Abstract
Purpose: Osteoarthritis (OA) is a common disease in the elderly population. Most of the previous OA-related researches focused on articular cartilage degeneration, osteophyte formation and synovitis etc. However, the role of the meniscus in these pathological changes has not been given enough attention. The goal of our study was to find the pathological changes of the meniscus in OA knee and determine their relationship. Method: 20 months old female Chinese rabbits received either knee damaging operations with articular cartilage scratch method or sham operation randomly on one of their knees. They were sacrificed after 1-6 weeks post-operation. Medial Displacement Index (MDI) for meniscus dislocation, hematoxylin and eosin (HE) for routine histological evaluation, Toluidine blue (TB) stains for evaluating proteoglycans were carried out. Immunohistochemical (IHC) staining was performed with a two-step detection kit. Results: Histological analysis showed chondrocyte clusters around cartilage lesions and moderate loss of proteoglycans in the operation model, as well as MDI increase and all characteristics of OA. High expression of MMP-3 and TIMP-1 also were found in both hyaline cartilage and meniscus. Conclusion: Biomechanical and biochemistry environment around the meniscus is altered when OA occur. If meniscus showed degeneration, subluxation and dysfunction, OA would be more severe. Prompt repair or reconstruction of hyaline cartilage in weight bearing area when it injured could prevent meniscus degeneration and subluxation, then prevent the development of OA.
Keywords: Cartilage injury, osteoarthritis, meniscus subluxation, MMP-3, TIMP-1
Introduction
Osteoarthritis (OA) is a degenerative joint disease for which no known cure exists. It is characterized by the degeneration of articular cartilage and changes in other joint tissues including subchondral (substance) bone and meniscus [1]. OA can be caused by many factors including diet, injury, strain and genetic abnormalities [2-5]. However, the molecular mechanisms driving the onset and progression of the disease are incompletely understood.
Nowadays, knowledge of biology, genetics, biochemistry and cytobiology has grown rapidly. More and more cutting edge biological techniques and methods in OA researches have been developed. In all these OA-related researches, most of them have been focused on articular cartilage degeneration, osteophyte formation and synovitis, etc. little attention has been given to the role of the meniscus in these pathological changes.
The meniscus is a fibrocartilaginous structure between the femur and tibia with the important task of load distribution [6,7]. About 70% of the load passes through the medial tibiofemoral compartment and 30% through the lateral compartment in normally aligned knees [8]. While very limited biomechanical studies on effects of different meniscus position have been done, there is evidence that a displaced meniscus, e.g., a root tear, and it will no longer provide optimal load transmission in the knee, thus result in increased cartilage contact stress in similar manner as that after partial or total meniscectomy [9,10].
The objective of our study is to find the pathological changes of the meniscus in OA knee and try to clarify the relationship between them.
Materials and methods
Ethics statement
All procedures in the study were authorized by the locally responsible ethics committee and authorities.
Animals
24 skeletally mature 20 months old female Chinese rabbits with a mean body weight of 2236 g (±188 g were acquired from a population kept at institute of Chinese laboratory animals. All animals were kept in individual cages (100 cm×70 cm×40 cm) at a room temperature of 20-22°C, starting two weeks preoperatively. The day/night period was 12 h. Animals had free access to water and food.
Surgical procedure
12 rabbits were randomly selected and were operated on one of the knees. The knee being operated on in each rabbit was also randomly selected. During the operation, the cartilage of the lateral and medial condyles was damagedwith a Kirschner-wire (1.5 mm diameter) that was bent 90° at 0.5 mm from the tip, which kept the depth of the grooves was restricted to 0.5 mm. In utmost flexion, ten longitudinal and diagonal grooves were made on the weight-bearing parts of femoral condyles without damaging the subchondral bone [11,12]. Each of the remaining 12 rabbits received sham operation in one randomly selected knee. The contralateral knees of all 24 rabbits were left alone. All the grooved knees were categorized as experimental knees (E). Sham operated knees were categorized as sham control knees (C). The rest, which had no surgeries at all, were categorized as normal knees (N).
Tissue harvesting
Rabbits were sacrificed by pentobarbital overdose at 1-6 weeks post-operation [13,14]. Each week, 2 rabbits with grooved knees and 2 sham operated rabbits were chosen randomly, accounting total 2 experimental knees, 2 sham control knees and 4 normal knees [15]. The morphology of knee cartilage and interior meniscus was observed first, then medial displacement index was measured, and after that, the medial tibial plateau and interior meniscus was put into 4% paraformaldehyde, and demineralized in 15% EDTA for histopathological analysis.
