Abstract
Many studies reported that DcR3 participated in the clinicopathological characteristics of gastrointestinal cancer, however, they all included few patients and had inconsistent results. So we conducted a meta-analysis to explore the correlation between overexpression of DcR3 and the clinicopathological characteristics of gastrointestinal cancer. Identical search strategies were used to search relevant literatures in PubMed, Web of Science and Chinese Biomedical Literature Database. The prognostic significances and clinicopathological differences of DcR3 in gastrointestinal cancer were analyzed. A total of 28 studies comprising 3294 gastrointestinal cancer patients met the inclusion criteria. Overexpression of DcR3 was closely related with these clinicopathological features, including TNM stages (OR = 1.63, 95% CI 1.35-1.98), grade of differentiation (OR = 1.31, 95% CI 1.10-1.56), lymph node metastasis (OR = 2.02, 95% CI 1.66-2.47), infiltration degree (OR = 1.72, 95% CI 1.38-2.12), and metastasis (OR = 1.66, 95% CI 1.27-2.16). DcR3 may play an important role in gastrointestinal cancer, and DcR3 indicated distinct clinicopathologic features.
Keywords: DcR3, gastrointestinal cancer, prognostic, metastasis
Introduction
Decoy receptor 3 (DcR3) is a newly discovered member of tumor necrosis factor receptor (TNFR) family; it is a soluble secretory protein lacking a transmembrane sequence [1].
DcR3 is mostly expressed in tumor cells and competitively inhibits TNF signaling. Overex-pression of DcR3 in tumor cells protects them from apoptosis. Studies have shown that DcR3 expression and amplification are closely correlated with the development of various malignant tumors as well as their immune escape [2,3]. Wu et al. [4] reported that DcR3 could not be detected in non-tumor patients, but could be detected in 98.8% (82/83) of patients with malignant cancers. However, the numbers of patients they included were small, and the conclusions they drew were inconsistent. For example, Meisongzhu Yang et al. [5] reported that there was no correlation between DcR3 expression and tumor cell differentiation; Guo-Hong Li [6] reported that DcR3 was correlated with tumor cell differentiation; Therefore, we made a meta-analysis from eligible studies to investigate the relationship between DcR3 expression and clinicopathological characteristics of gastrointestinal cancer patients.
Experimental procedure
Inclusion and exclusion criteria
Studies were included if they met the following criteria: (I) evaluating DcR3 expression in the gastrointestinal cancer tissues; (II) assessing the relationships between DcR3 expression with gastrointestinal cancer clinicopathologic features; (III) sufficient information provided to estimate odds ratio (OR) about clinicopathologic features; (IV) articles written in English or Chinese. The exclusion criteria were as follow: (I) letters, reviews, case reports, conference abstracts, or editorials; (II) articles had no sufficient data to calculate odds ratio (OR) or hazard ratio (HR); and (III) studies had duplication data.
Search strategy
PubMed, WanFang, CNKI and Web of Science databases were searched using the terms: “DcR3”or “TR6” or “M68”. Bibliographies, review articles and other pertinent studies were searched for additional eligible studies. In addition, colleagues in Gastroenterology field were involved for providing details of clinical trials. Two investigators conducted the research independently, and they also evaluated study quality using Cochrane recommendations. Disagreement was solved by discussion with others.
Study screening and data extraction
Two reviewers screened each study independently to determine whether it met the inclusion criteria, and resolved disagreementsby consensus. For each included study, Jing T and Ran Ao independently extracted the following data using a standard form: first author, year of publication, country, kind, number of patients, test method, clinicopathological parameters (TNM stage, differentiation, lymph node metastasis, infiltration degree, Metastasis).
Methodological assessment
Newcastle-Ottawa quality assessment scale (NOS) was used to assess the quality of each study [7]. The scale assigned 0-9 stars to each study based on three categories (selection, comparability and outcome).
Data analysis and statistical methods
Meta-analyses were carried out by using the Stata software version 12.0. There was no statistically significant heterogeneity in this analysis (I2 > 50%, P-Het > 0.1), so fixed effect model was applied. Statistical heterogeneity between studies was evaluated with the chi-square test and the I2 statistic. Potential causes of heterogeneity were explored by carrying out sensitivity and subgroup analyses. The odds ratio (OR) was calculated for dichotomous data with 95% confidence intervals (CIs) for all analyses. P values that were < 0.05 were considered statistically significant.
