Abstract
Aim: To investigate the clinical features of airway malacia in children. Material and methods: A comprehensive analysis was done on information of 459 young patients with airway malacia. Results: Number of children with tracheomalacia, tracheobronchomalacia, and bronchomalacia was 7 (1.5%), 17 (3.7%), and 435 (94.8%), respectively. Incidence of bronchomalacia in left lung was 11.0% (n=48), while that of right lung was 53.3% (n=232). Meanwhile, bronchomalacia of both lungs were noticed in 155 children (35.6%). With regards to the extent of malacia, number of children with slight, moderate and severe malacia was 226, 195, and 38, respectively. All the children enrolled in this study were diagnosed with pulmonary infection, among which 376 were diagnosed with ordinary pneumonia, 83 were diagnosed with severe pneumonia. 227 children showed a disease course of less than 1 month, while 201 children reported a disease course of 1~3 months, and 31 children reported a disease course of more than 3 months. Statistical difference was noticed in the disease condition of respiratory tract infection of patients with various malacia extent (P < 0.05). Re-check of fiberoptic bronchoscopy was performed in 19 patients, among which 14 patients (73.7%) showed improvement compared with the previous conditions. Conclusion: Airway malacia has been frequently noticed in male children aged ≤ 2 years old. Patients with severe airway malacia were apt to develop severe pneumonia compared with those with slight or moderate malacia. Improvements or even elimination of malacia were noticed with the aging of the children and the anti-infection therapy.
Keywords: Fiexible bronchofiberscope, airway malacia, clinical features, children
Introduction
Tracheomalacia (TM) is commonly acknowledged as a weakness of the trachea caused by reduction and/or atrophy of the cartilage integrity. If the mainstem bronchi are involved in the tracheomalacia, it is usually called tracheobronchomalacia (TBM). Bronchomalacia (BM) refers to the malacia of bronchi with no involvement of the trachea [1]. Airway malacia has been considered as a major cause for repeated or persistent gasping, chronic cough, and repeated respiratory tract infection in pediatrics [1]. Despite increasing recognition of airway malacia, diagnosis using routine examinations has been impossible as the clinical symptoms of airway malacia were vague [2]. Meanwhile, patients with airway malacia have been commonly misdiagnosed as bronchial asthma, respiratory tract infection, as well as foreign body in bronchus. To date, flexible bronchofiberscope has been considered as the golden standard for the diagnosis of airway malacia in pediatrics [3]. To our knowledge, two types of airway malacia (i.e. primary and secondary airway malacia) are available according to the cause of the disease. In clinical practices,many patients have been over-diagnosed with primary airway malacia due to a neglect of infection. Currently, most of the airway malacia studies focus on the clinical diagnosis, analysis of disease cause, and the treatment [4,5]. Few studies in a large sample size are carried out to investigate the clinical features of patients with airway malacia after flexible bronchofiberscope. There is also a lack of dynamic observation on the pathologic features of airway by multiple repeated bronchoscopies in children with airway malacia. Also, no direct correlation has been established between the respiratory tract infection and the airway malacia. In conclusion, this study aimed to further investigate general information, airway pathological features under fiberoptic bronchoscope, etiology, correlation with airway infection, treatment and prognosis, by clinical analysis on patients with airway malacia.
Material and methods
For these patients suffering from repeated or persistent gasping, chronic cough, repeated respiratory tract infection, atelectasis etc. that responded poorly to treatment, they were recommended to complete the consent form of flexible bronchofiberscope in hospitalization. Written informed consents were obtained from the next of kin, caretakers, or guardians on the behalf of the children involved in our study. This study was approved by the Ethics Committee of Children’s Hospital of Chongqing Medical University.
Patients
Bronchofiberscopy was performed on 2,749 children in total in Children’s Hospital of Chongqing Medical University from January 2009 to December 2012, because of chronic cough, recurrent wheeze, recurrent airway infections, atelectasis etc., among whom 459 children (male: 376, female: 83) were primarily diagnosed with airway malacia.
