Abstract
Objective: This study aimed to investigate the effects of Dexmedetomidine combined with Dezocine on the cognition and hippocampal microglia activation of rats. Methods: Laparotomy was successfully performed in 48 rats which were then divided into Dexmedetomidine+Dezocine group and Dezocine group. Rats in Dexmedetomidine+dezocine group were infused with Dexmedetomidine and dezocine via the tail vein after anesthesia; rats in Dezocine group were infused with dezocine via the tail vein. After surgery, rats underwent detection of learning and memory functions at 1, 3, and 7 days after surgery, and the neuroglobin and norepinephrine expression was detected in the hippocampal microglia at the same time points. Results: 1, 3 and 7 days after surgery, the latency to escape in Dexmedetomidine+Dezocine group was significantly shorter than that in Dezocine group, and the number of cells positive for neuroglobin or norepinephrine in the CAL region of hippocampus of Dexmedetomidine+Dezocine group was also markedly higher than that of Dezocine group (P < 0.05). Conclusion: Surgery and anesthesia have influence on the cognition of rats to a certain degree, and dexmedetomidine combined with dezocine can effectively improve the impaired cognition due to surgery and anesthesia, which may be attributed to the increase in the protective neuroglobin and norepinephrine in the hippocampus.
Keywords: Dexmedetomidine given, Dezocine, cognition, hippocampal microglia, norepinephrine
Introduction
Clinical epidemiological studies have shown that the incidence of impaired cognition is at a high level after surgery, especially after major surgeries such as brain surgery, heart surgery and chest/abdominal surgery [1,2]. The cognition is compromised gradually, accompanied by reduction in indicators of nervous system, and especially, the manifestations of hippocampus and temporal cortex are obvious. Biologically, hippocampus is important for the higher nervous activities such as learning and memory and a crucial component for the maintenance of central nervous system (CNS) function [3,4]. Different brain regions of the CNS are innervated by norepinephrine (NE). In patients with impaired cognition, the number of neurons reduces by more than 50%, which may directly influence the NE content and has detrimental effects on relevant activities such as learning and memory [2,5]. Dezocine is an alkyl derivative of benzene morphine and an antagonist of mixed opioid receptor excitation [6]. Dezocine has been used in the anesthesia under major surgeries. In animal studies, the anesthetic effect of dezocine is better than that of morphine, and has no adverse effects on the cardiovascular system and respiratory system. Dexmedetomidine hydrochloride is a new agonist of α2-adrenergic receptor (α2-AR) and may inhibit the release of NE and induce the sympathetic nervous system torpescence. It has relative selectivity and high efficiency and may exert sedative and antalgic effects [7]. In the study of Kat and Wacker, results showed the activation of α2-AR could influence the space navigation capability of animals in water maze [8,9]. On the basis of above findings, we investigated the influences of dexmedetomidine combined with dezocine on the cognition and hippocampal microglia activation, and explored the potential mechanisms. Our findings may provide evidence for the medication in clinical anesthesia.
Materials and methods
Animals
A total of 48 rats weighing 400-500 g were purchased and housed in a specific pathogen free and quiet environment with ventilation and 12/12 light/dark cycle at a temperature of 20°C-28°C and a humidity of 50%. Rats were given ad libitum access to water and food and 4 rats were housed in a cage.
Instrument and reagents
ZH0065 Morris water maze video analysis system (Beijing Shuolinyuan Technology Co., Ltd.), Ultra-low temperature refrigerator (SANY, Japan), light microscope (Olympus, Japan), dexmedetomidine hydrochloride injection (10110334; Jiangsu Hengrui Medicine Co., Ltd.), dezocine (13032221; Yangtze River Pharmaceutical Group Co., Ltd.), norepinephrine kit (Cusabio), neuroglobin (Ngb; Chinese Military Academy of Medical Sciences), standards, Horseradish peroxidase conjugated antibodies, Avidin-Biotin conjugated antibodies, and diluent for Biotin conjugated antibodies (Shanghai Sangon Biotech Co., Ltd.) were used in the present study.
Establishment of animal model
Rats received food deprivation for 12 h and water deprivation for 6 h. Animals were anesthetized intraperitoneally, and an access to tail vein was established. After routine sterilization, rats were fixed on a table, and a longitudinal incision was made. The skin, subcutaneous and peritoneum were separated, and the abdominal cavity was sprayed with normal saline at 37°C to assure the moisture. After surgery, the wound was closed. The success rate of surgery was 100% in this study, and there were no severe side effects. The time to regaining consciousness was not prolonged.