Result
Description of studies
As shown in Figure 1, 1346 published records were identified from a search of the above databases using the search strategy as described above. After exclusion of the studies that were out of the scope of our systematic review, a total of 28 eligible studies were included in the final meta-analysis. These 28 studies assessed the relationships between DcR3 expression and gastrointestinal cancer clinicopathologic features. The clinical features of these 28 included studies were summarized in Table 1. These studies were published from 2002 to 2013, and total 3294 gastrointestinal cancer patients were enrolled. Sample sizes ranged from 60 to 218 patients (mean 118). 13 of these studies enrolled less than 100 patients and 15 studies included more than 100 patients. 27 of these studies evaluated patients from China, 1 from Japan. 25 of these studies got 6 scores or more in methodological assessment, which meant they had high qualities.
Figure 1.
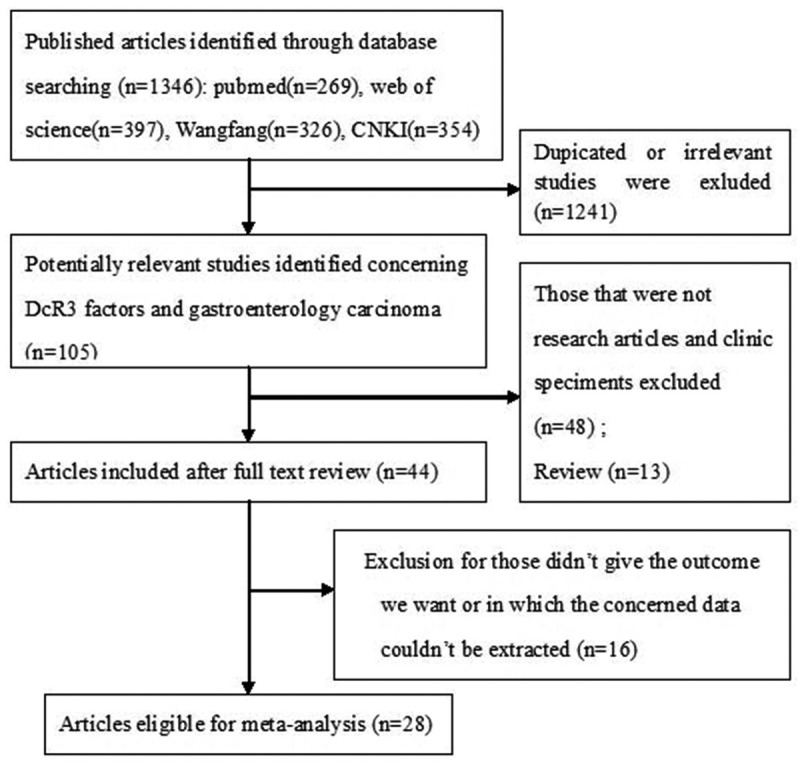
Flow diagram of study selection procedures.
Table 1.
Main characteristics of all studies included in Meta-analysis
| First author (Ref.) | Year | Country | Kind | NO of Case (control) | Method | Clinicopathological factors | Quality score |
|---|---|---|---|---|---|---|---|
| YANG [5] | 2010 | China | HCC | 67 (28) | SC | ①②⑤ | 7 |
| Qi [8] | 2009 | China | CRC | 100 (100) | IHC/RT-PCR | ⑤ | 7 |
| AO [9] | 2013 | China | EC | 150 (30) | IHC | ①②④⑤ | 7 |
| Li [10] | 2005 | China | EC | 28 (40) | IHC | ② | 6 |
| Xiong [11] | 2010 | China | EC | 109 (109) | RT-PCR | ②③④ | 7 |
| Yusushi [12] | 2002 | Japan | GC | 84 | RT-PCR | ③⑤ | 6 |
| Chen [13] | 2008 | China | GC | 79 (42) | IHC | ①②③⑤ | 8 |
| Yang [14] | 2012 | China | GC | 50 (50) | RT-PCR | ①② | 7 |
| Liu [15] | 2008 | China | CC | 66 | IHC | ①②③ | 5 |
| Zhu [16] | 2011 | China | CRC | 60 (10) | IHC | ②③④ | 6 |
| Li [6] | 2008 | China | CRC | 100 (100) | IHC | ②③ | 8 |
| Yang [17] | 2008 | China | CRC | 101 (21) | IHC | ②③④ | 8 |
| Cai [18] | 2010 | China | CG | 38 (42) | IHC | ①②③④ | 8 |
| Chen [19] | 2009 | China | HCC | 40 (20) | RT-PCR | ①③⑤ | 6 |
| Shen [20] | 2003 | China | HCC | 48 (48) | RT-PCR | ⑤ | 6 |
| Chen [21] | 2007 | China | HCC | 69 (69) | RT-PCR | ①②⑤ | 6 |
| Yu [22] | 2005 | China | GC | 62 (62) | RT-PCR | ②③④ | 8 |
| Zhang [23] | 2009 | China | CRC | 70 (10) | IHC | ② | 8 |
| Cui [24] | 2012 | China | HCC | 40 (40) | IHC | ② | 6 |
| Liu [25] | 2011 | China | EC | 84 (84) | IHC | ①②③④ | 5 |
