Abstract
The association between MMP1 -1607 1G>2G polymorphism and cancer risk has been reported, but results remained controversial and ambiguous. To assess the association between MMP1 -1607 1G>2G polymorphism and cancer risk, a meta-analysis was performed. Based on comprehensive searches of the PubMed, Elsevier Science Direct, Excerpta Medica Database (Embase), and Chinese Biomedical Literature Database (CBM), we identified outcome data from all articles estimating the association between MMP1 -1607 1G>2G polymorphism and cancer risk. The pooled odds ratio (OR) with 95% confidence intervals (CIs) were calculated. Thirty-eight studies involving 10178 cases and 9528 controls were included. Overall, significant association between MMP1 -1607 1G>2G polymorphism and cancer susceptibility was observed for additive model (OR = 1.21, 95% CI 1.09-1.35), for codominant model (OR = 1.34, 95% CI 1.10-1.63), for dominant model (OR = 1.17, 95% CI 1.01-1.34), for recessive model (OR = 1.31, 95% CI 1.14-1.52). In the subgroup analysis by ethnicity, the significant association was found among Asians but not among Caucasians. In the subgroup analysis by site of cancer, significant associations were found among lung cancer, colorectal cancer, head and neck cancer and bladder cancer. This meta-analysis demonstrated that the MMP1 -1607 1G>2G polymorphism was significantly associated with cancer risk.
Keywords: Cancer, MMP1, meta-analysis, genetics
Introduction
Cancer is a disease resulting from complex interactions between environmental and genetic factors [1,2]. Genetic factors, including the sequence alterations and organization aberrations of the cellular genome that range from single-nucleotide substitutions to gross chromosome, could modulate several important biological progress and alert susceptibility to cancer consequently.
Matrix metalloproteinase (MMP) is a family of zinc-dependent endopeptidases that are able to degrade essentially all extracellular matrix (ECM) components, such as basement membranes, collagen, and fibronectin [3,4]. The human MMPs family, which consists of at least 26 proteases, can be divided into several subgroups according to their structure and substrate specificity [5]. Among the MMPs, MMP1 is the most highly expressed interstitial collagenase degrading fibrillar collagens, which are major constituents of the extracellular matrix. The level of MMP1 expression can be affected by single nucleotide polymorphism (SNP). An SNP of the MMP1 gene occurs at position 1607 bp upstream of the transcriptional initiation site. An insertion of a guanine base (G) creates the sequence 5’-GGAT-3’, the core binding site for members of the EST family of transcription factors [6]. MMP1 -1607 2G allele has been associated with higher transcriptional activity of the gene [6].
To identify whether the MMP1 -1607 1G>2G polymorphism is involved in the pathogenesis of tumors in vivo, many case-control studies concerning this allelic variation and cancer risk have been broadly performed [7-44]. However, there is still uncertainty about the level of risk for a variety of cancers in a number of studies investigating the effect of -1607 1G>2G polymorphism on different types of cancers and ethnic populations. Therefore, we performed a meta-analysis to identify statistical evidence for an association between the MMP1 -1607 1G>2G polymorphism and cancer risk using all published data to date.
Methods
Publication search and inclusion criteria
Data were collected from the following electronic databases: PubMed, Elsevier Science Direct, Excerpta Medica Database (Embase), and Chinese Biomedical Literature Database (CBM). We searched the articles using the search terms “matrix metalloproteinase 1 or MMP1”, ”polymorphism or SNP”, ”cancer or neoplasm or carcinoma”. Additional studies were identified by a hand search of references of original studies and review articles. No languagerestrictions were applied. A study was included in the current meta-analysis if (1) it was published up to June, 2014; (2) it was a case-control study of the MMP1 -1607 1G>2G polymorphism and cancer risk. We excluded the study in which family members were studied. When there were multiple studies from the same population, only the largest study was included.
Data extraction
Two investigators independently extracted data from the included studies. Data extracted from eligible studies included the first author’s name, publication date, country origin, ethnicity, site of tumor, total numbers of cases and controls. The two investigators checked the data extraction results and reached consensus on all of the data extracted. If different results were generated, they would check the data and have a discussion to come to an agreement.