Grouping
A total of 48 rats receiving laparotomy was randomly divided into 2 groups: Dexmedetomidine+Dezocine group and Dezocine group. Rats in Dexmedetomidine+Dezocine group were injected with dexmedetomidine at 10 μg/kg/h for 1 h and dezocine at 5 μg/kg/h for 1 h via the tail vein. Rats in Dezocine group were injected with dezocine at 5 μg/kg/h for 1 h via the tail vein. At 1, 3 and 7 days, rats received cognition examination and then were sacrificed for biochemical analysis.
Examination of learning and memory
Morris water maze test was performed in these rats. The light intensity was moderate, and substances were placed at the same site during the examinations. One day before examination, rats were placed in water maze and allowed to find the platform within 120 s and stay on the platform for 30 s. At 24 h after surgery, examination was initiated. Water maze test was done at 8-9 am, and the swimming time and trajectory were recorded within 120 s. When the rats found, climbed and stayed on the platform for longer than 5 s, we acknowledged that these rats found the platform. The escape latency was recorded from investigators placing rats into water to rats finding the platform. When the rats did not find the platform within 120 s, investigators placed these rats onto the platform and allowed them to stay on the platform for 30 s, and the escape latency was recorded as 120 s.
Sample collection
At 2 h after examination of learning and memory, rats were intraperitoneally anesthetized and sacrificed by decapitation. The skull was removed, and the brain was exposed. The brain was harvested into and washed with cold normal saline. After removing cortex, the hippocampus was exposed, collected and stored at -80°C. Before detection, the hippocampus was homogenized in PBS (100 mg tissues/1 ml 1×PBS), followed by centrifugation at 5000 rpm/min for 5 min. The supernatant was collected for further use.
Detection of Ngb expression
Immunohistochemistry was performed to detect the Ngb expression in the CAL region of hippocampus according to manufacturer’s instructions. Determination: Ngb positive cells had brown-yellow granules in the cytoplasm or both cytoplasm and nucleus, and quantification was also done for Ngb expression.
Detection of NE expression
ELISA was employed to detect the NE content in the hippocampus. The optical density (OD) was measured at 450 nm and the concentration of NE was calculated according to the standard curve.
Statistical analysis
SPSS version 19.0 was employed for statistical analysis. Qualitative data were expressed as percentages and compared with chi square test. Quantitative data were expressed as mean±standard deviation and compared with t test. A value of P < 0.05 was considered statistically significant.
Results
Escape latency in different groups
Water maze test showed rats swam along the wall soon after placing into the water. After repeated examination, this phenomenon reduced and they began to find the platform, showing the reduction in escape latency. Statistical analysis showed the escape latency in Dexmedetomidine+Dezocine group was significantly shorter than that in Dezocine group at 1, 3 and 7 days after surgery (P < 0.05) (Table 1 and Figure 1).
Table 1.
Escape latency of rats at different time points after surgery
| Group | 1 d (n=8) | 3 d (n=8) | 7 d (n=8) | F | P |
|---|---|---|---|---|---|
| Dexmedetomidine+Dezocine | 88.09±11.23 | 75.87±18.23 | 55.32±7.45 | 10.346 | < 0.05 |
| Dezocine | 118.34±19.93 | 109.88±20.34 | 80.34±8.41 | 12.934 | < 0.05 |
| t | 6.430 | 7.409 | 8.334 | ||
| P | < 0.05 | < 0.05 | < 0.05 |
Figure 1.