| Zhang [26] | 2010 | China | GC | 60 (30) | IHC | ②③④ | 5 |
| Yue [27] | 2010 | China | EC | 98 (20) | IHC | ①②③④ | 6 |
| Li [28] | 2004 | China | GC | 98 (56) | IHC | ①②③④ | 7 |
| Zhao [29] | 2009 | China | GC | 75 (15) | IHC | ①③④ | 7 |
| Guo [30] | 2009 | China | GC | 86 (86) | RT-PCR | ①②③④ | 7 |
| Zhou et al. [31] | 2012 | China | PC | 50 (50) | RT-PCR | ①③ | 6 |
| Shen et al. [32] | 2005 | China | HCC | 48 (48) | RT-PCT | ①⑤ | 7 |
| Liu et al. [33] | 2013 | China | CRC | 62 (62) | IHC | ①②③④ | 6 |
HCC, hepatocellular carcinoma; CRC, colorectal cancer; EC, esophageal cancer; GC, gastric carcinoma; PC, pancreatic carcinoma; CG, carcinoma of gallbladder; IHC, immunohistochemistry; SC, serum concentration; ①TNM stage; ②differentiation; ③lymph node metastasis; ④infiltration degree; ⑤metastasis.
Correlation of DcR3 expression with clinicopathological parameters
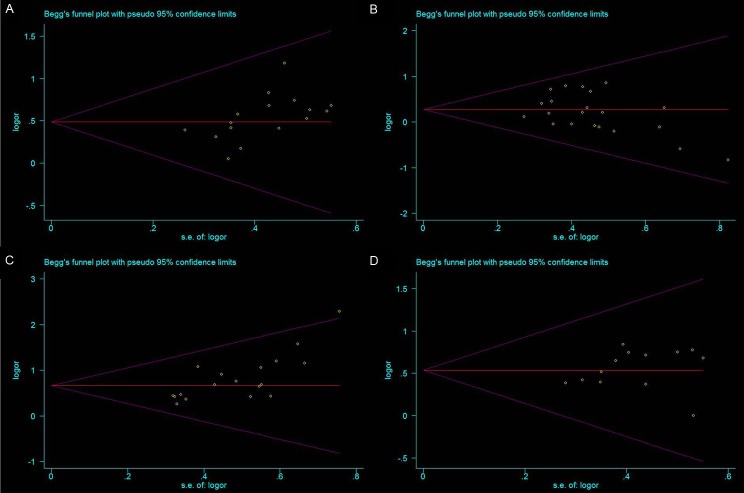
The meta-analysis was assessed the correlation between DcR3 expression and clinicopathological of gastroenterology carcinoma. As shown in Table 2, overexpression of DcR3 was significantly associated with TNM stages (OR, 1.63; 95% CI: 1.35, 1.98), differentiation (OR, 1.31; 95% CI: 1.10, 1.56), lymph node metastasis (OR, 2.02; 95% CI: 1.66, 2.47), infiltration degree (OR, 1.72; 95% CI: 1.38, 2.12), metastasis (OR, 1.66; 95% CI: 1.27, 2.16). The funnel plot for the outcome of correlation between DcR3 expression and TNM stage, differentiation, lymph node metastasis, infiltration degree, metastasis on gastroenterology carcinoma (Figures 2, 3, 4, 5 and 6). Egger’s test indicated that there was no evidence of significant publication bias after assessing the funnel plot (Figure 7) for the studies included in our meta-analysis. And the results of the sensitivity analysis are stable.
Table 2.
DcR3 clinicopathological features for gastroenterology carcinoma
| Heterogeneity | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Clinicopathological features | No. of studies | No. of patients | Pooled OR (95% CI) | PHet | I2 (%) | P value | Model used |
| TNM stage | 16 | 1862 | 1.63 [1.35, 1.98] | 0.969 | 0 | 0 | FEM |
| differentiation | 21 | 2568 | 1.31 [1.10, 1.56] | 0.771 | 0 | 0.003 | FEM |
| Lymph node metastasis | 18 | 2161 | 2.02 [1.66, 2.47] | 0.656 | 0 | 0 | FEM |
| Infiltration degree | 13 | 1710 | 1.72 [1.38, 2.12] | 0.993 | 0 | 0 | FEM |
| metastasis | 9 | 1070 | 1.66 [1.27, 2.16] | 0.568 | 0 | 0 | FEM |
REM, random-effects model; FEM, fixed-effects model; OR, odds ratio; CI, confidence interval.