Statistical analysis
Hardy-Weinberg equilibrium (HWE) in controls in each study was calculated by chi-squared test. P value < 0.05 was considered a departure from HWE. The association between MMP1 -1607 1G>2G polymorphism and cancer risk was estimated by the odds ratio (OR), together with the 95% confidence interval (95% CI). The significance of the pooled OR was determined by the Z test, with P < 0.05 considered significant. Stratified analysis was also performed by ethnicity and cancer site. We estimated the ORs in the dominant model, recessive model, codominant model, and additive model.
Heterogeneity between studies was assessed by Q test. If P < 0.1, the heterogeneity was considered statistically significant. The I2 values were used to quantify the percentage of the total variation among studies when heterogeneitywas assessed. When I2 < 50%, a fixed effects model was applied to estimate the pooled results. Otherwise, the random-effect model was used. Sensitivity analysis was carried out by removing each study at a time to evaluate the stability of the results. Publication bias was analyzed by performing Egger’s test quantitatively [45]. All statistical analysis was conducted using STATA software (version 11.0; STATA Corporation, College Station, TX). Two sided P-values < 0.05 were considered statistically significant.
Results
Characteristics of the included studies
A total of 322 articles were retrieved after first search in PubMed, Elsevier Science Direct, Embase, and CBM. As shown in Figure 1, after our selection, 38 case-control studies fulfilled the inclusion criteria [7-44]. Characteristics of included studies are summarized in Table 1. There were 16 studies used Caucasians and 22 studies used Asians. Thirteen studies investigated head and neck cancer, four studies investigated bladder cancer, four studies investigatedcolorectal cancer, and eight studies investigated lung cancer.
Figure 1.
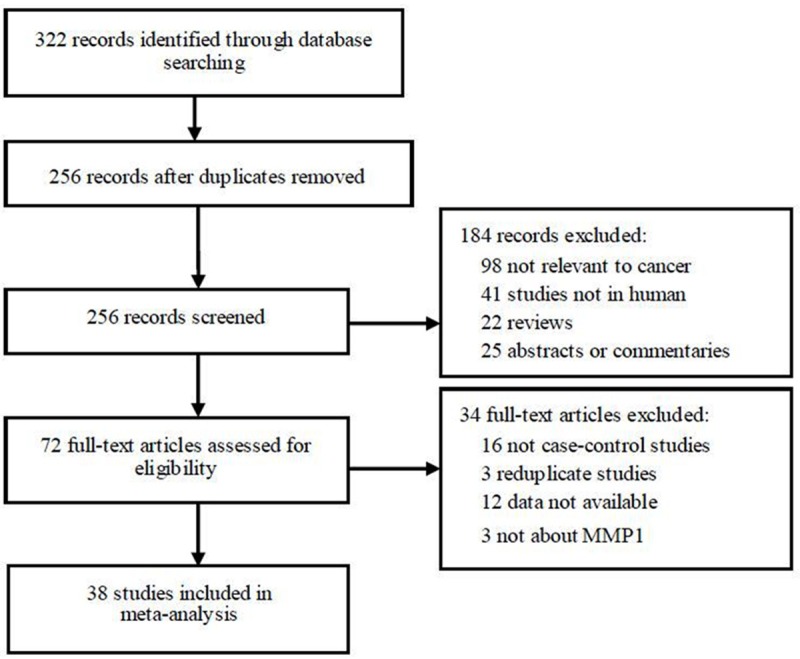
Flow diagram of study identification.
Table 1.
