–45. doi: 10.1007/s00234-011-0921-8. [DOI] [PubMed] [Google Scholar]
- 8.Ministry of Health of the People’s Republic of China. Guidelines for the diagnosis and treatment of hand, foot and mouth disease (2008 edition) Available at: http://www.chinacdc.net.cn/n272442/n272530/n275462/275477/n292888/23509.html. Accessed May 20, 2008. Chinese.
- 9.Shieh WJ, Jung SM, Hsueh C, Kuo TT, Mounts A, Parashar U, Yang CF, Guarner J, Ksiazek TG, Dawson J, Goldsmith C, Chang GJ, Oberste SM, Pallansch MA, Anderson LJ, Zaki SR. Pathologic studies of fatal cases in outbreak of hand, foot, and mouth disease, Taiwan. Emerg Infect Dis. 2001;7:146–8. doi: 10.3201/eid0701.700146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y, Wang H, Gong E, Du J, Zhao X, McNutt MA, Wang S, Zhong Y, Gao Z, Zheng J. Neuropathology in 2 cases of fatal enterovirus type 71 infection from a recent epidemic in the People’s Republic of China: a histopathologic, immunohistochemical, and reverse transcription polymerase chain reaction study. Hum Pathol. 2009;40:1288–95. doi: 10.1016/j.humpath.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Wang YF, Chou CT, Lei HY, Liu CC, Wang SM, Yan JJ, Su IJ, Wang JR, Yeh TM, Chen SH, Yu CK. A mouse-adapted enterovirus 71 strain causes neurological disease in mice after oral infection. J Virol. 2004;78:7916–24. doi: 10.1128/JVI.78.15.7916-7924.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong KT, Munisamy B, Ong KC, Kojima H, Noriyo N, Chua KB, Ong BB, Nagashima K. The distribution of inflammation and virus in human enterovirus 71 encephalomyelitis suggests possible viral spread by neural pathways. J Neuropathol Exp Neurol. 2008;67:162–9. doi: 10.1097/nen.0b013e318163a990. [DOI] [PubMed] [Google Scholar]
- 13.Peng BW, Du ZH, Li XJ, Lin HS, Liu HS, Chen WX, Mai JN, Liang HC. Evolution and prognosis of the acute flaccid paralysis associated with enterovirus 71 infection evaluated through a clinical and magnetic resonance imaging follow-up study. Zhonghua Er Ke Za Zhi. 2012;50:255–60. [PubMed] [Google Scholar]
- 14.Zeng H, Wen F, Gan Y, Huang W. MRI and associated clinical characteristics of EV71-induced brainstem encephalitis in children with hand-foot-mouth disease. Neuroradiology. 2012;54:623–30. doi: 10.1007/s00234-011-0979-3. [DOI] [PubMed] [Google Scholar]