Fiberoptic bronchoscopy
Fiberoptic bronchoscopy was performed using Olympus BF bronchoscope introduced through transnasal route or epiglottis after proper lubrication with xylocaine ointment. All procedures were carried out as per the International recommendations [6]. Briefly, the secretion in the airway was removed after administration of penehyclidine hydrochloride injection (0.01 mg/kg). Subsequently, balanced anesthesia was carried out using propofol (1-1.5 mg/kg) plus midazolam (0.05-0.075 mg/kg) and Fentanyl (1-2 mg/kg). After that, surface anesthesia was performed to the throat and trachea under a condition of consistent pumping of propofol (6-8 ug/kg/h). During the whole procedure,clinical monitoring was carried out including blood oxygen saturation, respiration, heart rate, and facial expressions.
Type of airway malacia
The category of airway malacia was carried out as previous described [3]. Those with entrapment of tracheal diameter of ≥ 1/3 in expiratory phase was considered as slight, while those with entrapment of tracheal diameter of ≥ 1/2 was considered as moderate. Severe airway malacia was considered in those with entrapment of tracheal diameter of ≥ 4/5.
Statistical analysis
Microsoft Excel were used to collect data of characteristics of airway pathological changes under fiberoptic bronchoscope, general information of patients, clinical manifestation, other auxiliary examinations, treatments and re-check of bronchoscopy. SAS 9.2 software was used for the data analysis. Spearman rank correlation analysis was performed to investigate the correlation between airway malacia and infection. P < 0.05 demonstrated significant difference.
Results
Patient characteristics
459 patients, who were diagnosed as airway malacia, covered a proportion of 16.70% of all the patients taking bronchoscopy out of chronic cough, recurrent wheeze, recurrent airway infection and atelectasis etc. The distribution of gender and age of patients was shown in Figure 1.
Figure 1.
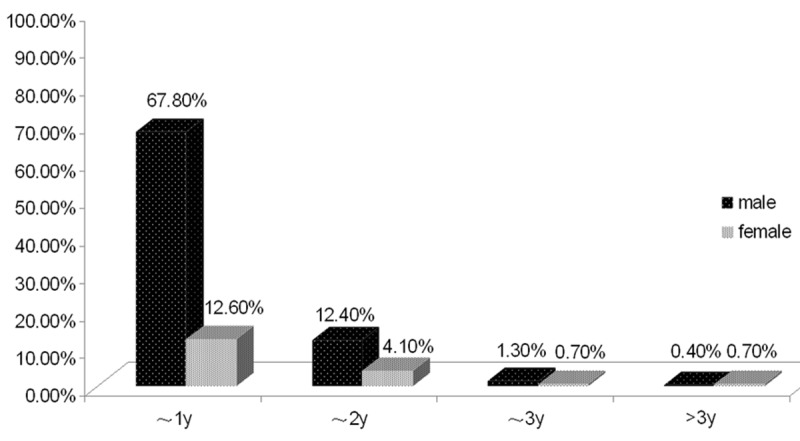
The distribution of the age of airway malacia. The patients were aged from 1 month to 101 months, among which 369 children (male: 311, female: 58) were aged less than 12 months, 76 children (male: 57, female: 19) were aged 1~2 years, 9 children (male: 6, female: 3) were aged 2~3 years, and 5 children (male: 2, female: 3) aged more than 3 years old.