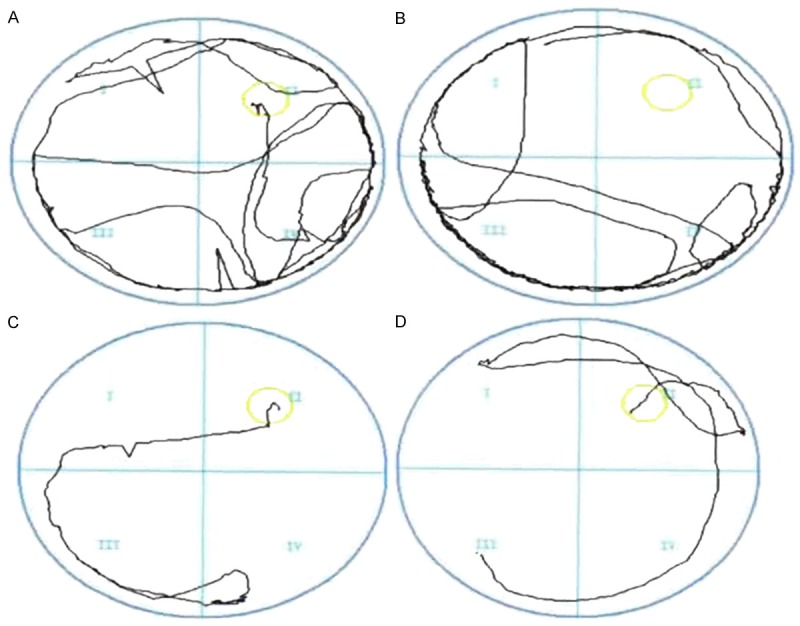
Examination of learning and memory of rats in two groups. A. Swimming trajectory on day 1 in Dezocine group; B. Swimming trajectory on day 1 in Dexmedetomidine+Dezocine group; C. Swimming trajectory on day 7 in Dezocine group; D. Swimming trajectory on day 7 in Dexmedetomidine+Dezocine group.
Expression of Ngb in the hippocampus
Results showed the number of Ngb positive cells in the CAL region of hippocampus in Dexmedetomidine+Dezocine was significantly higher than that in Dezocine group at 1, 3 and 7 days after surgery (P < 0.05) (Table 2).
Table 2.
Ngb expression in the hippocampus at different time points (n/mm2)
| Group | 1 d (n=8) | 3 d (n=8) | 7 d (n=8) | F | P |
|---|---|---|---|---|---|
| Dexmedetomidine+Dezocine | 68.50±4.37 | 91.17±3.60 | 97.83±3.54 | 8.347 | < 0.05 |
| Dezocine | 34.67±6.12 | 44.83±2.48 | 55.33±3.27 | 6.443 | < 0.05 |
| t | 6.440 | 18.345 | 12.981 | ||
| P | < 0.05 | < 0.05 | < 0.05 |
NE concentration
According to the OD value of standards, the standard curve was delineated and the NE concentration of the hippocampus was calculated. Statistical analysis showed the NE concentration in the Dexmedetomidine+Dezocine group was significantly higher than that in the Dezocine group at 1, 3 and 7 days after surgery (P < 0.05) (Table 3).
Table 3.
NE concentration of the hippocampus at different time points
| Group | 1 d (n=8) | 3 d (n=8) | 7 d (n=8) | F | P |
|---|---|---|---|---|---|
| Dexmedetomidine+Dezocine | 134.34±33.43 | 167.34±29.88 | 199.78±30.24 | 9.004 | < 0.05 |
| Dezocine | 91.78±28.99 | 115.60±30.20 | 136.89±32.32 | 8.457 | < 0.05 |
| t | 7.884 | 8.456 | 10.389 | ||
| P | < 0.05 | < 0.05 | < 0.05 |
Discussion
Surgery and anesthesia may induce intense stress for the body and cause significant change in the homeostasis, which may promote the impairment of cognition [10]. In respect of the mechanisms, the number of neurons in the brain reduces and the growth of neurons and synapses decreases during the peri-operative period. In addition, the accumulation of byproducts due to stress may significantly reduce the ability of the brain to combat with stress, causing cognition impairment [11].
Morris water maze test is a widely accepted tool for the evaluation of learning and memory of rodents in studies. In this test, animals are obligated to find the platform hidden in the water, aiming to examine the spatial position sense and direction sense [12]. In the present study, the Morris water maze system was connected to a computer which could automatically and synchronously record the trajectory and the time to finding the platform, which is helpful for the determination of cognition function. In our study, rats swam along the wall in early phase of examination. After repeated examinations, the swimming along the wall reduced, and rats tended to find the platform, showing the shortened escape latency.