Figure 2.
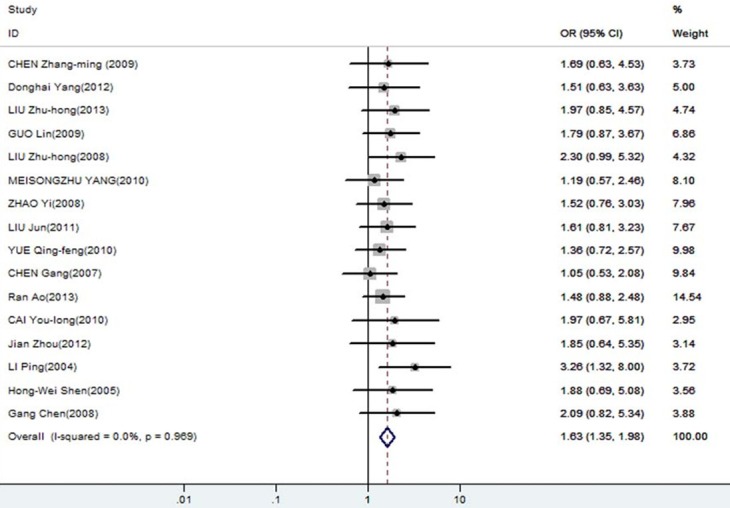
Forest plot of odds ratios (ORs) with corresponding 95% CIs for the association of DcR3 expression with TNM stages.
Figure 3.
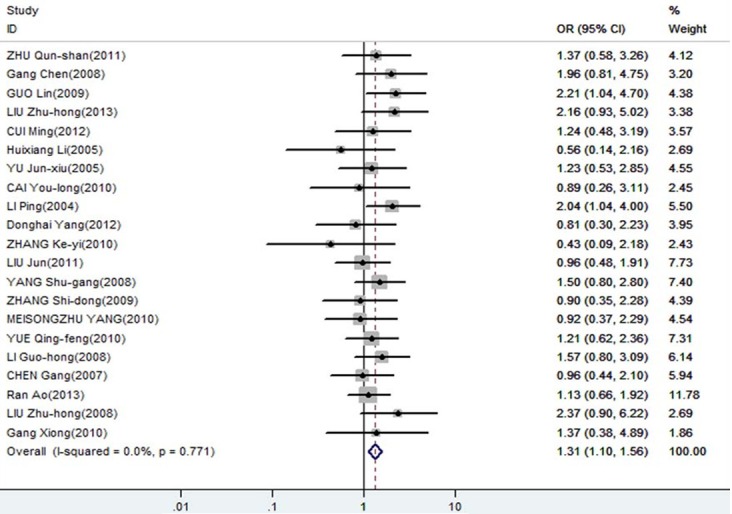
Forest plot of odds ratios (ORs) with corresponding 95% CIs for the association of DcR3 expression with differentiation.
Figure 4.
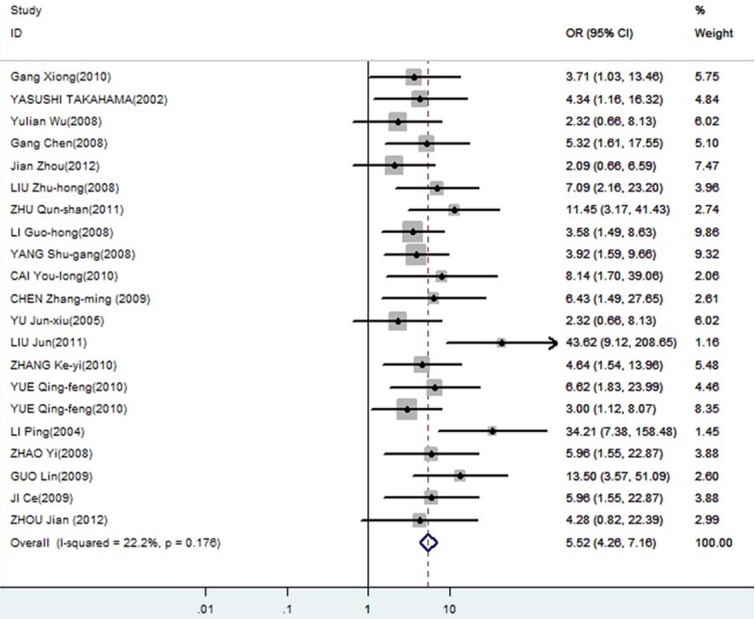
Forest plot of odds ratios (ORs) with corresponding 95% CIs for the association of DcR3 expression with lymph node metastasis.
