Abstract
In the present study, the aim was to reveal the relationship of serum IL-6, IL-10 and IL-13 levels in patient with Enterovirus 71 (EV71) infection. To elucidate the role of immune mechanisms in the pathogenesis of Hand, foot, and mouth disease (HFMD), we analyzed the cytokine of 112 EV71-infected patients. A significant elevation of serum (interleukin) IL-6, IL-10 and IL-13 levels in patients with EV71 infection compare with Un-EV71 infection HFMD patient and Healthy individuals. The production of inflammatory cytokines was increased with disease clinical stage. In addition, the immunological consequences of these cytokine in patient with EV71 infection showed a downward trend after cure. These data suggested that EV71 infection significantly increased the release of circulating IL-6, IL-10 and IL-13. The systemic inflammatory response may play an important role in the pathogenesis of HFMD. Moreover, this study may be designed to evaluate the potential therapeutic of medicine in the treatment of patients with EV71 infection.
Keywords: Enterovirus 71, neurologic pulmonary edema, Hand food mouth disease (HFMD), pro-inflammatory
Introduction
Hand, foot, and mouth disease (HFMD), a common infectious disease in young children (mostly under 5 years), typically characterized by mucocutaneous papulovesicular rashes on hands, feet, mouth, and buttocks, was first reported in New Zealand in 1957 [1]. Coxsackievirus A16 (CVA16) and human Enterovirus 71 (EV71) are the two major causative agents of HFMD [2]. CVA16-associated HFMD has a milder outcome with much lower incidence of severe complications [3]. In contrast, EV71-associated HFMD has a higher incidence of severe complications with a variety of neurological diseases, including aseptic meningitis, encephalitis, poliomyelitis-like paralysis, menigoencephalitis, myocarditis, and neonatal sepsis [4,5]. Systemic inflammatory response syndrome caused by virus infection is a typical consequence and persistent hypercytokinemia may result in progression to multiple organ failure.
The pathogenesis of HFMD has been studied all the time, with some breakthrough. There is increasing evidence that proinflammatory and anti-inflammatory cytokine may play a critical role in EV71 infection. Previous studies showed that EV71-infected mice exhibited a subtle and transient elevation of IL-6, IL-10 and INF-γ [6]. EV71 infection dramatically increased the release of IL-6, and anti-IL-6 treated mice showed reduced tissue damage, viral loads and B cells activation [7]. At the same time, anti-IL6 treatment significantly increased the IL-10/IL-6 ratios, suggesting that the release of IL-10 likely helped balance between proinflammatory and anti-inflammatory cytokines and clear the immune response to EV71. Patients with pulmonary edema caused by EV71 infection were found to have higher IL-13 levels than those with uncomplicated brain stem encephalitis. Furthermore, overproduction of IL-13 might contribute to the pathogenesis of pulmonary edema, because exogenous treatment of IL-6, IL-13 and INF-γ exacerbate pulmonary abnormality of EV71-infected mice [6]. The study was aimed at analyzing IL-6, IL-10 and IL-13 levels in children with Enterovirus 71 infection and their association with disease clinical stage.
Material and methods
Subjects
A total of 112 HFMD patients between years 2011 and 2013 in HFMD and ICU (Intensive Care Unit) departments of Dongguan People’s Hospital were included in this study. The diagnostic criteria of HFMD disease with EV71 infection according to file: “Hand, foot and mouth disease treatment guidelines” published by Health department of China in 2010 and stool test positive for EV71 virus. (http://www.moh.gov.cn/mohyzs/s3586/201004/46884.shtml). Patients were divi-ded into clinical stage II (Involvement of the nervous system) 30 cases, stage III (Early cardiopulmonary failure) 30 cases and stage IV (Cardiopulmonary failure) 22 cases (6 cases deaths) according to file: “The expert consensus of clinical treatment in severe Enterovirus 71 (EV71) infection disease” also published by Health department of China (http://www.moh.gov.cn/mohyzs/s3585/201105/51750.shtml). 30 un-EV71 infection HMFD patients and 30 healthy uninfected individuals serve as controls. The study was approved by the Internal Review and the Ethics Boards of Guangdong Medical College and Dongguan People’s Hospital, and informed consent was obtained from all study subjects.