Medial displacement index (MDI)
Conditions of hyaline cartilage and medial meniscus were observed, and the meniscus MDI were measured, the brief procedure was shown in Figure 1 and referred to the previous study [6], the MDI=(B+C-A)/B, the representationof A-C were shown in Figure 1.
Figure 1.
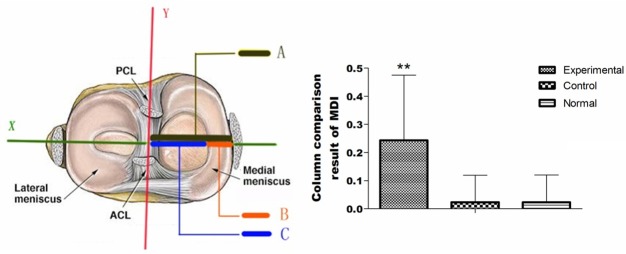
The meniscus dislocation index measurement and result. ACL: anterior cruciate ligament; PCL: after cruciate ligament; Reference line Y: through tibial tubercle midpoint and tibial condyle spines midpoint between attachment; Reference line X: the condyle spines midpoint between straight line perpendicular to the Y. A: condyle spines midpoint between the distance to the edge of the medial tibial plateau. B: the width of meniscus; C: between condyle spines point to the distance of the medial meniscus free edge. Column Chart: The MDI, experimental group compared with sham control group and normal group is higher, and there were significant difference (P < 0.01); There was no significant difference between sham control group and normal group (P > 0.05).
Histopathologic staining
Paraffin-embedded knee blocs were sagittally cut in serial sections at 5-mm thick. Sections were stained with Hematein and Eosin (HE) for routine histological evaluation. Toluidine blue (TB) strains were used to evaluate proteoglycans in the cartilage matrix [16].
Immunohistochemical (IHC) staining
Paraffin-embedded tibial plateau cartilage and meniscus was used for IHC staining. IHC staining was performed with a two-step detection kit (Zhongshan Golden Bridge Biotechnology) as described previously [16,17]. The primary antibodies were matrix metalloproteinase-3 (MMP-3) and tissue inhibitor of metalloproteinase-1 (TIMP-1) (Abcam, 1:50 dilution).
Statistical analysis
Statistical analysis was performed using SPSS version 11.0 for Windows. The datum is expressed as the mean standard deviation of the various measurements. The statistical significance of intragroup differences was analyzed using one-way analysis of variance (ANOVA) and the Duncan post-hoc test. Differences with a P value less than 0.05 were considered statistically significant.
Results and discussion
Time-dependent MDI changes in OA
Knee joints in experimental group exhibited typical OA symptoms (Figure 2B). Cartilage surface fibrillation, softening and ulcers were found. Finally, the entire hyaline cartilage was damaged. Meniscus degenerates gradually, resulted in subluxation and MDI increase (Figure 1).
Figure 2.
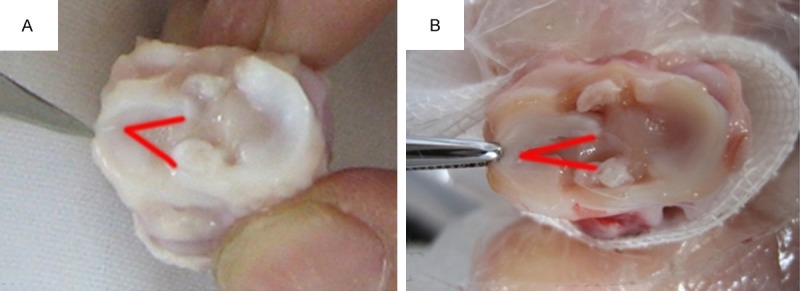
The knee joint meniscus. A. The normal group, the meniscus is not slack, and red line shows the meniscus free edge. B. The experimental group, the meniscus flabby dislocation, and red line shows the meniscus free edge.
Time-dependent HE staining changes in OA
HE staining of sham control group and the normal group were consistent, and four layers, namely, surface, transitional layer, columnar layer and calcified layer are visible in articular cartilage. The surface of spindle cells arranged in parallel with the articular cartilage surface. Transitional layer cells were round and arranged in irregular, while slightly larger volume than the surface. Columnar layer cells were larger and arranged in columnar joints perpendicular to the surface. The calcified layer of the largest cell volume, arranged in irregular, and there is calcification within the cartilage matrix. Meniscal (Meniscium) chondrocytes were normal morphology, neatly arranged along the directionof the meniscus surface, and regular structure of collagen fibers (Figure 3A).