Fiberoptic bronchoscopy observation
The distribution of airway malacia is shown in Table 1. The number of children with TM, TBM, and BM was 7 (1.5%), 17 (3.7%), and 435 (94.8%), respectively. Among these patients, the incidence of BM on left lung was 11.0% (n=48), while that of right lung was 53.3% (n=232), and BM on both sides were noticed in 155 children (35.6%). For the scale of disease, focal malacia was noticed in 246 children (53.6%), while multiple malacia was noticed in 213 children (46.4%). With regards to the extent of malacia, the number of children with slight, moderate and severe malacia was 226, 195, and 38, respectively. Besides malacia, the combined lesions included laryngomalacia (n=14), tracheostenosis or bronchial stenosis (n=271, including inflammatory stenosis and congenital stenosis), variation of bronchial opening (n=52), airway hyper-reactivity (n=4), purulent endotracheitis (n=3), abnormal passagesof trachea (n=3), granulation tissue formation in bronchus (n=2), and nodule-like lesions in the posterior wall of the trachea (n=1).
Table 1.
Distribution of Airway malacia
| Position | N (%) |
|---|---|
| Trachea | 24 (5.2%) |
| Right principal bronchus | 9 (2.0%) |
| Left principal bronchus | 63 (13.7%) |
| Bronchus lobaris superior dexter | 376 (81.9%) |
| Right middle lobe bronchus | 79 (17.2%) |
| Bronchus lobaris inferior dexter | 85 (18.5%) |
| Bronchus lobaris superior sinister | 68 (14.8%) |
| Bronchus lobaris inferior sinister | 150 (32.7%) |
Clinical features
Table 2 summarized the association between the disease course of respiratory tract infection and the extent of malacia. As indicated in Table 2, 227 children showed a disease course of less than 1 month, while 201 children reported a disease course of 1~3 months, and at the same time, 31 children reported a disease course of more than 3 months. Table 3 summarized the association between the disease course of respiratory tract infection and the scale of the malacia. The clinical manifestations of the patients included cough (n=449), gasping (n=411), dyspnea (n=83), fever (n=70), wheezy phlegm (n=11), vomiting or bucking during feeding (n=4). For the pulmonary signs, moist rales (n=440), wheezing sound (n=427), and laryngeal stridor (n=6) were noticed. All the children enrolled in this study were diagnosed with pulmonary infection according to the clinical symptoms, vital signs, chest X-ray, computedtomography scanning proposed by the Society of Pediatrics, Chinese Medical Association [7], among which 83 were diagnosed with severe pneumonia (Tables 4, 5). Forty-seven of the children were premature infants, while 412 of them were term infants. For the past medical history, 216 children reported histories of gasping (containing repeated gasping in 119 patients). Additionally, chronic cough (n=35), respiratory tract infection (n=263), feeding intolerance (n=12), short breath and cyanosis after exercise (n=2), wheezy phlegm (n=3), and assisted ventilation using respirators (n=4) were reported, respectively. No malacia was noticed previously in 3 patients using fiberoptic bronchoscopy. Other complications included bronchial asthma (n=28), atelectasis (n=32), gastroesophageal reflux (n=12), atrial septal defect (n=20), ventricular septal defect (n=1), patent ductus arteriosus (n=5), pulmonary hypertension (n=3), aberrant right subclavian artery (n=1), trachea depression induced by left superior pulmonary vein (n=1), pulmonary hernia (n=1), cystic disease of lung (n=1), thoracic or spinal deformity (n=3), eventration of diaphragm (n=1), zenetrale koonlination storung (n=5), cerebral palsy (n=3), epilepsy (n=2), hydrocephalus (n=2), congenital chilopalatognathus (n=4), and Pierre Robin syndrome (n=1).
Table 2.
Association between airway malacia extent and the disease course of respiratory tract infection
| Extent | Disease course | rs | p value | ||
|---|---|---|---|---|---|
|
| |||||
| < 1 month | 1-3 months | > 3 months | |||
| Slight | 109 | 104 | 13 | 0.00691 | 0.8827 |
| Moderate | 103 | 78 | 14 | ||
| Severe | 15 | 19 | 4 | ||
Table 3.
Association between airway malacia scale and the disease course of respiratory tract infection
| Scale | Disease course | rs | p value | ||
|---|---|---|---|---|---|
|
| |||||
| < 1 month | 1-3 months | > 3 months | |||
| Local malacia | 127 | 105 | 14 | 0.05348 | 0.2529 |
| Multiple malacia | 100 | 96 | 17 | ||
Table 4.