Assessments
Serum samples of the subjects were collected at regular intervals. Briefly, three time points were settled for serum collection, the day of admission (named TP0), the day of disease improved (about 5~8 days, named TP1) and the day of disease recovered (about 10~14 days, named TP2). The criteria for delimiting the day of disease improved was spirit improved obviously, body temperature dropped below 38°C, nervous system involvement significantly alleviated, WBC counts and blood glucose level decreased. The criteria for delimiting the day of disease recovered was body temperature, WBC counts, blood glucose, nervous system involvement, and heart and lung function recovered to normal (http://www.moh.gov.cn/mohyzs/s3585/201105/51750.shtml). Levels of the IL-6, IL-10 and IL-13 were measured with ELISA kit (Ray Biotech, Inc.), respectively.
Statistical analysis
GraphPad Prism version 5.0 (GraphPad software, USA) was used for statistical analysis. For the comparison of normally distribution, Student’s t-test for independent samples or one-way analysis of variance was used. ANOVA LSD-t test was used for the comparison among groups. Chi-square test was used for non-parametric test. In addition, correlations between cytokines levels and clinical stage were examined using Spearman’s test Pearson correlation was used to measure the degree of dependency between variables. P < 0.05 was considered with statistical significance.
Results
One hundred-Twelve HFMD patients were enrolled in the study. All characteristics for patients and normal controls are summarized in Table 1. The patients were subdivided into 3 stages, as described above. The median age did not differ significantly among patients with EV71 infection. Among EV71 infection HFMD patients, the frequency of high fever (> 39°C) > 72 h at admission, depression, vomit, easily frightened, limb jitter, abnormal breathing, increased heart rate, blood pressure, capillary filling time (CRT) > 2 s, white blood cells (WBC) > 15 × 109/L and glutamate (GLU) > 8.3 mmol/L in stage IV patients were increased remarkably, seen in Table 1.
Table 1.
The clinical data of studied subjects
| Groups | EV71 infection HFMD patient | Un-EV71 infection HFMD patient (n = 30) | Healthy individuals (n = 30) | ||
|---|---|---|---|---|---|
|
| |||||
| Stage II (n = 30) | Stage III (n = 30) | Stage IV (n = 22) | |||
| Female/Male | 10/20 | 11/19 | 8/14 | 12/18 | 12/18 |
| Age (months) Mean ± SD | 24.73 ± 11.04 | 27.73 ± 11.93 | 25.91 ± 12.42 | 22.57 ± 10.89 | 26.90 ± 11.45 |
| High fever (> 39°C) > 72 h at admission, % | 60.00 | 63.33 | 95.45 | 16.67 | 0 |
| Depression, % | 26.6 | 43.33 | 77.27 | 3.33 | 0 |
| Vomit, % | 6.67 | 50.00 | 72.72 | 6.67 | 0 |
| Easily frightened, % | 13.33 | 66.67 | 81.81 | 0 | 0 |
| Limb jitter, % | 73.33 | 90.00 | 100 | 6.67 | 0 |
| Abnormal breathing, % | 13.33 | 13.33 | 45.45 | 0 | 0 |
| Increased heart rate, % | 31.3 | 43.33 | 86.36 | 0 | 0 |
| Blood pressure, % | 26.67 | 63.33 | 68.18 | 0 | 0 |
| CRT > 2 s,% | 3.33 | 56.67 | 63.64 | 0 | 0 |
| WBC > 15 × 109/L, % | 40.00 | 40.00 | 77.27 | 20.00 | 0 |
| GLU > 8.3 mmol/L, % | 53.33 | 56.67 | 77.27 | 13.33 | 0 |
Next, we measured serum IL-6, IL-10 and IL-13 in patients infected with EV71. Interestingly, the mean serum IL-6, IL-10 and IL-13 levels of EV71 infection patients were increased with the severity of the disease, and also higher than un-EV71 infection HFMD patients or healthy individuals groups respectively (Figure 1A). Especially, the serum IL-6, IL-10 and IL-13 levels of clinical stage IV EV71 infection HFMD patients were (149.94 ± 43.54) pg/ml, (97.95 ± 31.86) pg/ml and (50.80 ± 19.21) pg/ml respectively, correspondingly about two times higher than clinical stage II EV71 infection HFMD patients (Figure 1B).