Figure 5.
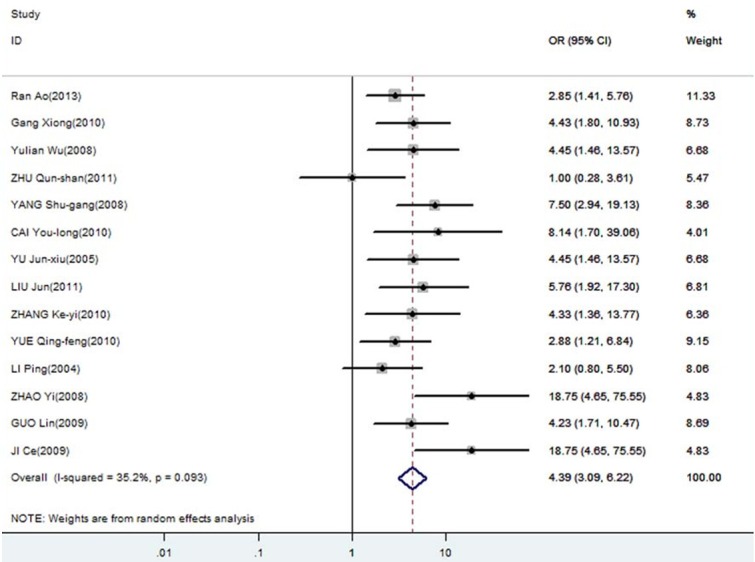
Forest plot of odds ratios (ORs) with corresponding 95% CIs for the association of DcR3 expression with infiltration degree.
Figure 6.
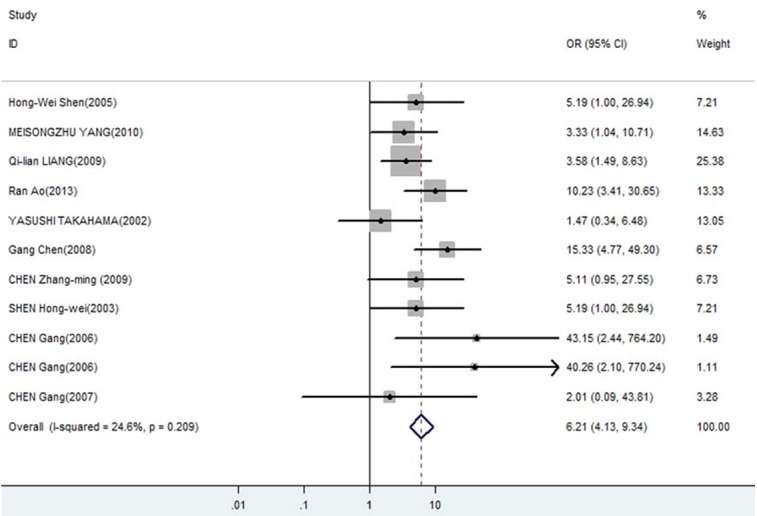
Forest plot of odds ratios (ORs) with corresponding 95% CIs for the association of DcR3 expression with metastasis.
Figure 7.
A. Egger’s publication bias plot showed no publication bias for studies regarding overexpressed DcR3 and TNM stage in the meta-analysis; B. Egger’s publication bias plot showed no publication bias for studies regarding overexpressed DcR3 and differentiation in the meta-analysis; C. Egger’s publication bias plot showed no publication bias for studies regarding overexpressed DcR3 and lymph node metastasis in the meta-analysis; D. Egger’s publication bias plot showed no publication bias for studies regarding overexpressed DcR3 and infiltration degree in the meta-analysis.
Discussion
Decoy receptor 3 (DcR3) is a member of the tumor necrosis factor receptor (TNFR) superfamily. It has been shown to be the decoy receptor for Fas ligand (FasL), LIGHT and TL1A [34-36]. As DcR3 lacks a transmembrane structure in its amino acid sequence, it belongs to a kind of secretory protein. DcR3 is not expressed or only slightly expressed in normal tissues and serum, but highly expressed in malignant tumor tissues. Overexpression of DcR3 in tumor cells protects them from apoptosis. DcR3 protects tumor cells from immune surveillance as it contributes to the suppression of the host anti-tumor immunity. The overexpression of DcR3 can be found in multiple human malignant tumors such as gastric carcinoma [12], colon carcinoma [4], esophageal carcinoma [10], lung carcinoma [34], hepatic carcinoma [37,38], spongioblastoma [39], oophoroma [40,41], etc.