Association between extent of airway malacia and disease condition
| Extent | Disease conditions | rs | p value | |
|---|---|---|---|---|
|
| ||||
| Ordinary pneumonia | Severe pneumonia | |||
| Slight and moderate | 351 | 70 | 0.12589 | 0.0069 |
| Severe | 25 | 13 | ||
Table 5.
Association between airway malacia scale and disease condition
| Scale | Disease condition | rs | p value | |
|---|---|---|---|---|
|
| ||||
| Ordinary pneumonia | Severe pneumonia | |||
| Local malacia | 204 | 42 | -0.02819 | 0.5469 |
| Multiple malacia | 172 | 41 | ||
Imaging results
Expect for 106 patients were diagnosed with pulmonary infections via imageological examinations in other hospitals, 212 patients were confirmed with pulmonary infections according to X-ray tests in our hospital, among whom consolidation shadow or atelectasis (n=17), airway obstruction (n=4), deformity of thoracic bones (n=1), and cystic disease of lung (n=1). Routine chest CT scans of 195 patients all suggested pulmonary infections, among whom consolidation shadow or atelectasis (n=39), lymphadenectasis (n=12), deformity of thoracic bones (n=3), and pulmonary arterial ligament (n=1). Airway reconstructions was performed based on CT scans in 140 patients, which revealed localized stenosis or narrowing of airway (n=40), tracheal bronchus (n=2), foreign body in trachea or sputum obstruction (n=3), irregular anatomical structure of airway (n=1), and vague airway or obstruction (n=5). Moreover, 25 patients received vascular reconstruction examinations based on CT scans. The results indicated cardiovascular abnormalities were noticed in 5 patients (20%), among which patent ductus arteriosus (n=2), atrial septal defect (n=1), aberrant right subclavian artery (n=1), and trachea depression induced by left superior pulmonary vein (n=1).
Cardiac color Doppler ultrasound detection
In total, cardiac color Doppler ultrasound detection was carried out in 92 patients, which revealed atrial septal defect (n=16), ventricular septal defect (n=1), patent ductus arteriosus (n=5), patent oval foramen (n=52), and pulmonary hypertension (n=3).
Pulmonary function test
In total, pulmonary function determination was conducted in 178 patients. Among these patients, 46 patients were diagnosed with slight infarction of respiratory flow volume, while those of moderate and severe infarction of respiratory flow volume were noticed 68 patients and 21 patients, respectively. Meanwhile, obstructive and restrictive ventilatory functional disturbance was noticed in 43 patients.
Treatment and outcomes
All the patients received cough relieving, sputum elimination and excretion, and oxygen therapy. In addition, administration of antibiotics was carried out in 445 patients. During the fiberoptic bronchoscopy, local lavage or drug administration was given accordingly. Among these patients, 4 received mechanical ventilation due to deterioration of conditions. Improvements of the symptoms were noticed after treatment with the mean hospitalization duration of 9.7 days. Forty-one patients were discharged due to personal reasons after informing the potential dangers even though in the presence of symptoms and pulmonary signs. One patient received pulmonary lobectomy due to bronchiectasis induced by repeated respiratory tract infections.
Re-check of fiberoptic bronchoscopy
Re-check of fiberoptic bronchoscopy was performed in 19 patients, among which 14 patients (73.7%) showed improvement compared with the previous conditions (Table 6). No evidence of malacia was noticed in 8 patients, while improvements were noticed in 6 patients. No significant improvement was noticed in 4 patients, while 1 patient showed deterioration in malacia extent.
Table 6.