Figure 1.
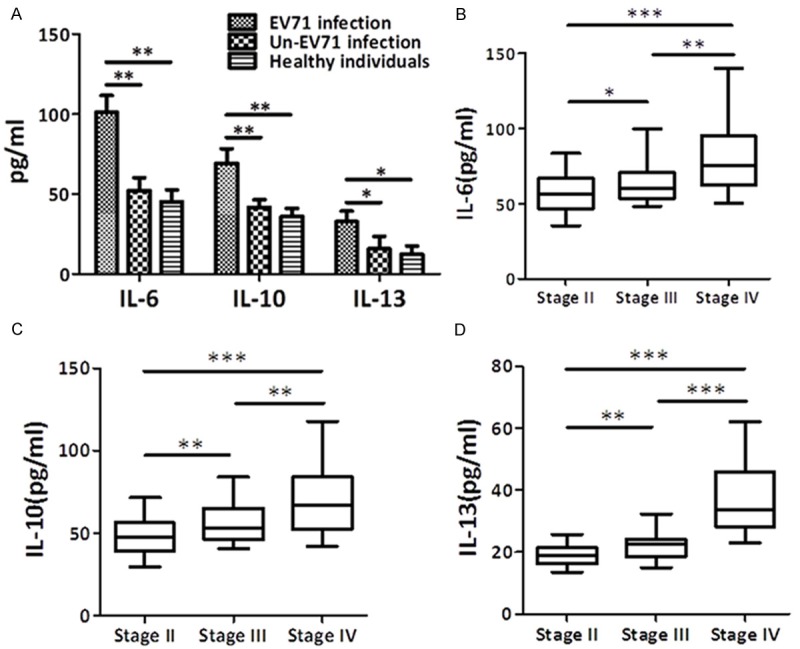
Serum IL-6, IL-10 and IL-13 levels of patients at the day of admission (Mean ± SD). (A) The mean serum IL-6, IL-10 and IL-13 levels of EV71 infection patients, un-EV71 infection HFMD patients and healthy individuals were summarized. (B-D) Means and 95% confidence interval of IL-6 (B), IL-10 (C) and IL-13 (D) change by different stage in untreated patients. P values are for different subtype. *p < 0.05, **p < 0.01.
In this study, our clinical data suggested that increases in serum IL-6, IL-10 and IL-13 were associated with disease clinical stage in human EV71 patients. No matter which clinical stage II, III or IV patients, these cytokines levels of EV71 infection HFMD patients were decreased after treatment. Especially in clinical stage IV patients, serum IL-6 levels were decreased from (149.94 ± 43.54) pg/ml to (74.15 ± 19.1) pg/ml, serum IL-10 levels were decreased from (97.95 ± 31.86) pg/ml to (53.48 ± 17.07) pg/ml, and serum IL-13 levels were decreased from (50.80 ± 19.21) pg/ml to (21.12 ± 11.27) pg/ml, after treatment at the day of disease recovered (Figure 2).
Figure 2.
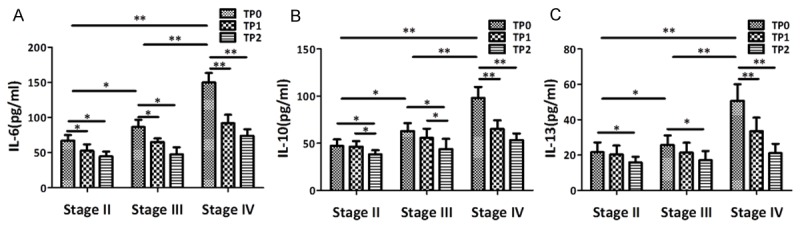
Serum IL-6, IL-10 and IL-13 levels of different clinical stage EV71 infection HFMD patients after treatment (Mean ± SD). The mean serum IL-6 (A), IL-10 (B) and IL-13 (C) levels of EV71 infection patients with different clinical stage in different time points (TP) of disease recovered. *p < 0.05. **p < 0.05.