Dezocine is an agonist of κ receptor and an antagonist of μ receptor. Its receptor is widely distributed in the brain, brainstem and spinal cord and dezocine may induce spinal analgesia and mild sedation, leading to the impairment of cognition. Dezocine has mild addiction and thus has been classified as a non-narcotic [13]. Although dexmedetomidine may blunt the sympathetic nervous system and induce related effects, it has relatively high safety [14]. In addition, dexmedetomidine may bind to the glucuronide in the liver and is involved in the redox reaction in the presence of cytochrome P-450 enzyme system. Thus, dexmedetomidine may attenuate the hemodynamic disorders (such as increase in heart rhythm) due to tracheal intubation and provide favorable sedation when the consciousness is required during surgery or non-invasive operation, which may provide a better anesthesia [15]. In the present, results showed the escape latency in Dezocine group at 1, 3 and 7 days after surgery was significantly longer than that in Dexmedetomidine+Dezocine group (P < 0.05). This suggests that dexmedetomidine combined with dezocine may attenuate the detrimental influence of anesthesia on the memory of rats.
Learning and memory are usually employed to evaluate the development of intelligence in humans. Currently, studies have shown that the hippocampus of the limbic system is important for the generation of higher nervous activities (such as learning and memory) [16]. NE is the first identified monoamine neurotransmitter. When the nerve impulses reach the presynaptic membrane, NE may be excreted into synaptic clefts via the synaptic vesicles, inducing a series of biological responses such as learning and memory. In the presence of surgery induced stress, the NE concentration reduces in the hippocampus, which may cause presynaptic inhibition, resulting in reduction in synaptic activity. This may influence the release of downstream neurotransmitters, which may impair the memory of animals. This suggests that NE in the central nervous system is involved in the learning and memory [17]. In our study, the NE concentration of the CAL region of hippocampus in Dexmedetomidine+Dezocine group was significantly higher than that in Dezocine group at 1, 3 and 7 days after surgery (P < 0.05). This suggests that dexmedetomidine may attenuate the reduction in NE concentration of the hippocampus and relatively increase the NE concentration to affect the release of downstream neurotransmitters.
Ngb is a new endogenous neuroprotective factor and has a potent neuroprotective effect. In recent years, Ngb has been extensively studied because it has a high affinity to oxygen and is specifically expressed in the nervous system. Studies have confirmed that the extent of Ngb expression in different regions of the brain is negatively related to the extent of ischemia/hypoxia [18]. Dexmedetomidine may activate the NE receptor in the nucleus ceruleus and is closely associated with the activation of receptor coupled G1/G0 signaling system. In the present study, the number of cells positive for Ngb in the CAL region of the hippocampus in Dexmedetomidine+Dezocine group was significantly higher than that in Dezocine group at 1, 3 and 7 days after surgery (P < 0.05). This suggests that dexmedetomidine can elevate the Ngb expression to reduce the incidence of neurological sequelae. Taken together, surgery and anesthesia has influence on the cognition of rats to different extents, and dexmedetomidine combined with dezocine may attenuate the cognition impairment due to anesthesia, which is attributed to the increases in the Ngb expression and NE concentration in the hippocampus.
Disclosure of conflict of interest
None.
References
- 1.Sanders RD, Xu J, Shu Y, Januszewski A, Halder S, Fidalgo A, Sun P, Hossain M, Ma D, Maze M. Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology. 2009;110:1077–1085. doi: 10.1097/ALN.0b013e31819daedd. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg DA, Jin K, Khan AA. Neuroglobin: an endogenous neuroprotectant. Curr Opin Pharmacol. 2008;8:20–24. doi: 10.1016/j.coph.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo J, Guo J, Han D, Li H. [Comparison of dexmedetomidine and midazolam on neurotoxicity in neonatal mice] . Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2013;30:607–610. [PubMed] [Google Scholar]