Characteristics of the studies included in this meta-analysis
| First author | Year | Country | Race | Site | Case | Control | HWE |
|---|---|---|---|---|---|---|---|
| Ye | 2001 | UK | Caucasian | Mixed | 142 | 139 | Yes |
| Hinoda | 2002 | Japan | Asian | Colorectal | 127 | 101 | Yes |
| Ghilardi | 2002 | Italy | Caucasian | Mixed | 110 | 86 | Yes |
| Hirata | 2003 | Japan | Asian | Mixed | 210 | 119 | Yes |
| Hashimoto | 2004 | Japan | Asian | Head and neck | 568 | 140 | Yes |
| Zinzindohoue | 2004 | France | Caucasian | Head and neck | 249 | 129 | Yes |
| Lin | 2004 | China | Asian | Head and neck | 147 | 121 | Yes |
| Matsumura | 2004 | Japan | Asian | Mixed | 166 | 215 | Yes |
| Ju | 2005 | Korea | Asian | Mixed | 332 | 232 | Yes |
| Su | 2005 | USA | Caucasian | Lung | 1323 | 2014 | Yes |
| Kondo | 2005 | Japan | Asian | Head and neck | 82 | 83 | Yes |
| McCready | 2005 | USA | Caucasian | Head and neck | 57 | 81 | Yes |
| Lai | 2005 | China | Asian | Mixed | 197 | 197 | Yes |
| Cao | 2006 | China | Asian | Head and neck | 120 | 96 | Yes |
| Zhang | 2006 | China | Asian | Lung | 200 | 150 | Yes |
| O-charoenrat | 2006 | Thailand | Asian | Head and neck | 300 | 300 | Yes |
| Elander | 2006 | Sweden | Caucasian | Colorectal | 208 | 127 | Yes |
| Woo | 2006 | Korea | Asian | Colorectal | 304 | 185 | Yes |
| Przybylowska | 2006 | Poland | Caucasian | Mixed | 129 | 141 | Yes |
| Kader | 2006 | USA | Caucasian | Bladder | 555 | 556 | Yes |
| Cheng | 2007 | China | Asian | Lung | 130 | 127 | Yes |
| Ju | 2007 | Korea | Asian | Mixed | 332 | 133 | Yes |
| Wei | 2007 | China | Asian | Lung | 75 | 71 | Yes |
| Vairaktaris | 2007 | Greece | Caucasian | Head and neck | 141 | 156 | Yes |
| Tasci | 2007 | Turkey | Asian | Bladder | 94 | 102 | Yes |
| Shimizu | 2008 | Japan | Asian | Head and neck | 91 | 69 | Yes |
| Patricia | 2008 | Spain | Asian | Lung | 510 | 501 | Yes |
| Kouhkan | 2008 | Iran | Asian | Colorectal | 100 | 150 | Yes |
| Penelope | 2009 | USA | Caucasian | Head and neck | 455 | 313 | Yes |
| Dos Reis | 2009 | Brazil | Caucasian | Mixed | 100 | 100 | Yes |
| Vairaktaris | 2009 | Greece | Caucasian | Head and neck | 168 | 162 | Yes |
| Srivastava | 2010 | India | Asian | Bladder | 200 | 200 | Yes |
| Chaudhary | 2010 | India | Asian | Head and neck | 426 | 422 | Yes |
| Liu | 2011 | China | Asian | Lung | 825 | 825 | Yes |
| Hart | 2011 | Norway | Caucasian | Lung | 434 | 436 | Yes |
| Cheung | 2012 | Canada | Caucasian | Head and neck | 279 | 309 | Yes |
| Fakhoury | 2012 | USA | Asian | Lung | 51 | 41 | Yes |
| Wieczorek | 2013 | Poland | Caucasian | Bladder | 241 | 199 | Yes |
HWE, Hardy-Weinberg equilibrium.
Results of meta-analysis
The overall OR for 2G versus 1G (additive model) was 1.21 (95% CI 1.09-1.35). This result suggested that individuals who carry the 2G allele may have a 21% increased cancer risk compared with 1G allele carrier. When all the studies were pooled into meta-analysis using other genetic models (Table 2), there was also significant association between MMP1 -1607 1G>2G polymorphism and cancer risk (for codominant model: OR = 1.34, 95% CI 1.10-1.63; for dominant model: OR = 1.17, 95% CI 1.01-1.34; for recessive model: OR = 1.31, 95% CI 1.14-1.52).
Table 2.