From a clinical perspective, therefore, it is of great significance to identify the most useful biomarkers, which can help clinicians to adopt preventive strategies for patients at high risk and further improve outcome of patient with gastrointestinal cancer. Although the association of DcR3 with gastrointestinal cancer and clinicopathologic features has been explored for several years, the available data have not yet been fully analyzed Considering that meta-analysis is a valuable tool in biomarker validation [42], here we conducted a meta-analysis to investigate the association between DcR2 expression of gastrointestinal cancer patients and clinicopathologic features.
In this meta-analysis, we first assessed the association between DcR3 expression of gastrointestinal cancer patients and clinicopathologic features. We analyzed the data of 3294 gastrointestinal cancer patients from 28 individualstudies, and showed that overexpression of DcR3 was significantly associated with TNM stage, differentiation, lymph node metastasis, infiltration degree, metastasis. Thus, DcR3 indicate distinct clinicopathologic features. Additionally, the results of sensitivity analysis showed that the association was not changed after removing any study.
In summary, we showed that both overexpressed DcR3 were significantly associated with TNM stage, differentiation, lymph node metastasis, infiltration degree, metastasis on gastroenterology carcinoma patients. But our study had some limitations. Firstly, given all the included studies investigated gastrointestinal cancer patients from China and Japan, the results just represent the correlation between DcR3 expression and gastrointestinal cancer patients from Asia. Secondly, only 28 studies (including 3294 gastrointestinal cancer patients) had available data to calculate HRs, in which just 9 studies (including 1070 gastrointestinal cancer patients) investigated the correlation between DcR3 expression and metastasis. The sample size was not big enough so that the association of DcR3 expression and metastasis was not significant. Third, the study included in our meta-analysis was restricted only to articles published in English or Chinese, which probably provided additional bias. So large, well-designed prospective studies are required to investigate the precise clinicopathologic differences of DcR3 expression.
Disclosure of conflict of interest
None.
References
- 1.Chen J, Zhang L, Kim S. Quantification and detection of DcR3, a decoy receptor in TNFR family. J Immunol Methods. 2004;285:63–70. doi: 10.1016/j.jim.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Chen L, Tian X, Li W, Agarwal A, Zhuang G. Expressions of Fas/DcR3 and RGD-FasL mediated apoptosis in pituitary adenomas. Neurol India. 2009;57:28–30. doi: 10.4103/0028-3886.48808. [DOI] [PubMed] [Google Scholar]
- 3.Mueller AM, Pedre X, Killian S, David M, Steinbrecher A. The Decoy Receptor 3 (DcR3, TNFRSF6B) suppresses Th17 immune responses and is abundant in human cerebrospinal fluid. J Neuroimmunol. 2009;209:57–64. doi: 10.1016/j.jneuroim.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 4.Wu Y, Han B, Sheng H, Lin M, Moore PA, Zhang J, Wu J. Clinical significance of detecting elevated serum DcR3/TR6/M68 in malignant tumor patients. Int J Cancer. 2003;105:724–732. doi: 10.1002/ijc.11138. [DOI] [PubMed] [Google Scholar]
- 5.Yang M, Chen G, Dang Y, Luo D. Significance of decoy receptor 3 in sera of hepatocellular carcinoma patients. Ups J Med Sci. 2010;115:232–237. doi: 10.3109/03009734.2010.516410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li GH. Thesis. China: Guangdong Medical College; 2008. The expression and significance of DcR3 and Survivin in colorectal carcinoma. [Google Scholar]