Discussion
Human enterovirus type 71 (EV71) has emerged as a major cause of viral encephalitis and neurologic pulmonary edema (NPE), the two reasons of death in children with severe HFMD, in children worldwide [8]. The identified EV71 receptors provide a useful information for understanding EV71 replication and tissue infection. However, the serological characteristics of the EV71 infection on child remain unclear. This study examined IL-6, IL-10 and IL-13 levels in children with EV71 infection and their association with disease clinical stage. To the best of our knowledge, this study for the first time examined the relations between inflammatory cytokines and clinical stage of HFMD with EV71 infection.
IL-6, an important pro-inflammatory cytokines, is found low expression on healthy individuals’ blood, but high expression on patients with pathogens infection or inflammation. Several studies compared IL-6 levels between healthy individuals and HFMD patients with EV71 infection [9,10]. The study by Zhang et al., compared 99 patients with EV71 infection and 19 healthy individuals and found an elevated IL-6 levels may relate on HFMD patients with EV71 infection [9]. However, the study by Han et al., compared 91 severe or mild EV71 infection HFMD patients and found the elevated IL-6 levels was no correlated with immunoglobulin M levels by the end of the disease course [1]. While these previous studies provide fundamental and useful information on EV71-mediated inflammation, the field currently lacks a more comprehensive in EV71 infection HFMD patients with different clinical stage. Similarly, this study also found a high expression of IL-6 levels on EV71 infection HFMD patients, especially in clinical stage IV EV71 infection HFMD patients, when they compared with un-EV71 infection HFMD patients or healthy individuals. Actually, IL-6 is a marker of non-specific pathogens infection or inflammation. Thus, the rise of this marker levels only in a proportion of patients especially in severe patients, suggests that IL-6 may play role in EV71 infection HFMD patients.
IL-10, the most well-known endogenous inflammatory cytokine [11], have been found to play a double-edged role in the pathogenesis of pulmonary edema [12]. IL-10 inhibits antigen presentation to T cells, and suppresses phagocytosis, the oxidative burst, and intracellular killing. Here, we found markedly elevated IL-10 levels on EV71 infection HFMD patients, especially in clinical stage IV HFMD patients with EV71 infection. IL-10 can be modulated in several acute and chronic pathogens infection [13]. A close relationship between clinical stage and IL-10 release were existed in our study. This suggests that IL-10 plays a role in the immune regulatory functions of the EV71 infection. Thus, the IL-10 levels increase in patients with EV71 infection appears to be triggered by the virus. Up-regulated IL-10 levels may have a protective effect in the development of EV71 infection by influencing the neurologic pulmonary edema. The protective effects of IL-10 are mediated via inhibiting TNF-α, IL-1, IL-6, and IL-8 secretion [14].
IL-13, as with IL-10, is another cytokine produced by T cells that has potential anti-inflammatory activity and suppresses the cytotoxic functions of monocytes and macrophages [15,16]. An exaggerated production of IL-13, similarly to IL-6 or IL-10, was observed in our patients and usually peaked during the early phase of hospitalization, especially in clinical stage IV EV71 infection HFMD patients. IL-13 levels were consistently elevated in all our patients during the early phase of hospitalization and decreased after conventional therapy. We considered that overproduction of IL-13 might contribute to the pathogenesis of EV71 infection by increasing pulmonary vascular permeability and smooth muscle hyperplasia and cause airway hyperresponsiveness [17].
In conclusion, IL-6, IL-10 and IL-13 levels are affected by the EV71 infection in HFMD patients. And, this marker may have the potential to be used for the follow-up of treatment response, particularly in HFMD patients with EV71 infection.
Acknowledgements
This work was supported by the Science and Technology Key Project of Dongguan (2011105102009) and the Science and Technology Fund of Guangdong Medical College (XK1454).
Disclosure of conflict of interest
None.