Comparison of fiexible bronchofiberscope results
| NO | The age at diagnosis | Follow up period | Comparison of flexible bronchofiberscope results |
|---|---|---|---|
| 01 | 13 months | 11 months | Improvement |
| 02 | 5 months | < 1 month | No improvement |
| 03 | 4 months | 5 months | Improvement |
| 04 | 40 months | 34 months | No improvement |
| 05 | 7 months | < 1 month | Improvement |
| 06 | 7 months | 4 months | Improvement |
| 07 | 5 months | 1 month | Deterioration |
| 08 | 3 months | 3 months | No improvement |
| 09 | 4 months | 7 months | Improvement |
| 10 | 4 months | 1 month | Improvement |
| 11 | 6 months | 3 months | No improvement |
| 12 | 32 months | 11 months | Improvement (no malacia) |
| 13 | 24 months | 1 month | Improvement (no malacia) |
| 14 | 8 months | < 1 month | Improvement (no malacia) |
| 15 | 5 months | < 1 month | Improvement (no malacia) |
| 16 | 101 months | 2 months | Improvement (no malacia) |
| 17 | 4 months | 5 months | Improvement (no malacia) |
| 18 | 11 months | 8 months | Improvement (no malacia) |
| 19 | 12 months | 3 months | Improvement (no malacia) |
Discussion
Primary airway malacia is not rare in the general population, with an estimated incidence of at least 1 in 2,100 children. Meanwhile the detection rate of airway malacia was 31.25% among the patients who underwent bronchoscopy [8]. In addition, it has been considered as the major cause for repeated and persistent gasping in children of less than 6 months old [9]. However, the detection rate was 16.70% in this study.
Our results indicated airway malacia was frequently noticed in male children aged less than 2 years old, which was consistent with the incidence rate reported in the previous results [1,10]. Nevertheless, Boogaard et al reported that airway malacia was diagnosed in 160 children (94 male children) with a median age of 4.0 years [8]. We speculated that may be associated with the early application of fiberoptic bronchoscopy in our department. Or, it may be related with the races. Still, our data could not explain the higher incidence rate of airway malacia in male children. The incidence of BM was comparatively lower than that of the TBM or TM [1,5], however, the opposite factwas identified in our study. As indicated by fiberoptic bronchoscopy, BM has been frequently noted and most of the patients were diagnosed with BM in right lung. On the contrary, Boogaard et al reported BM had no significantdifferences in distribution in both lungs [8]. Additionally, BM of right upper lobe was the most frequently observed type. Moreover, most of the patients were suffered from slight and moderate malacia, which was consistent with the previous report [10].
Our study demonstrated that patients with severe airway malacia were apt to develop severe pneumonia compared with those with slight or moderate malacia (Table 4). According to the data in Tables 2 and 3, no correlation was identified between the disease course of respiratory tract infection and extent/scale of the malacia. Additionally, no correlation was observed between the extent of pneumonia and the scale of the malacia (Table 5). However, Masters et al reported that neither the sites nor the severity of malacia exhibited a dose effect on rates or severity of illness [11]. The clinical manifestations of malacia vary widely. All these symptoms may be associated with the decrease of anterior cartilaginous ring/membrane structures. Thus, the children with airway malacia showed significant airway collapse in the expiratory phase, which may induce remarkable increase of airway resistance, and finally leading to (i) Air current that induce stridor in the presence of secretion in the airway, or low pitch stridor in the absence of secretion; (ii) Severe cough due to stimulation or tremor of the airway in the expiration phase, and at the same time, induced by pulmonary infection; (iii) Respiratory tract infection due to obstruction of trachea combined with squamous metaplasia and reduced function of mucociliary clearance [12].