Main results of meta-analysis
| No. of study | Case/control | 2G vs. 1G | 2G2G vs. 1G1G | 2G2G+1G2G vs. 1G1G | 2G2G vs. 2G1G+1G1G | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| OR (95% CI) | Pheterogeneity | OR (95% CI) | Pheterogeneity | OR (95% CI) | Pheterogeneity | OR (95% CI) | Pheterogeneity | |||
| Overall | 38 | 10178/9528 | 1.21 (1.09-1.35) | < 0.001 | 1.34 (1.10-1.63) | < 0.001 | 1.17 (1.01-1.34) | < 0.001 | 1.31 (1.14-1.52) | < 0.001 |
| Site | ||||||||||
| Head and neck cancer | 13 | 3083/2381 | 1.20 (1.03-1.31) | < 0.001 | 1.27 (0.80-2.00) | < 0.001 | 1.07 (0.77-1.50) | < 0.001 | 1.34 (1.02-1.67) | < 0.001 |
| Lung cancer | 8 | 3548/4165 | 1.16 (1.01-1.34) | 0.004 | 1.28 (1.00-1.63) | 0.027 | 1.12 (1.00-1.25) | 0.189 | 1.22 (1.01-1.49) | 0.004 |
| Bladder cancer | 4 | 1103/1053 | 1.59 (0.88-2.86) | < 0.001 | 2.18 (0.77-6.16) | < 0.001 | 1.62 (0.74-3.57) | < 0.001 | 1.44 (1.05-1.97) | < 0.001 |
| Colorectal cancer | 4 | 739/563 | 1.59 (1.34-1.88) | 0.812 | 2.22 (1.52-3.24) | 0.902 | 1.67 (1.19-2.34) | 0.965 | 1.85 (1.46-2.34) | 0.784 |
| Race | ||||||||||
| Caucasian | 15 | 5101/5449 | 1.04 (0.90-1.19) | < 0.001 | 1.09 (0.84-1.41) | < 0.001 | 1.08 (0.91-1.28) | 0.001 | 1.03 (0.84-1.26) | < 0.001 |
| Asian | 22 | 5077/4079 | 1.36 (1.17-1.58) | < 0.001 | 1.59 (1.19-2.13) | < 0.001 | 1.27 (1.01-1.61) | < 0.001 | 1.56 (1.30-1.87) | < 0.001 |
In the subgroup analyses by ethnicity, the significant association was found among Asians (OR = 1.36, 95% CI 1.17-1.58) but not among Caucasians (OR = 1.04, 95% CI 0.90-1.19) in additive model (2G vs. 1G). In the subgroup analysis by site of cancer, MMP1 -1607 1G>2G polymorphism was significantly associated with lung cancer and colorectal cancer in each genetic models. In addition, this polymorphism increased bladder cancer risk in the recessive model (OR = 1.44, 95% CI 1.05-1.97) and head and neck cancer risk in the recessive model and additive model (Table 2).
Every single study involved in this meta-analysis was deleted each time to examine the influence of the individual data set to the pooled ORs. Elimination of each study made no qualitative difference on the pooled OR values, which indicated that the final results of the meta-analysis were stable (data not shown). Egger’s test further confirmed the absence of publication bias in this meta-analysis (P > 0.05).
Discussion
This current meta-analysis of 38 studies including 10178 cases and 9528 controls systematically evaluated the association between MMP1 -1607 1G>2G polymorphism and cancer risk. The results indicated that -1607 1G>2G polymorphism was a conspicuous high risk factor for developing cancer in the overall study populations. In the subgroup analysis by ethnicity, no significant association was found in Caucasians. However, cancer risk was increased in Asians who carried 2G allele, suggesting a possible influence among environmental exposures and different genetic backgrounds. After stratification by site, this association remained significant in lung cancer, colorectal cancer, head and neck cancer and bladder cancer. This result indicated that MMP1 -1607 1G>2G polymorphism might play a same role in the etiology of different cancers.
Functional analyses have shown that the expression level of MMP1 depends on the genetic variation within the promoter of the MMP1 gene. The -1607 2G allele is thought to form the core of a consensus DNA element recognizedby the Ets transcription factor, which up-regulates MMP1 transcription [6]. Further investigations of association of -1607 1G>2G with allelic expression imbalance suggest that this polymorphism does not account for all differencesin allelic expression observed [46]. Transcription of a gene is more likely to be influenced by multiple polymorphisms, and these are hypothesized by some authors to be located in the promoter of that gene, which acts in concert to exert a haplotype effect [47]. Pearce et al. [48] investigated the promoter region in detail and found that the -1607 1G>2G deletion alone cannot fully segregate the various MMP1 haplotypes that differ in promoter activity. Thus, the mechanism was still unclear and this issue should be investigated in the future studies.