References
- 1.Robinson CR, Doane FW, Rhodes AJ. Report of an outbreak of febrile illness with pharyngeal lesions and exanthem: Toronto, summer 1957; isolation of group A Coxsackie virus. Can Med Assoc J. 1958;79:615–621. [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu Z, Zhu S, Guo X, Wang J, Wang D, Yan D, Tan X, Tang L, Zhu H, Yang Z, Jiang X, Ji Y, Zhang Y, Xu W. Retrospective seroepidemiology indicated that human enterovirus 71 and coxsackievirus A16 circulated wildly in central and southern China before large-scale outbreaks from 2008. Virol J. 2010;7:300. doi: 10.1186/1743-422X-7-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang LY, Lin TY, Huang YC, Tsao KC, Shih SR, Kuo ML, Ning HC, Chung PW, Kang CM. Comparison of enterovirus 71 and coxsackie-virus A16 clinical illnesses during the Taiwan enterovirus epidemic, 1998. Pediatr Infect Dis J. 1999;18:1092–1096. doi: 10.1097/00006454-199912000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Chong CY, Chan KP, Shah VA, Ng WY, Lau G, Teo TE, Lai SH, Ling AE. Hand, foot and mouth disease in Singapore: a comparison of fatal and non-fatal cases. Acta Paediatr. 2003;92:1163–1169. [PubMed] [Google Scholar]
- 5.McMinn PC. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol Rev. 2002;26:91–107. doi: 10.1111/j.1574-6976.2002.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 6.Huang SW, Lee YP, Hung YT, Lin CH, Chuang JI, Lei HY, Su IJ, Yu CK. Exogenous interleukin-6, interleukin-13, and interferon-γ provoke pulmonary abnormality with mild edema in enterovirus 71-infected mice. Respir Res. 2011;12:147. doi: 10.1186/1465-9921-12-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khong WX, Foo DG, Trasti SL, Tan EL, Alonso S. Sustained high levels of interleukin-6 contribute to the pathogenesis of enterovirus 71 in a neonate mouse model. J Virol. 2011;85:3067–3076. doi: 10.1128/JVI.01779-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nolan MA, Craig ME, Lahra MM, Rawlinson WD, Prager PC, Williams GD, Bye AM, Andrews PI. Survival after pulmonary edema due to enterovirus 71 encephalitis. Neurology. 2003;60:1651–1656. doi: 10.1212/01.wnl.0000066810.62490.ff. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Liu H, Wang L, Yang F, Hu Y, Ren X, Li G, Yang Y, Sun S, Li Y, Chen X, Li X, Jin Q. Comparative study of the cytokine/chemokine response in children with differing disease severity in enterovirus 71-induced hand, foot, and mouth disease. PLoS One. 2013;8:e67430. doi: 10.1371/journal.pone.0067430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han J, Wang Y, Gan X, Song J, Sun P, Dong XP. Serum cytokine profiles of children with human enterovirus 71-associated hand, foot, and mouth disease. J Med Virol. 2014;86:1377–1385. doi: 10.1002/jmv.23929. [DOI] [PubMed] [Google Scholar]
- 11.Enk AH, Angeloni VL, Udey MC, Katz SI. Inhibition of Langerhans cell antigen-presenting function by IL-10: a role for IL-10 in induction of tolerance. J Immunol. 1993;151:2390–2398. [PubMed] [Google Scholar]
- 12.Wang SM, Lei HY, Huang KJ, Wu JM, Wang JR, Yu CK, Su IJ, Liu CC. Pathogenesis of enterovirus 71 brainstem encephalitis in pediatric patients: roles of cytokines and cellular immune activation in patients with pulmonary edema. J Infect Dis. 2003;188:564–570. doi: 10.1086/376998. [DOI] [PubMed] [Google Scholar]
- 13.Blackburn SD, Wherry EJ. IL-10, T cell exhaustion and viral persistence. Trends Microbiol. 2007;15:143–146. doi: 10.1016/j.tim.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Cassatella MA, Meda L, Bonora S, Ceska M, Constantin G. Interleukin-10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leukocytes: evidence for an autocrine role of tumor necrosis factor and IL-1 beta in mediating the production of IL-8 triggered by lipopolysaccharide. J Exp Med. 1993;178:2207–2211. doi: 10.1084/jem.178.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minty A, Chalon P, Derocq JM, Dumont X, Guillemot JC, Kaghad M, Labit C, Leplatois P, Liauzun P, Miloux B. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993;362:248–250. doi: 10.1038/362248a0. [DOI] [PubMed] [Google Scholar]
- 16.De Vries JE. The role of IL-13 and its receptor in allergy and inflammatory responses. J Allergy Clin Immunol. 1998;102:165–169. doi: 10.1016/s0091-6749(98)70080-6. [DOI] [PubMed] [Google Scholar]
- 17.Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, Elias JA, Sheppard D, Erle DJ. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002;8:885–889. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]