- 4.Hundahl CA, Allen GC, Nyengaard JR, Dewilde S, Carter BD, Kelsen J, Hay-Schmidt A. Neuroglobin in the rat brain: localization. Neuroendocrinology. 2008;88:173–182. doi: 10.1159/000129698. [DOI] [PubMed] [Google Scholar]
- 5.Kose EA, Bakar B, Kasimcan O, Atilla P, Kilinc K, Muftuoglu S, Apan A. Effects of intracisternal and intravenous dexmedetomidine on ischemia-induced brain injury in rat: a comparative study. Turk Neurosurg. 2013;23:208–217. doi: 10.5137/1019-5149.JTN.6757-12.0. [DOI] [PubMed] [Google Scholar]
- 6.van Oostrom H, Stienen PJ, Doornenbal A, Hellebrekers LJ. The alpha(2)-adrenoceptor agonist dexmedetomidine suppresses memory formation only at doses attenuating the perception of sensory input. Eur J Pharmacol. 2010;629:58–62. doi: 10.1016/j.ejphar.2009.11.062. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Liu J, Zhu H, Tejima E, Tsuji K, Murata Y, Atochin DN, Huang PL, Zhang C, Lo EH. Effects of neuroglobin overexpression on acute brain injury and long-term outcomes after focal cerebral ischemia. Stroke. 2008;39:1869–1874. doi: 10.1161/STROKEAHA.107.506022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kat MG, Vreeswijk R, de Jonghe JF, van der Ploeg T, van Gool WA, Eikelenboom P, Kalisvaart KJ. Long-term cognitive outcome of delirium in elderly hip surgery patients. A prospective matched controlled study over two and a half years. Dement Geriatr Cogn Disord. 2008;26:1–8. doi: 10.1159/000140611. [DOI] [PubMed] [Google Scholar]
- 9.Wacker P, Nunes PV, Cabrita H, Forlenza OV. Post-operative delirium is associated with poor cognitive outcome and dementia. Dement Geriatr Cogn Disord. 2006;21:221–227. doi: 10.1159/000091022. [DOI] [PubMed] [Google Scholar]
- 10.Jeon YT, Hwang JW, Lim YJ, Park SK, Park HP. Postischemic sevoflurane offers no additional neuroprotective benefit to preischemic dexmedetomidine. J Neurosurg Anesthesiol. 2013;25:184–190. doi: 10.1097/ANA.0b013e3182764d2a. [DOI] [PubMed] [Google Scholar]
- 11.Shehabi Y, Grant P, Wolfenden H, Hammond N, Bass F, Campbell M, Chen J. Prevalence of delirium with dexmedetomidine compared with morphine based therapy after cardiac surgery: a randomized controlled trial (DEXmedetomidine COmpared to Morphine-DEXCOM Study) Anesthesiology. 2009;111:1075–1084. doi: 10.1097/ALN.0b013e3181b6a783. [DOI] [PubMed] [Google Scholar]
- 12.Tachibana K, Hashimoto T, Kato R, Uchida Y, Ito R, Takita K, Morimoto Y. Neonatal administration with dexmedetomidine does not impair the rat hippocampal synaptic plasticity later in adulthood. Paediatr Anaesth. 2012;22:713–719. doi: 10.1111/j.1460-9592.2012.03810.x. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Wu Y, Ren C, Lu Y, Gao Y, Zheng X, Zhang C. The activity of recombinant human neuroglobin as an antioxidant and free radical scavenger. Proteins. 2011;79:115–125. doi: 10.1002/prot.22863. [DOI] [PubMed] [Google Scholar]
- 14.Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 15.Hwang L, Choi IY, Kim SE, Ko IG, Shin MS, Kim CJ, Kim SH, Jin JJ, Chung JY, Yi JW. Dexmedetomidine ameliorates intracerebral hemorrhage-induced memory impairment by inhibiting apoptosis and enhancing brain-derived neurotrophic factor expression in the rat hippocampus. Int J Mol Med. 2013;31:1047–1056. doi: 10.3892/ijmm.2013.1301. [DOI] [PubMed] [Google Scholar]
- 16.Li E, Kim DH, Cai M, Lee S, Kim Y, Lim E, Hoon Ryu J, Unterman TG, Park S. Hippocampus-dependent spatial learning and memory are impaired in growth hormone-deficient spontaneous dwarf rats. Endocr J. 2011;58:257–267. doi: 10.1507/endocrj.k11e-006. [DOI] [PubMed] [Google Scholar]
- 17.Krugers HJ, Karst H, Joels M. Interactions between noradrenaline and corticosteroids in the brain: from electrical activity to cognitive performance. Front Cell Neurosci. 2012;6:15. doi: 10.3389/fncel.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohman RA, Tarr AJ, Byler SL, Boehm GW. Age increases vulnerability to bacterial endotoxin-induced behavioral decrements. Physiol Behav. 2007;91:561–565. doi: 10.1016/j.physbeh.2007.03.032. [DOI] [PubMed] [Google Scholar]