Some limitations should be addressed. First, in this meta-analysis, we found obvious heterogeneity across studies. Importantly, it should be acknowledged that potential heterogeneity and bias may distort the results. Therefore, results from this meta-analysis should be interpreted with caution. Second, due to lacking of the original data of the eligible studies, we could not perform other subgroup analyses based on age, smoking, and so on. Third, cancer is a multifactorial disease and potential interactions among gene-gene and gene-environment should be considered.
In conclusion, a significant association was detected between the MMP1 -1607 1G>2G polymorphism and cancer risk. Moreover, further studies with large sample size of different ethnic populations and cancer types will be necessary to validate this result.
Disclosure of conflict of interest
None.
References
- 1.Bredberg A. Cancer: more of polygenic disease and less of multiple mutations? A quantitative viewpoint. Cancer. 2011;117:440–5. doi: 10.1002/cncr.25440. [DOI] [PubMed] [Google Scholar]
- 2.Pharoah PD, Dunning AM, Ponder BA, Easton DF. Association studies for finding cancer-susceptibility genetic variants. Nat Rev Cancer. 2004;4:850–60. doi: 10.1038/nrc1476. [DOI] [PubMed] [Google Scholar]
- 3.Stetler-Stevenson WG, Liotta LA, Kleiner DE. Extra cellular matrix 6: role of matrix metalloproteinases in tumor invasion and metastasis. Faseb J. 1993;7:1434–341. doi: 10.1096/fasebj.7.15.8262328. [DOI] [PubMed] [Google Scholar]
- 4.Stetler-Stevenson WG, Yu AE. Proteases in invasion: matrix metalloproteinases. Semin Cancer Biol. 2001;11:143–52. doi: 10.1006/scbi.2000.0365. [DOI] [PubMed] [Google Scholar]
- 5.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 6.Rutter JL, Mitchell TI, Buttice G, Meyers J, Gusella JF, Ozelius LJ, Brinckerhoff CE. A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter creates an Ets binding site and augments transcription. Cancer Res. 1998;58:5321–5. [PubMed] [Google Scholar]
- 7.Ye S, Dhillon S, Turner SJ, Bateman AC, Theaker JM, Pickering RM, Day I, Howell WM. Invasiveness of cutaneous malignant melanoma is influenced by matrix metalloproteinase 1 gene polymorphism. Cancer Res. 2001;61:1296–8. [PubMed] [Google Scholar]
- 8.Hinoda Y, Okayama N, Takano N, Fujimura K, Suehiro Y, Hamanaka Y, Hazama S, Kitamura Y, Kamatani N, Oka M. Association of functional polymorphisms of matrix metalloproteinase (MMP)-1 and MMP-3 genes with colorectal cancer. Int J Cancer. 2002;102:526–9. doi: 10.1002/ijc.10750. [DOI] [PubMed] [Google Scholar]
- 9.Ghilardi G, Biondi ML, Caputo M, Leviti S, DeMonti M, Guagnellini E, Scorza R. A single nucleotide polymorphism in the matrix metalloproteinase-3 promoter enhances breast cancer susceptibility. Clin Cancer Res. 2002;8:3820–3. [PubMed] [Google Scholar]
- 10.Hirata H, Naito K, Yoshihiro S, Matsuyama H, Suehiro Y, Hinoda Y. A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter is associated with conventional renal cell carcinoma. Int J Cancer. 2003;106:372–4. doi: 10.1002/ijc.11229. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto T, Uchida K, Okayama N, Imate Y, Suehiro Y, Hamanaka Y, Ueyama Y, Shinozaki F, Yamashita H, Hinoda Y. Association of matrix metalloproteinase (MMP)-1 promoter polymorphism with head and neck squamous cell carcinoma. Cancer Lett. 2004;211:19–24. doi: 10.1016/j.canlet.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 12.Zinzindohoue F, Blons H, Hans S, Loriot MA, Houllier AM, Brasnu D, Laccourreye O, Tregouet DA, Stucker I, Laurent-Puig P. Single nucleotide polymorphisms in MMP1 and MMP3 gene promoters as risk factor in head and neck squamous cell carcinoma. Anticancer Res. 2004;24:2021–6. [PubMed] [Google Scholar]