Figure 3.
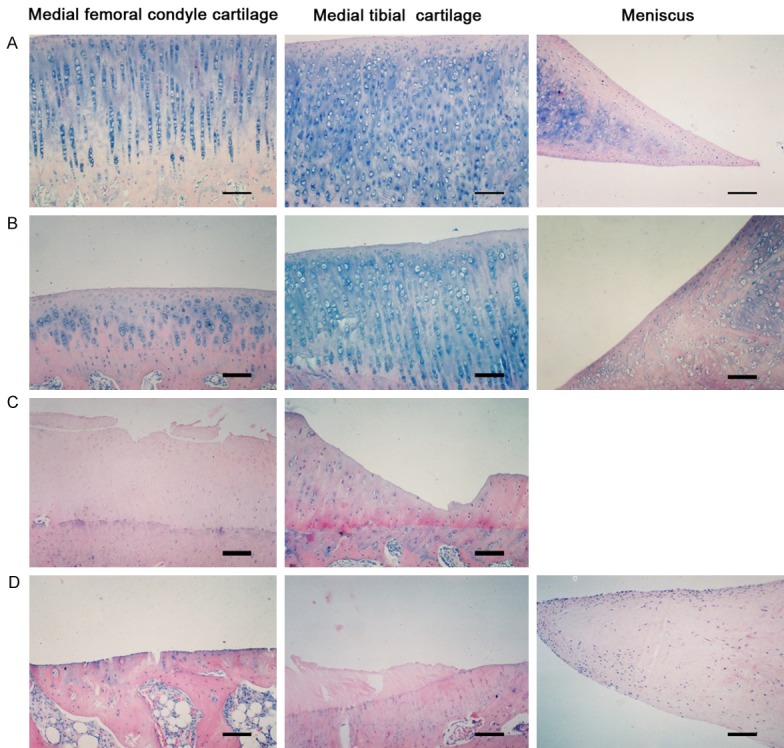
Hematein and Eosin (HE) of cartilage and meniscus (Bar=200 μm). A. HE staining of sham control group, articular cartilage can be broadly divided into four layers. Meniscal chondrocytes were of normal morphology, neatly arranged along the direction of the meniscus surface, and regular structure of collagen fibers. B. 2 weeks after operation, the articular cartilage surface showed tiny fissures, and layers of chondrocytes increased significantly, while chondrocytes began to appear irregular, poorly, and even necrosis. The chondrocytes of meniscal were proliferation, the meniscus edge aggregation and collagen thickening. C. 4 weeks after operation, the cartilage surface cracks increased and deepen, and cartilage cells arranged in disorder and depression fossa vacuolar change, tide line thickening forward, non-calcified layer thinning, while calcified layer is relatively thick. D. 6 weeks after operation, cartilage thickness was thin, and some parts of full-thickness cartilage loss, and chondrocyte number decreased, nucleus were hypertrophy or condensation and appeared disintegration and death. The meniscal chondrocytes are further aggregated to the edge, and a large number of chondrocyte degeneration necrosis, collagen fibers disorders, multiple internal tear visible traces.
In the experimental group, after one week or two weeks, appearance of tiny fissures in the articular cartilage surface, and significant increase in layers of chondrocytes were observed, while chondrocytes began to show irregularity, and even necrosis. There were also proliferation in chondrocytes of the meniscus, aggregation of the meniscus edge and collagen thickening (Figure 3B). After three weeks or four weeks, the cartilage surface cracks increased and deepened. Cartilage cells were arranged in disorder and depression fossa vacuolar (vascular) were altered. The tide line was thickened forward; non-calcified layer was thinning; while the calcified layer became relatively thick (Figure 3C). After 5 weeks or 6 weeks, cartilage thickness was low, with complete cartilage loss in some parts, and chondrocyte number was decreased. Nucleus was hypertrophied or condensed and appeared disintegrated with cell death occurring. The meniscal (meniscus) chondrocytes are further aggregated to the edge, and a large number of chondrocyte degeneration necrosis, collagen fibers disorders, as well as visible traces of multiple internal tear were observed (Figure 3D).