Currently, chest CT has been commonly used in the diagnosis of respiratory diseases in pediatrics as it shows higher sensitivity and safety, as well as non-invasive features. The airway conditions could be clearly displayed by multi-slice spiral CT and airway reconstruction technique. For the diagnosis of airway malacia, images obtained in the expiratory and inhale phases should be combined. Nowadays, CT has been commonly used in the diagnosis of airway malacia in adults, however, the dynamic airway imaging of the expiratory and inhale phases were hard to obtain in children [1]. According to the results of our study, chest array, CT and echocardiography could assist to find the secondary factors causing malacia, while pulmonary function had no significant diagnostic value. Two types of airway malacia (i.e. primary and secondary airway malacia) were available. The primary airway malacia was induced by developmental immaturity or loss of tracheal cartilage in an independent or congenital manner [1,12]. To date, the onset of primary airway malacia has been combined with congenital disorders, such as premature birth, congenital syndromes such as Hunter’s syndrome [13] or Crouzon syndrome [14] or deformities [1,12]. In 2011, Nelson et al. reported a child with multiple congenital anomalies containing TBM combined with development lag was associated with the deletion of 555 kb on chromosome 16p13.3, 444 kb telomeric to the CREBBP gene and 623kb centromeric of PKD1 [15]. Meanwhile, Chetcuti-Ganado reported a patient with congenital cardiac disease combined with TBM was associated with partial trisomy for the long arms chromosomes 11 and 22 [16]. It has been proposed that maldevelopment of foregut in the embryonic stage was related with the pathogenesis of airway malacia, thus, patients with airway malacia always combined with maldevelopment of esophagus [17]. Blair et al reported that more cells were apt to form trachea rather than esophagus during the development of embryonic stage was associated with airway malacia [18]. In our study, abnormal passages were noticed in the airway of 3 patients by means of fiberoptic bronchoscopy, however, tracheoesophageal fistula was not diagnosed as esophagography was not carried out. Our results indicated 47 children were preterm infants (10.2%), and we speculated that this may be associated with the collapse of tracheal cartilage induced by maldevelopment of tracheal cartilage in these patients. Besides airway malacia, partial of the patients showed other conditions, among which tracheal or bronchial stenosis has been commonly noticed.
According to the previous literatures, the risk factors for secondary airway malacia were mainly consisted of long-term chatheterization, tracheotomy, severe tracheobronchitis, and depression caused by cardiovascular deformity,lymphadenectasis, enlargement of thymus gland, thymic cyst, carcinoma or skeletal malformation [1,2]. Meanwhile, gastroesophageal reflux was also a risk factor for airway malacia as the reflux of acid containing liquid may disruptthe mucous membrane of the trachea surface [19]. And airway malacia may be associated with the nervous system diseases as the disorder of nervous system may affect the dominance of the airway [19]. The malacia induced by tracheal cannula and tracheotomy may be related with the compression of air sac, and the tracheal injury induced by the impact of the air current at the end of the endotracheal tube [1,2]. For the malacia caused by extravascular compression, the major causes were the disrupture of the airway integrity and the increased compliance of the position underwent compression [1]. In our study, part of the patients also combined with one or more secondaryfactors mentioned above. Fiberoptic bronchoscopy was carried out in the previous days in 3 patients, and revealed no malacia, and improvement was noticed in 5 patients by re-check of fiberoptic bronchoscopy ≤ 1 month, demonstrating a partial of the patients with acute inflammation may develop airway malacia. On this basis, it is hard to identify whether the airway malacia is primary even though no other secondary reasons are noticed as acute inflammation may induce malacia, as a consequence, further fiberoptic bronchoscopy should be carried out for the final diagnosis. Excluding the secondary factors except inflammation, the malacia is secondary to acute inflammation if re-check of bronchoscopy shows improvement or no malacia within a short time, while continuous existence of malacia suggests primary malacia. The patients usually took bronchodilators duo to wheeze before the malacia had been definitely diagnosed, however the response was unsatisfactory. Some studies, in which bronchodilators were used on malacia patients, reported that the PEF decreased significantly when examining the pulmonary function after taking the medicine. It was considered that the obstruction aggravated due to the dilating of the airway smooth muscle [2].