- 13.Lin SC, Chung MY, Huang JW, Shieh TM, Liu CJ, Chang KW. Correlation between functional genotypes in the matrix metalloproteinases-1 promoter and risk of oral squamous cell carcinomas. J Oral Pathol Med. 2004;33:323–6. doi: 10.1111/j.1600-0714.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 14.Matsumura S, Oue N, Nakayama H, Kitadai Y, Yoshida K, Yamaguchi Y, Imai K, Nakachi K, Matsusaki K, Chayama K, Yasui W. A single nucleotide polymorphism in the MMP-9 promoter affects tumor progression and invasive phenotype of gastric cancer. J Cancer Res Clin Oncol. 2005;131:19–25. doi: 10.1007/s00432-004-0621-4. [DOI] [PubMed] [Google Scholar]
- 15.Ju W, Kang S, Kim JW, Park NH, Song YS, Kang SB, Lee HP. Promoter polymorphism in the matrix metalloproteinase-1 and risk of cervical cancer in Korean women. Cancer Lett. 2005;217:191–6. doi: 10.1016/j.canlet.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 16.Su L, Zhou W, Asomaning K, Lin X, Wain JC, Lynch TJ, Liu G, Christiani DC. Genotypes and haplotypes of matrix metalloproteinase 1, 3 and 12 genes and the risk of lung cancer. Carcinogenesis. 2006;27:1024–9. doi: 10.1093/carcin/bgi283. [DOI] [PubMed] [Google Scholar]
- 17.Kondo S, Wakisaka N, Schell MJ, Horikawa T, Sheen TS, Sato H, Furukawa M, Pagano JS, Yoshizaki T. Epstein-Barr virus latent membrane protein 1 induces the matrix metalloproteinase-1 promoter via an Ets binding site formed by a single nucleotide polymorphism: enhanced susceptibility to nasopharyngeal carcinoma. Int J Cancer. 2005;115:368–76. doi: 10.1002/ijc.20849. [DOI] [PubMed] [Google Scholar]
- 18.McCready J, Broaddus WC, Sykes V, Fillmore HL. Association of a single nucleotide polymorphism in the matrix metalloproteinase-1 promoter with glioblastoma. Int J Cancer. 2005;117:781–5. doi: 10.1002/ijc.21207. [DOI] [PubMed] [Google Scholar]
- 19.Lai HC, Chu CM, Lin YW, Chang CC, Nieh S, Yu MH, Chu TY. Matrix metalloproteinase 1 gene polymorphism as a prognostic predictor of invasive cervical cancer. Gynecol Oncol. 2005;96:314–9. doi: 10.1016/j.ygyno.2004.09.065. [DOI] [PubMed] [Google Scholar]
- 20.Cao ZG, Li CZ. A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter enhances oral squamous cell carcinoma susceptibility in a Chinese population. Oral Oncol. 2006;42:32–8. doi: 10.1016/j.oraloncology.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Zhang WQ, Lin H, Zhou YA, Wang YJ, Cheng QS. Association of MMP1-1607 (1G>2G) single nucleotide polymorphism with susceptibility to lung cancer in Northwestern Chinese population of Han nationality. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2006;23:313–5. [PubMed] [Google Scholar]
- 22.O-charoenrat P, Leksrisakul P, Sangruchi S. A functional polymorphism in the matrix metalloproteinase-1 gene promoter is associated with susceptibility and aggressiveness of head and neck cancer. Int J Cancer. 2006;118:2548–53. doi: 10.1002/ijc.21644. [DOI] [PubMed] [Google Scholar]
- 23.Elander N, Soderkvist P, Fransen K. Matrix metalloproteinase (MMP) -1, -2, -3 and -9 promoter polymorphisms in colorectal cancer. Anticancer Res. 2006;26:791–5. [PubMed] [Google Scholar]