Time-dependent TB staining changes in OA
The sham control group showed purple matrix and each layer stained uniform, with surface layer being shallow, while transitional layers deep and columnar layer the deepest. Calcified layer was significantly lighter stained since calcinosis (Figure 4A). In the experimental group, 1 week or 2 weeks after, staining is visibly uneven with no dye retained in the upper layer and only partially stained in deep layer (Figure 4B). After 3 weeks, staining in deep layers also decreased. After 5 weeks, the majority of the region was not stained except the cluster poly cells, which still appeared dark after staining (Figure 4C).
Figure 4.

The medial tibial plateau cartilage toluidine blue staining (Bar=80 μm). A. The sham control group showed purple matrix and each layer stained uniform, and surface shallow, while transitional layers deep, and columnar layer deepest. Calcified layer was significantly lighter stain since calcinosis. B. 2 weeks after operation, it visible staining unevenly and dyed almost full-thickness loss in shallow cartilage, but lost in the deep part of the dye and some stained, especially around the stained chondrocytes cluster together more apparent. C. 5 weeks after operation, the loss of many specimens stained in most regions, but still observed around the cluster poly darker staining.
Time-dependent IHC staining changes in OA
MMP-3 and TIMP-1 showed weak expression in the sham control group and normal group, in the experimental group, high levels of MMP-3 (Figure 5) and TIMP-1 were detected in cartilage matrix and cartilage cytoplasmic and lacunae at 2 weeks post-operation. MMP-3 level peaked at 4 weeks before gradually weakened. At six weeks, MMP-3 in chondrocytes is unevenly expressed, but still at far higher levels than that in the sham controls. TIMP-1 expression was significant increase at 4 weeks (Figure 6) and 6 weeks (Table 1).
Figure 5.
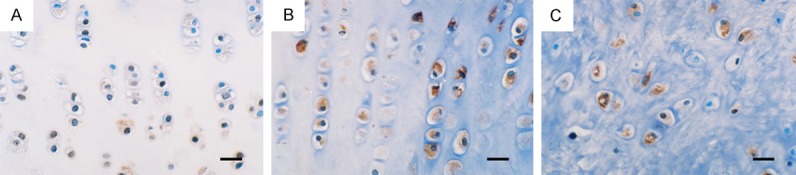
Immunohistochemical (IHC) Staining for MMP-3 of cartilage and meniscus (Bar=40 μm). A. 2 weeks after operation, MMP-3 positive expression in the medial femoral condyle cartilage. B. 2 weeks after operation, MMP-3 positive expression in the medial tibial plateau cartilage. C. 2 weeks after operation, MMP-3 positive expression in the medial meniscus.
Figure 6.
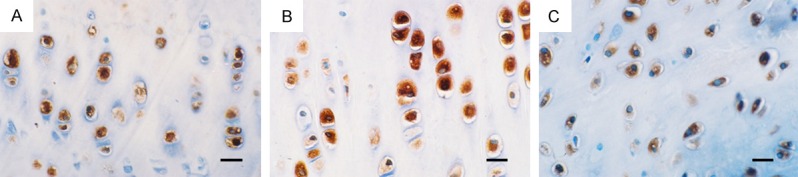
Immunohistochemical (IHC) Staining for TIMP-1 of cartilage and meniscus (Bar=40 μm). A. 4 weeks after operation, TIMP-1 positive expression in the medial femoral condyle cartilage. B. 4 weeks after operation, TIMP-1 positive expression in the medial tibial plateau cartilage. C. 4 weeks after operation, TIMP-1 positive expression in the medial meniscus.
Table 1.
Groups of MMP3/TIMP-1 expression
| Classes | Meniscus of experimental | Meniscus of sham control | Meniscus of normal | Tibial plateau of experimental | Tibial plateau of control | tibial plateau of normal |
|---|---|---|---|---|---|---|
| MMP-3 | 128.76±6.81* | 96.36±2.14 | 96.63±2.81 | 128.73±16.57* | 90.8±5.89 | 90.81±5.21 |
| TIMP-1 | 135.81±17.03* | 91.11±6.66 | 91.08±6.92 | 137.17±9.87* | 108.25±7.22 | 109.81±6.37 |
| MMP-3/TIMP-1 | 0.96±0.08 | 1.06±0.08 | 1.07±0.07 | 0.95±0.17 | 0.84±0.09 | 0.83±0.07 |
P < 0.01.