- 7.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 8.Liang QL, Wang BR, Li GH. DcR3 and survivin are highly expressed in colorectal carcinoma and closely correlated to its clinicopathologic parameters. J Zhejiang Univ Sci B. 2009;10:675–682. doi: 10.1631/jzus.B0920077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ao R, Du YQ, Wang Y, Chen YS, Wang BY. MMP-2 and DcR3 expression in esophageal cancer tissue and correlation with patient survival. Int J Clin Exp Med. 2013;6:700–705. [PMC free article] [PubMed] [Google Scholar]
- 10.Li H, Zhang L, Lou H, Ding I, Kim S, Wang L, Huang J, Di Sant’Agnese PA, Lei JY. Overexpression of decoy receptor 3 in precancerous lesions and adenocarcinoma of the esophagus. Am J Clin Pathol. 2005;124:282–287. doi: 10.1309/XK59-4E4B-5WU8-2QR6. [DOI] [PubMed] [Google Scholar]
- 11.Xiong G, Guo H, Ge X, Xu X, Yang X, Yang K, Jiang Y, Bai Y. Decoy receptor 3 expression in esophageal squamous cell carcinoma: correlation with tumour invasion and metastasis. Biomarkers. 2011;16:155–160. doi: 10.3109/1354750X.2010.536258. [DOI] [PubMed] [Google Scholar]
- 12.Takahama Y, Yamada Y, Emoto K, Fujimoto H, Takayama T, Ueno M, Uchida H, Hirao S, Mizuno T, Nakajima Y. The prognostic significance of overexpression of the decoy receptor for Fas ligand (DcR3) in patients with gastric carcinomas. Gastric Cancer. 2002;5:61–68. doi: 10.1007/s101200200011. [DOI] [PubMed] [Google Scholar]
- 13.Chen G, Luo D. Over-expression of decoy receptor 3 in gastric precancerous lesions and carcinoma. Ups J Med Sci. 2008;113:297–304. doi: 10.3109/2000-1967-240. [DOI] [PubMed] [Google Scholar]
- 14.Yang D, Fan X, Yin P, Wen Q, Yan F, Yuan S, Liu B, Zhuang G, Liu Z. Significance of decoy receptor 3 (Dcr3) and external-signal regulated kinase 1/2 (Erk1/2) in gastric cancer. BMC Immunol. 2012;13:28. doi: 10.1186/1471-2172-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu ZH, Chen SH, Chen YJ, Xu WB, Xie LX, Shen JH, Ni YP. Expression of TR6 and survivin gene in the tissue of cardiac caner and its clinical significance. Journal of Chinese Physician. 2008;10:1336–1338. [Google Scholar]
- 16.Zhu QS, Chen P, Zhao W. The Expression of DcR3 and HER-2/ neu in 60 Cases with Colorectal Cancer and its Clinical Significance. Journal of Chinese Oncology. 2011;17:610–613. [Google Scholar]
- 17.Yang SG. Thesis. China: Fujian Medical University; 2008. Expression of Dcr3, EGFR and Ki67 In colorectal carcinoma and its clinical significance. [Google Scholar]
- 18.Cai YL. Thesis. China: Tianjin Medical University; 2010. Expression DcR3 of in carcinoma of gallbladder. [Google Scholar]
- 19.Chen ZM, Ren SL, Zhang L. Expression of decay receptor 3 and the effect on angiogenesis in hepatic cell carcinoma. China Journal of Modern Medicine. 2009;19:1790–1796. [Google Scholar]
- 20.Shen HW, Wu YL, Peng SY. Overexpression and genomic amplification of decoy receptor 3 in hepatocellular carcinoma and significance thereof. Zhonghua Yi Xue Za Zhi. 2003;83:744–747. [PubMed] [Google Scholar]
- 21.Chen G. PhD thesis. China: Guangxi Medical University; 2007. Expression of DcR3 and its function on the Growth, Migrating and apoptosis in Hepatocel1u1ar Carcinoma. [Google Scholar]
- 22.Yu JX. PhD thesis. China: Zhejiang University School of Medicine; 2005. The study on the relationship between DcR3 expression and lymph node metastasis, VEGF-C, VEGF-D expression in gastric cancer. [Google Scholar]
- 23.Zhang JS. Thesis. China: Hebei Medical University; 2009. The Level of DcR3 gene in the colorectal carcinoma and the effects on the immunologic balance. [Google Scholar]
- 24.Cui M, Guo YZ, Hu XY, Zhou ZW, Xu W. Expression and clinical significance of decoy receptor 3 and Caveolin-1 in hepatocellular carcinoma. The Journal of Practical Medicine. 2012;28:3902–3904. [Google Scholar]
- 25.Liu J. Thesis. China: Soochow University; 2011. Clinical significance of DcR3, bcl-2 and P53 protein expressinon in esophageal carcinoma. [Google Scholar]
- 26.Zhang KJ, Wu ZY, Zhang SY, Shen JH. Expression and significance of Survivin and DcR3 in gastric carcinoma. Guangdong Medical Journal. 2010;31:1157–1158. [Google Scholar]