- 24.Woo M, Park K, Nam J, Kim JC. Clinical implications of matrix metalloproteinase-1, -3, -7, -9, -12, and plasminogen activator inhibitor-1 gene polymorphisms in colorectal cancer. J Gastroenterol Hepatol. 2007;22:1064–70. doi: 10.1111/j.1440-1746.2006.04424.x. [DOI] [PubMed] [Google Scholar]
- 25.Przybylowska K, Kluczna A, Zadrozny M, Krawczyk T, Kulig A, Rykala J, Kolacinska A, Morawiec Z, Drzewoski J, Blasiak J. Polymorphisms of the promoter regions of matrix metalloproteinases genes MMP-1 and MMP-9 in breast cancer. Breast Cancer Res Treat. 2006;95:65–72. doi: 10.1007/s10549-005-9042-6. [DOI] [PubMed] [Google Scholar]
- 26.Kader AK, Shao L, Dinney CP, Schabath MB, Wang Y, Liu J, Gu J, Grossman HB, Wu X. Matrix metalloproteinase polymorphisms and bladder cancer risk. Cancer Res. 2006;66:11644–8. doi: 10.1158/0008-5472.CAN-06-1212. [DOI] [PubMed] [Google Scholar]
- 27.Cheng XL. Correlation of single nucleotide polymorphism in them atrixmetalloproteinase-1 gene promoter (-1607) 1G/2G with risk of lung cancer. J Shanxi Med Univ. 2007:777–9. [Google Scholar]
- 28.Ju W, Kim JW, Park NH, Song YS, Kim SC, Kang SB, Lee HP. Matrix metalloproteinase-1 promoter polymorphism and epithelial ovarian cancer: does ethnicity matter? J Obstet Gynaecol Res. 2007;33:155–60. doi: 10.1111/j.1447-0756.2007.00511.x. [DOI] [PubMed] [Google Scholar]
- 29.Wei WQ, Liang XQ, Wen XP. The Association of MMP-1-1607 (1G>2G) single nucleotide polymorphism with the susceptibility to non-small cell lung carcinoma in Guizhou Han nationality. J Guiyang Med Coll. 2007:267–9. [Google Scholar]
- 30.Vairaktaris E, Yapijakis C, Derka S, Serefoglou Z, Vassiliou S, Nkenke E, Ragos V, Vylliotis A, Spyridonidou S, Tsigris C, Yannopoulos A, Tesseromatis C, Neukam FW, Patsouris E. Association of matrix metalloproteinase-1 (-1607 1G/2G) polymorphism with increased risk for oral squamous cell carcinoma. Anticancer Res. 2007;27:459–64. [PubMed] [Google Scholar]
- 31.Tasci AI, Tugcu V, Ozbek E, Ozbay B, Simsek A, Koksal V. A single-nucleotide polymorphism in the matrix metalloproteinase-1 promoter enhances bladder cancer susceptibility. Bju Int. 2008;101:503–7. doi: 10.1111/j.1464-410X.2007.07315.x. [DOI] [PubMed] [Google Scholar]
- 32.Shimizu Y, Kondo S, Shirai A, Furukawa M, Yoshizaki T. A single nucleotide polymorphism in the matrix metalloproteinase-1 and interleukin-8 gene promoter predicts poor prognosis in tongue cancer. Auris Nasus Larynx. 2008;35:381–9. doi: 10.1016/j.anl.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Arriaga P, Lopez-Cima MF, Fernandez-Somoano A, Pascual T, Marron MG, Puente XS, Tardón A. Polymorphism +17 C/G in matrix metalloprotease MMP8 decreases lung cancer risk. BMC Cancer. 2008;8:378. doi: 10.1186/1471-2407-8-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kouhkan F, Motovali-Bashi M, Hojati Z. The influence of interstitial collagenase-1 genotype polymorphism on colorectal cancer risk in Iranian population. Cancer Invest. 2008;26:836–42. doi: 10.1080/07357900801953204. [DOI] [PubMed] [Google Scholar]
- 35.Bradbury PA, Zhai R, Hopkins J, Kulke MH, Heist RS, Singh S, Zhou W, Ma C, Xu W, Asomaning K, Ter-Minassian M, Wang Z, Su L, Christiani DC, Liu G. Matrix metalloproteinase 1, 3 and 12 polymorphisms and esophageal adenocarcinoma risk and prognosis. Carcinogenesis. 2009;30:793–8. doi: 10.1093/carcin/bgp065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dos Reis ST, Pontes J Jr, Villanova FE, Borra PM, Antunes AA, Dall’oglio MF, Srougi M, Leite KR. Genetic polymorphisms of matrix metalloproteinases: susceptibility and prognostic implications for prostate cancer. J Urol. 2009;181:2320–5. doi: 10.1016/j.juro.