No special treatment is necessary for the patients with primary airway malacia as the tracheal cartilage may enhance with the aging of the children, and most of the children with airway malacia will recover at the age of 2 years [1,2]. In our study, the patients were mostly aged less than 2 years old, which supported that view, so the principle of treatment was keeping the airway unobstructed. For the airway malacia patients combined with pulmonary infection, conservative therapy could be carried out preferentially such as infection control, oxygen inhale and sputum excretion. In addition, lavage and meditation could be used during the process of bronchoscopy. With regard to the secondary airway malacia, etiological treatment should be carried out to eliminate the compression and inflammation of the trachea and bronchus, and malacia could be improved when airway compression was removed or inflammation was controlled promptly [2]. For severe airway malacia children with poor outcomes after conventional therapy, insertion of stents should be considered, however, complications have been reported such as granulationtissues formation, shifting of stents, as well as the metallic stent fracture sometimes requiring removal [1,20]. As demonstrated in the results of our study, most airway malacia improved or even disappeared as patients aging or inflammation controlled, while for the few of unimproved or even worsened, it was considered that the follow-up time was not long enough or recurrent infections etc. caused the continuous malacia. There are some limitations in our study. For example, fiberoptic bronchoscopy was only carried out in a small number of children during the follow up. Additionally, interval of the fiberoptic bronchoscopy was different among the children received fiberoptic bronchoscopy in the follow up period.
Conclusions
In conclusion, airway malacia has been frequently noticed in male infants aged ≤ 2 years old, among which malacia in bronchus lobaris superior dexter has been frequently reported. Patients with severe airway malacia were apt to develop severe pneumonia compared with those with slight or moderate malacia. However, no correlation was identified between the disease course of respiratory tract infection and extent/scale of the malacia. Additionally, no correlation was observed between the extent of pneumonia and the scale of the malacia. Most of the children with airway malacia will recover with the aging and infection control. We concluded that fiberoptic bronchoscopy should be carried out in children with repeated cough, gasping and respiratory tract infection and respond poorly to bronchodilators in a promptly manner. Airway malacia should be confirmed and relevant treatments should be taken according to the causes. Moreover, single fiberoptic bronchoscopy was inadequate for the distinct of primary or secondary airway malacia, if necessary, re-check of fiberoptic bronchoscopy should be carried out to observe the anatomical structure of the airway.
Acknowledgements
This study was financially supported by grants from the National Clinical Key Specialty Construction Projects.
Disclosure of conflict of interest
None.
References
- 1.Carden KA, Boiselle PM, Waltz DA, Ernst A. Tracheomalacia and tracheobronchomalacia in children and adults: an in-depth review. Chest. 2005;127:984–1005. doi: 10.1378/chest.127.3.984. [DOI] [PubMed] [Google Scholar]
- 2.Tan JZ, Ditchfield M, Freezer N. Tracheobronchomalacia in children: review of diagnosis and definition. Pediatr Radiol. 2012;42:906–915. doi: 10.1007/s00247-012-2367-5. quiz 1027-1028. [DOI] [PubMed] [Google Scholar]