Meniscus and tibial plateau of experimental group significantly increased MMP-3 positive cells than sham control group and normal group (P < 0.01), but MMP-3 positive cells was no significant difference between sham control group and normal group (P > 0.05). Meniscus and tibial plateau of experimental group significantly increased TIMP-1 positive cells than sham control group and normal group (P < 0.01), but TIMP-1 positive cells was no significant difference between sham control group and normal group (P > 0.05).
Meniscus and tibial plateau of experimental group significantly increased MMP-3 and TIMP-1 positive cells than sham control group and normal group (P < 0.01), but MMP-3 and TIMP-1 positive cells was no significant difference between sham control group and normal group (P > 0.05) (Table 1).
Clinical study in our hospital found that although some patients had intact meniscal (meniscus), but there were serious degeneration and loss of normal anatomical position from the inside edge of the tibia part or complete dislocation were detected by Magnetic Resonance Imaging (MRI), arthroscopic knee surgery and total knee arthroplasty. These symptoms were not obvious, and patients had no history of trauma or previous treatment of other symptoms of knee osteoarthritis. The early lesions of the meniscus were not a serious obstacle to the tibiofemoral articulation, and it is usually not found until the MRI examination or surgery [18,19]. Some patients had moderate pain, without interlocking, and no or only very accumulation of fluid. However, bending of the knee to certain angle stimulus meniscus injury and pain, and leg supination can cause or aggravate the pain. Physical examination showed tenderness in the central part of tibial collateral ligament and the nearby area, but valgus stress test reported no pain [20]. Currently, multiple human studies on meniscal (meniscus) subluxation were based on the observation on osteoarthritis patients only [18,19]. There are also studies focusing on the normal population or specific population groups (e.g., athletes) [21-23], including the use of corpses as a model of meniscal (meniscus) knee dislocation phenomena [24]. Not many studies using animal models of mensical (mental) dislocation have been reported. Using patients as the research subjects may introduce uncontrollable factors. Therefore, we used a rabbit model of osteoarthritis to accurately simulate and study meniscal (meniscus) dislocations.
Scratch method is used to prepare the articular cartilage of knee osteoarthritis models. This method is a simple process and cause direct damage to the articular cartilage surface preparation [25], which was good simulation of osteoarthritis that is gradually progressing from mild to severe pathological changes [26]. Moreover, this method does not affect the integrity of tissue around the knee, which can better simulate meniscal (meniscus) biomechanicalenvironment. Due to the limited size of rabbit knee joints, the accuracy of the measurement is not guaranteed if imaging methods were used, therefore we directly measured the rabbit knees in the from raw specimen, and then calculate MDI. Specific measure was referenced with Kenny [18] methods to measure the distance changed in the MRI image into the corresponding gross specimen vernier caliper measurement site.
Data show that there were significant differences between the experimental groups and the sham control groups with MDI, but no significant differences between the sham control groups and normal groups with MDI. MDI increased gradually with prolonging postoperative specimen, and experimental group osteoarthritis performance increased gradually. Gross and histological observations indicate that in the experimental knees, femoral condyle and tibial plateau showed changes in the cartilage over time that are typical of osteoarthritis. Symptoms like softening, erosion and cracking of surface cartilage, proteoglycan metabolism and fracture in the network of collagen fibers appeared gradually. And proliferation and gradual accumulation of chondrocytes after operation led to degeneration and necrosis, which ultimately destroyed the whole cartilage layer covering the bone. Medial meniscus degenerative changes have occurred, and the cilia surface becomes worn and torn. Stromal (scrotal) edema chondrocyte proliferations after necrosis are visible. There are apparent degeneration, twisting and breakage in collagen. In our study, loss of elasticity and relaxation, and dislocation of the entire meniscus were also observed. These show that the degree of meniscal (mental) dislocation gradually increased as osteoarthritis progressed. The results demonstrate that the operation method positioned precisely and produced accurate results. This simple procedure can well simulate the changes of osteoarthritis and is suitable for studying changes in the meniscus of the condition.
Studies have shown that the process of normal cartilage aging and osteoarthritis cartilage degeneration was concurrent with increased metal protease expression, which suggest that matrix metalloproteinases (MMPs) and osteoarthritis cartilage degeneration are closely linked [27]. MMPs were a series of zinc containing protease including MMP-3, MMP-10, MMP-11 and MMP-7. Their activities are dependent on calcium ions, and their main biological effects were degradation of the extracellular matrix. MMP-3 has high cracking activity on proteoglycan. It also activates MMP-1 to accelerate the degradation of the pathological collagen while disconnecting with hyaluronic aggregation (hypertonic aggregate) at the same time [28]. Ribbons and other reported, with articular cartilage erosion in inflammatory arthritis, MMP-3 in synovial fluid levels was elevated, so MMP-3 can be used to monitor the development of the disease process and its active form may be used to indicate extent of damage of joints [29].