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Vairaktaris E, Serefoglou Z, Avgoustidis D, Yapijakis C, Critselis E, Vylliotis A, Spyridonidou S, Derka S, Vassiliou S, Nkenke E, Patsouris E. Gene polymorphisms related to angiogenesis, inflammation and thrombosis that influence risk for oral cancer. Oral Oncol. 2009;45:247–53. doi: 10.1016/j.oraloncology.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Srivastava P, Gangwar R, Kapoor R, Mittal RD. Bladder cancer risk associated with genotypic polymorphism of the matrix metalloproteinase-1 and 7 in North Indian population. Dis Markers. 2010;29:37–46. doi: 10.3233/DMA-2010-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaudhary AK, Singh M, Bharti AC, Asotra K, Sundaram S, Mehrotra R. Genetic polymorphisms of matrix metalloproteinases and their inhibitors in potentially malignant and malignant lesions of the head and neck. J Biomed Sci. 2010;17:10. doi: 10.1186/1423-0127-17-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu L, Wu J, Wu C, Wang Y, Zhong R, Zhang X, Tan W, Nie S, Miao X, Lin D. A functional polymorphism (-1607 1G-->2G) in the matrix metalloproteinase-1 promoter is associated with development and progression of lung cancer. Cancer. 2011;117:5172–81. doi: 10.1002/cncr.26154. [DOI] [PubMed] [Google Scholar]
- 41.Hart K, Landvik NE, Lind H, Skaug V, Haugen A, Zienolddiny S. A combination of functional polymorphisms in the CASP8, MMP1, IL10 and SEPS1 genes affects risk of non-small cell lung cancer. Lung Cancer. 2011;71:123–9. doi: 10.1016/j.lungcan.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 42.Cheung WY, Zhai R, Bradbury P, Hopkins J, Kulke MH, Heist RS, Asomaning K, Ma C, Xu W, Wang Z, Hooshmand S, Su L, Christiani DC, Liu G. Single nucleotide polymorphisms in the matrix metalloproteinase gene family and the frequency and duration of gastroesophageal reflux disease influence the risk of esophageal adenocarcinoma. Int J Cancer. 2012;131:2478–86. doi: 10.1002/ijc.27541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fakhoury H, Noureddine S, Chmaisse HN, Tamim H, Makki RF. MMP1-1607 (1G>2G) polymorphism and the risk of lung cancer in Lebanon. Ann Thorac Med. 2012;7:130–2. doi: 10.4103/1817-1737.98844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wieczorek E, Reszka E, Jablonowski Z, Jablonska E, Beata Krol M, Grzegorczyk A, Gromadzinska J, Sosnowski M, Wasowicz W. Genetic polymorphisms in matrix metalloproteinases (MMPs) and tissue inhibitors of MPs (TIMPs), and bladder cancer susceptibility. BJU Int. 2013;112:1207–14. doi: 10.1111/bju.12230. [DOI] [PubMed] [Google Scholar]
- 45.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heighway J, Bowers NL, Smith S, Betticher DC, Koref MF. The use of allelic expression differences to ascertain functional polymorphisms acting in cis: analysis of MMP1 transcripts in normal lung tissue. Ann Hum Genet. 2005;69:127–33. doi: 10.1046/j.1529-8817.2004.00135.x. [DOI] [PubMed] [Google Scholar]
- 47.Terry CF, Loukaci V, Green FR. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J Biol Chem. 2000;275:18138–44. doi: 10.1074/jbc.M000379200. [DOI] [PubMed] [Google Scholar]
- 48.Pearce EG, Laxton RC, Pereira AC, Ye S. Haplotype effects on matrix metalloproteinase-1 gene promoter activity in cancer cells. Mol Cancer Res. 2007;5:221–7. doi: 10.1158/1541-7786.MCR-06-0139. [DOI] [PubMed] [Google Scholar]