- 27.Yue QF, Xiang M, Li FM, Liu H, Yue FT, Liu JJ, Wang WQ, Ji CH. Study on the expression level of DcR3 and MMP-2 in esophageal carcinoma and its impact on survival of the patients. China Oncology. 2010;20:745–750. [Google Scholar]
- 28.Li P, Huang CM, Zhang XF. Expression of DcR3 and Ki-67 in human gastric carcinoma and its clinical significance. J Fujian Med Univ. 2004;38:39–42. [Google Scholar]
- 29.Zhao Y, Deng X, Ma Y, Wang P. Expression and clinical significance of PTEN Fas/FasL and DCR3 in gastric cancer. Modern Oncology. 2009;17:1124–1129. [Google Scholar]
- 30.Guo L, Gao X, Lu RQ. The expression and clinical significance of DcR3 and Smad4 in gastric cancer. Laboratory Medicine. 2009;24:823–827. [Google Scholar]
- 31.Zhou J, Song SD, Li DC, Zhu DM, Zheng SY. Clinical significance of expression and amplification of the DcR3 gene in pancreatic carcinomas. Asian Pac J Cancer Prev. 2012;13:719–724. doi: 10.7314/apjcp.2012.13.2.719. [DOI] [PubMed] [Google Scholar]
- 32.Shen HW, Gao SL, Wu YL, Peng SY. Overexpression of decoy receptor 3 in hepatocellular carcinoma and its association with resistance to Fas ligand-mediated apoptosis. World J Gastroenterol. 2005;11:5926–5930. doi: 10.3748/wjg.v11.i38.5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu ZH, Ni YP, Huang QG, Xie LX, Xu WB, Zhong XY. Relation of expression of DcR3 and livin and prognosis in colorectal carcinoma. Guangdong Medical Journal. 2013;34:1055–1057. [Google Scholar]
- 34.Pitti RM, Marsters SA, Lawrence DA, Roy M, Kischkel FC, Dowd P, Huang A, Donahue CJ, Sherwood SW, Baldwin DT, Godowski PJ, Wood WI, Gurney AL, Hillan KJ, Cohen RL, Goddard AD, Botstein D, Ashkenazi A. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature. 1998;396:699–703. doi: 10.1038/25387. [DOI] [PubMed] [Google Scholar]
- 35.Yu KY, Kwon B, Ni J, Zhai Y, Ebner R, Kwon BS. A newly identified member of tumor necrosis factor receptor superfamily (TR6) suppresses LIGHT-mediated apoptosis. J Biol Chem. 1999;274:13733–13736. doi: 10.1074/jbc.274.20.13733. [DOI] [PubMed] [Google Scholar]
- 36.Migone TS, Zhang J, Luo X, Zhuang L, Chen C, Hu B, Hong JS, Perry JW, Chen SF, Zhou JX, Cho YH, Ullrich S, Kanakaraj P, Carrell J, Boyd E, Olsen HS, Hu G, Pukac L, Liu D, Ni J, Kim S, Gentz R, Feng P, Moore PA, Ruben SM, Wei P. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity. 2002;16:479–492. doi: 10.1016/s1074-7613(02)00283-2. [DOI] [PubMed] [Google Scholar]
- 37.Chen G, Luo DZ, Wang Y, Liao ZL, Zhang MY. Relationship between expression of decoy receptor 3 and apoptosis in hepatocellular carcinoma. Zhonghua Bing Li Xue Za Zhi. 2007;36:113–117. [PubMed] [Google Scholar]
- 38.Chen G, Luo D. Expression of decoy receptor 3 in liver tissue microarrays. Natl Med J India. 2008;21:275–278. [PubMed] [Google Scholar]
- 39.Arakawa Y, Tachibana O, Hasegawa M, Miyamori T, Yamashita J, Hayashi Y. Frequent gene amplification and overexpression of decoy receptor 3 in glioblastoma. Acta Neuropathol. 2005;109:294–298. doi: 10.1007/s00401-004-0956-6. [DOI] [PubMed] [Google Scholar]
- 40.Connor JP, Felder M. Ascites from epithelial ovarian cancer contain high levels of functional decoy receptor 3 (DcR3) and is associated with platinum resistance. Gynecol Oncol. 2008;111:330–335. doi: 10.1016/j.ygyno.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 41.Anderson GL, McIntosh M, Wu L, Barnett M, Goodman G, Thorpe JD, Bergan L, Thornquist MD, Scholler N, Kim N, O’Briant K, Drescher C, Urban N. Assessing lead time of selected ovarian cancer biomarkers: a nested case-control study. J Natl Cancer Inst. 2010;102:26–38. doi: 10.1093/jnci/djp438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang CH, Xu GL, Jia WD, Ge YS, Li JS, Ma JL, Ren WH. Prognostic significance of osteopontin in hepatocellular carcinoma: a meta-analysis. Int J Cancer. 2012;130:2685–2692. doi: 10.1002/ijc.26301. [DOI] [PubMed] [Google Scholar]