- 3.Jiang QB, Liu XC, Jiang ZF, Jiang Y. A study on tracheobronchomalacia in children. Chin J Prac Pedi. 2002;17:277–279. [Google Scholar]
- 4.Lee EY, Boiselle PM. Tracheobronchomalacia in infants and children: multidetector CT evaluation. Radiology. 2009;252:7–22. doi: 10.1148/radiol.2513081280. [DOI] [PubMed] [Google Scholar]
- 5.Xia Y, Huang Y, Li QB, Luo ZX, Liu EM, Chen KH, Tang W, Bo N, Zhao H, Yuan XP. Analysis of 53 cases with bronchoscopically confirmed pediatric tracheobronchomalacia. Chin J Pedi. 2007;45:96–99. [PubMed] [Google Scholar]
- 6.Brimacombe J, Berry A. Guidelines for care during bronchoscopy. Thorax. 1994;49:528. doi: 10.1136/thx.49.5.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chinese Medical Association. Guideline for the management of children with community acquired pneumonia. Chin J Pedi. 2007;45:83–90. [Google Scholar]
- 8.Boogaard R, Huijsmans SH, Pijnenburg MW, Tiddens HA, de Jongste JC, Merkus PJ. Tracheomalacia and bronchomalacia in children: incidence and patient characteristics. Chest. 2005;128:3391–3397. doi: 10.1378/chest.128.5.3391. [DOI] [PubMed] [Google Scholar]
- 9.Luo ZX, Liu EM, Li QB, Huang Y, Fu Z. nalysis on the causes of recurrent and persistent wheezing in 58 infants. J of Clin Pedi. 2006;24:478–479. [Google Scholar]
- 10.Yalcin E, Dogru D, Ozcelik U, Kiper N, Aslan AT, Gozacan A. Tracheomalacia and bronchomalacia in 34 children: clinical and radiologic profiles and associations with other diseases. Clin Pediatr (Phila) 2005;44:777–781. doi: 10.1177/000992280504400905. [DOI] [PubMed] [Google Scholar]
- 11.Masters IB, Zimmerman PV, Pandeya N, Petsky HL, Wilson SB, Chang AB. Quantified tracheobronchomalacia disorders and their clinical profiles in children. Chest. 2008;133:461–467. doi: 10.1378/chest.07-2283. [DOI] [PubMed] [Google Scholar]
- 12.Ridge CA, O’Donnell CR, Lee EY, Majid A, Boiselle PM. Tracheobronchomalacia: current concepts and controversies. J Thorac Imaging. 2011;26:278–289. doi: 10.1097/RTI.0b013e3182203342. [DOI] [PubMed] [Google Scholar]
- 13.Morehead JM, Parsons DS. Tracheobronchomalacia in Hunter’s syndrome. Int J Pediatr Otorhinolaryngol. 1993;26:255–261. doi: 10.1016/0165-5876(93)90096-l. [DOI] [PubMed] [Google Scholar]
- 14.Beck R, Sertie AL, Brik R, Shinawi M. Crouzon syndrome: association with absent pulmonary valve syndrome and severe tracheobronchomalacia. Pediatr Pulmonol. 2002;34:478–481. doi: 10.1002/ppul.10176. [DOI] [PubMed] [Google Scholar]
- 15.Nelson M, Quinonez S, Ackley T, Iyer RK, Innis JW. Multiple congenital anomalies and developmental delay in a boy associated with a de novo 16p13.3 deletion. Am J Med Genet A. 2011;155a:612–617. doi: 10.1002/ajmg.a.33808. [DOI] [PubMed] [Google Scholar]
- 16.Chetcuti-Ganado C, Grech V. Complex congenital cardiac disease in a patient with partial trisomy for the long arms of chromosomes 11 and 22. Cardiol Young. 2003;13:481–483. [PubMed] [Google Scholar]
- 17.Wailoo MP, Emery JL. The trachea in children with tracheo-oesophageal fistula. Histopathology. 1979;3:329–338. doi: 10.1111/j.1365-2559.1979.tb03014.x. [DOI] [PubMed] [Google Scholar]
- 18.Blair GK, Cohen R, Filler RM. Treatment of tracheomalacia: eight years’ experience. J Pediatr Surg. 1986;21:781–785. doi: 10.1016/s0022-3468(86)80366-9. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs IN, Wetmore RF, Tom LW, Handler SD, Potsic WP. Tracheobronchomalacia in children. Arch Otolaryngol Head Neck Surg. 1994;120:154–158. doi: 10.1001/archotol.1994.01880260026006. [DOI] [PubMed] [Google Scholar]
- 20.Ranu H, Madden BP. Endobronchial stenting in the management of large airway pathology. Postgrad Med J. 2009;85:682–687. doi: 10.1136/pgmj.2009.089011. [DOI] [PubMed] [Google Scholar]