TIMP is naturally occurring inhibitor of MMPs and is an important regulatory factor for maintaining normal metabolism of cartilage matrix and conversion. In normal cartilage tissue, the complexes formed between MMPs and TIMPs are kept in a state of balance to prevent the MMPs in the degradation of articular cartilage proteoglycan. Some studies have shown that osteoarthritis synovial membrane and cartilage cells have high expression of MMPs, but TIMP increased very little, and cartilage damage and MMPs/TIMPs imbalance have a positive correlation [30,31].
Studies performed by Janusz (Janus) et al. indicate that MMP inhibitors can reduce the articular cartilage damage in animal models of osteoarthritis. Inferences MMP inhibitors for the treatment of human osteoarthritis, and inhibitingMMPs activity may be an important prevention method and a potential treatment for osteoarthritis [32].
Under experimental conditions and meniscal (meniscus) cartilage of the tibial plateau cartilage type, TIMP-1 level is greater than the elevated levels of MMP-3 the result is MMP-3/TIMP-1 ratio remained unchanged, even at a later stage becomes a small trend Tips cartilage compared with the tibial plateau, TIMP-1 provided strong protective effect in the meniscal (meniscus) cartilage, which may be caused by differences in meniscal (meniscus) cartilage and cartilage cell platform, the specific reasons still need further study.
The experimental group showed significantly increased tibial cartilage MMP-3 levels. We detected that the medial femoral condyle cartilage was destructed by surgery. There are also femoral condyle cartilage cell damage, release of lysosome and MMPs and other lysosomal proteolytic enzymes, degradation of cartilage matrix, collagen network faults, and proteoglycan degradation. These changes cause the femoral condyle cartilage structure and metabolism abnormalities, as well as formation of osteoarthritis change. Femoral condyle cartilage damage breaks the appropriate fitting of the tibiofemoral articular surface, resulting in abnormally high levels of stress on the medial tibiofemoral joint, which in turn increased stress on the medial tibial cartilage. When the rough surface of the femoral condyle and knee tibial cartilage contact directly, the friction caused wearing in tibial cartilage. Synovial fluid released from the femoral condyle cartilage degrading enzymes also acts on a variety of tibial cartilage, and further damage the cartilage. As the cartilage cell are being damaged, above the femoral condyle rickets the same pathological changes were observed in the tibial plateau cartilage, then significant increase in MMP-3 expression were detected.
In summary, our results indicate that osteoarthritis of the knee medial meniscus changes the surrounding environment as well as the biomechanical and biochemical factors in the area. Such changes increase the driving force applied to the outside of the meniscus. As a result, the meniscus degenerates and stress on the medial tibiofemoral joint increased, which can lead to meniscal (meniscus) subluxation. We found that osteoarthritis MMP-3/TIMP-1 expression is an important factor affecting the abnormal meniscus and cartilage degeneration. MMP-3/TIMP-1 abnormal expression of the meniscal (meniscus) and articular cartilage structural damage, with changes in biomechanical properties, the meniscus surface wear, collagen disorders and less flexibility, place medial tibiofemoral joint under more pressure and prone to form a subluxation. Inhibiting the activity of MMPs to intervene above process may reduce the meniscus and cartilage damage, thus delay or even prevent development of the meniscus dislocation.
Acknowledgements
The authors wish to thank all the study participants, research staff and students who participated in this work.
Disclosure of conflict of interest
None.
References
- 1.Solomon LA, Russell BA, Makar D, Berube NG, Beier F. Loss of ATRX does not confer susceptibility to osteoarthritis. PLoS One. 2013;8:e85526. doi: 10.1371/journal.pone.0085526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aigner T, Haag J, Martin J, Buckwalter J. Osteoarthritis: aging of matrix and cells--going for a remedy. Curr Drug Targets. 2007;8:325–331. doi: 10.2174/138945007779940070. [DOI] [PubMed] [Google Scholar]
- 3.Aspden RM. Osteoarthritis: a problem of growth not decay? Rheumatology (Oxford) 2008;47:1452–1460. doi: 10.1093/rheumatology/ken199. [DOI] [PubMed] [Google Scholar]
- 4.Goldring MB, Marcu KB. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther. 2009;11:224. doi: 10.1186/ar2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang M, Shen J, Jin H, Im HJ, Sandy J, Chen D. Recent progress in understanding molecular mechanisms of cartilage degeneration during osteoarthritis. Ann N Y Acad Sci. 2011;1240:61–69. doi: 10.1111/j.1749-6632.2011.06258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruns K, Svensson F, Turkiewicz A, Wirth W, Guermazi A, Eckstein F, Englund M. Meniscus body position and its change over four years in asymptomatic adults: a cohort study using data from the Osteoarthritis Initiative (OAI) BMC Musculoskelet Disord. 2014;15:32. doi: 10.1186/1471-2474-15-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurosawa H, Fukubayashi T, Nakajima H. Load-bearing mode of the knee joint: physical behavior of the knee joint with or without menisci. Clin Orthop Relat Res. 1980:283–290. [PubMed] [Google Scholar]
- 8.Hsu RW, Himeno S, Coventry MB, Chao EY. Normal axial alignment of the lower extremity and load-bearing distribution at the knee. Clin Orthop Relat Res. 1990:215–227. [PubMed] [Google Scholar]
- 9.Bae JY, Park KS, Seon JK, Kwak DS, Jeon I, Song EK. Biomechanical analysis of the effects of medial meniscectomy on degenerative osteoarthritis. Med Biol Eng Comput. 2012;50:53–60. doi: 10.1007/s11517-011-0840-1. [DOI] [PubMed] [Google Scholar]
- 10.Harner CD, Mauro CS, Lesniak BP, Romanowski JR. Biomechanical consequences of a tear of the posterior root of the medial meniscus. Surgical technique. J Bone Joint Surg Am. 2009;91(Suppl 2):257–270. doi: 10.2106/JBJS.I.00500. [DOI] [PubMed] [Google Scholar]
- 11.Marijnissen AC, van Roermund PM, TeKoppele JM, Bijlsma JW, Lafeber FP. The canine ‘groove’ model, compared with the ACLT model of osteoarthritis. Osteoarthritis Cartilage. 2002;10:145–155. doi: 10.1053/joca.2001.0491. [DOI] [PubMed] [Google Scholar]
- 12.Mastbergen SC, Marijnissen AC, Vianen ME, van Roermund PM, Bijlsma JW, Lafeber FP. The canine ‘groove’ model of osteoarthritis is more than simply the expression of surgically applied damage. Osteoarthritis Cartilage. 2006;14:39–46. doi: 10.1016/j.joca.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Mainil-Varlet P, Schiavinato A, Ganster MM. Efficacy Evaluation of a New Hyaluronan Derivative HYADD 4-G to Maintain Cartilage Integrity in a Rabbit Model of Osteoarthritis. Cartilage. 2013;4:28–41. doi: 10.1177/1947603512455193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang XD, Kou XX, He DQ, Zeng MM, Meng Z, Bi RY, Liu Y, Zhang JN, Gan YH, Zhou YH. Progression of cartilage degradation, bone resorption and pain in rat temporomandibular joint osteoarthritis induced by injection of iodoacetate. PLoS One. 2012;7:e45036. doi: 10.1371/journal.pone.0045036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hulmes DJ, Marsden ME, Strachan RK, Harvey RE, McInnes N, Gardner DL. Intra-articular hyaluronate in experimental rabbit osteoarthritis can prevent changes in cartilage proteoglycan content. Osteoarthritis Cartilage. 2004;12:232–238. doi: 10.1016/j.joca.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Bar-Yehuda S, Rath-Wolfson L, Del Valle L, Ochaion A, Cohen S, Patoka R, Zozulya G, Barer F, Atar E, Pina-Oviedo S, Perez-Liz G, Castel D, Fishman P. Induction of an antiinflammatory effect and prevention of cartilage damage in rat knee osteoarthritis by CF101 treatment. Arthritis Rheum. 2009;60:3061–3071. doi: 10.1002/art.24817. [DOI] [PubMed] [Google Scholar]
- 17.Chen DY, Yao L, Chen YM, Lin CC, Huang KC, Chen ST, Lan JL, Hsieh SL. A potential role of myeloid DAP12-associating lectin (MDL)-1 in the regulation of inflammation in rheumatoid arthritis patients. PLoS One. 2014;9:e86105. doi: 10.1371/journal.pone.0086105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kenny C. Radial displacement of the medial meniscus and Fairbank’s signs. Clin Orthop Relat Res. 1997:163–173. doi: 10.1097/00003086-199706000-00022. [DOI] [PubMed] [Google Scholar]
- 19.Sugita T, Kawamata T, Ohnuma M, Yoshizumi Y, Sato K. Radial displacement of the medial meniscus in varus osteoarthritis of the knee. Clin Orthop Relat Res. 2001:171–177. doi: 10.1097/00003086-200106000-00023. [DOI] [PubMed] [Google Scholar]
- 20.Martin JA, Buckwalter JA. Roles of articular cartilage aging and chondrocyte senescence in the pathogenesis of osteoarthritis. Iowa Orthop J. 2001;21:1–7. [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson DR, Newman AP, Daniels AU. In vitro load transmission in the canine knee: the effect of medial meniscectomy and varus rotation. Knee Surg Sports Traumatol Arthrosc. 1993;1:44–50. doi: 10.1007/BF01552158. [DOI] [PubMed] [Google Scholar]
- 22.Voloshin AS, Wosk J. Shock absorption of meniscectomized and painful knees: a comparative in vivo study. J Biomed Eng. 1983;5:157–161. doi: 10.1016/0141-5425(83)90036-5. [DOI] [PubMed] [Google Scholar]
- 23.Cicuttini FM, Forbes A, Yuanyuan W, Rush G, Stuckey SL. Rate of knee cartilage loss after partial meniscectomy. J Rheumatol. 2002;29:1954–1956. [PubMed] [Google Scholar]
- 24.Verdonk PC, Verstraete KL, Almqvist KF, De Cuyper K, Veys EM, Verbruggen G, Verdonk R. Meniscal allograft transplantation: long-term clinical results with radiological and magnetic resonance imaging correlations. Knee Surg Sports Traumatol Arthrosc. 2006;14:694–706. doi: 10.1007/s00167-005-0033-2. [DOI] [PubMed] [Google Scholar]
- 25.Wilson AS, Legg PG, McNeur JC. Studies on the innervation of the medial meniscus in the human knee joint. Anat Rec. 1969;165:485–491. doi: 10.1002/ar.1091650404. [DOI] [PubMed] [Google Scholar]
- 26.Annandale T. An Operation for Displaced Semilunar Cartilage. Br Med J. 1885;1:779. doi: 10.1136/bmj.1.1268.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baratz ME, Fu FH, Mengato R. Meniscal tears: the effect of meniscectomy and of repair on intraarticular contact areas and stress in the human knee. A preliminary report. Am J Sports Med. 1986;14:270–275. doi: 10.1177/036354658601400405. [DOI] [PubMed] [Google Scholar]
- 28.Messner K, Fahlgren A, Ross I, Andersson B. Simultaneous changes in bone mineral density and articular cartilage in a rabbit meniscectomy model of knee osteoarthrosis. Osteoarthritis Cartilage. 2000;8:197–206. doi: 10.1053/joca.1999.0290. [DOI] [PubMed] [Google Scholar]
- 29.Messner K, Fahlgren A, Persliden J, Andersson BM. Radiographic joint space narrowing and histologic changes in a rabbit meniscectomy model of early knee osteoarthrosis. Am J Sports Med. 2001;29:151–160. doi: 10.1177/03635465010290020701. [DOI] [PubMed] [Google Scholar]
- 30.McNicholas MJ, Rowley DI, McGurty D, Adalberth T, Abdon P, Lindstrand A, Lohmander LS. Total meniscectomy in adolescence. A thirty-year follow-up. J Bone Joint Surg Br. 2000;82:217–221. [PubMed] [Google Scholar]
- 31.Petersen MM, Olsen C, Lauritzen JB, Lund B, Hede A. Late changes in bone mineral density of the proximal tibia following total or partial medial meniscectomy. A randomized study. J Orthop Res. 1996;14:16–21. doi: 10.1002/jor.1100140105. [DOI] [PubMed] [Google Scholar]
- 32.Quinby JS, Golish SR, Hart JA, Diduch DR. All-inside meniscal repair using a new flexible, tensionable device. Am J Sports Med. 2006;34:1281–1286. doi: 10.1177/0363546505286143. [DOI] [PubMed] [Google Scholar]


