Abstract
Objective: The study aimed to observe the complications after the bilateral internal iliac arteries and the median sacral artery embolization by different severity and combinations of gelfoam particles. Methods: Sixteen healthy adult dogs were randomly divided into five groups. Under the monitoring of digital subtraction angiography (DSA), gelfoam particles with diameter of 50-150 μm were applied. In group A, embolization was performed up to the trunk of bilateral internal iliac arteries and the median sacral artery; in group B, embolization was up to the trunk of bilateral internal iliac arteries; in group C, embolization was up to the first branch of bilateral internal iliac arteries and the median sacral artery; in group D, embolization was up to the trunk of unilateral internal iliac artery and the median sacral artery; in group E embolization was performed up to the trunk of unilateral internal iliac artery. Results: Seven dogs died within 48 hours after embolization. In the dead animals of groups A, C and D, there were rectum necrosis and lamellar obfuscation and hemorrhage edema in bladder. In the histological examination, there are rectum and bladder cell dissociation, inflammatory cell infiltration and epithelial cell ablating in the dead animals. The embolization mainly presented in arterioles with a diameter of 100-200 μm. Conclusion: When gelfoam particles of 50-150 μm in diameter were applied for embolization in the internal iliac artery and median sacral artery, at least unilateral internal iliac artery should be preserved when embolization is performed in the proximal artery and the trunk.
Keywords: Canine, internal iliac artery, embolization, digital subtraction angiography, complications
Introduction
At present, the gelfoam particles have become the common embolic agents to block target vessels in bleeding due to sacral tumors and pelvic fracture [1]. However, it is difficult to achieve super selective embolization in sacral region, and in compromise blood vessels in normal tissue will be blocked too, which may lead to pelvic organ necrosis. In order to avoid these problems, the size and applications of gelfoam particles should be defined to ensure the safety, but not yet [2-6]. At the meantime, even the size of particles is clarified, there is few reports regarding the relationship of embolization range and degree with pelvic organ pathological changes. In this study, gelfoam particlesof 50~150 μm in diameter were applied in different combinations and range to embolize the bilateral internal iliac artery and the middle sacral artery and the related changes of pelvic organs were observed, which provide theoretical evidences to ensure safe application of gelfoam particles in clinical.
Materials and methods
Animals, reagents and instruments
It was approved by ethics review committee of the first affiliated hospital of Anhui Medical University. Sixteen regular healthy adult dogs, including 14 males and 2 females at weight of 10-10 kg, were provided by the Experimental Animal Center of Anhui Medical University. Gelfoam particles (Jinling Pharmaceutical Co., Ltd.) were ground and filtered by grinding machine, measured to be 50-150 μm in diameter under microscope, and sterilized by ethylene oxide. Sodium pentobarbital (Shanghai Chemical Reagent Co.). Meglumine diatrizoate mucilage (Shanghai Xudong Haipu Pharmaceutical Co., Ltd.). 5F needles, 18G needles, 5F-C2 catheter, 5F vascular sheath, and TERUMO guidewire (Cook Inc., USA). Angiography system (GE, USA).
Experimental groups and methods
The dogs were anesthetized with 3% sodium pentobarbital (1 mL/kg) through hind limb superficial vein injection. Skin was cleaned in the right groin area, femoral artery was separated from surrounding tissue for about 2.5 cm in length and ligated at the distal end, and 5F puncture needle was pierced into femoral artery at an angle of 30° and was then inserted into sheath through guide wire. Under the monitoring of angiography, 5F-C2 catheter was inserted into the contralateral iliac artery through proximal end of femoral artery and formed a loop. The catheter was pulled down and 2-3 cm contrast agent was injected to form “smoke” tracer. DSA was performed at the distal end of abdominal aorta. The left and right external iliac arteries, the distal end of abdominal aorta and its branches-bilateral internal iliac artery and middle sacral artery were monitored. The catheter tip was inserted into the target vessels,and the left and right internal iliac arteries, median sacral artery and their branches were displayed. 50% diatrizoate was used as contrast agent and the flow was controlled at 4~5 mL/s, and in total 10~15 mL of contrast agent was used.
The gelfoam particles were mixed with contrast agent and applied to block internal iliac arteries and median sacral artery under the surveillance of DSA. Sixteen experimental dogs were divided into five groups according to embolism location. In the group A (n = 3), embolization was performed up to the trunk of bilateral internal iliac arteries and median sacral artery; in the group B (n = 3), embolization was up to the trunk of bilateral internal iliac arteries; in the group C (n = 3), embolization was up to the first branch of bilateral internal iliac arteries and median sacral artery; in the group D (n = 4), embolization was up to the trunk of unilateral internal iliac artery and median sacral artery; in the group E (n = 3) embolization was performed up to the trunk of unilateral internal iliac artery (as shown in Figure 1). To block unilateral iliac artery, 2.0 mL of embolization mixture was applied, and to block its branches, 1.0 mL of embolization mixture was applied. To block the trunk of median sacral artery, 0.8 mL of embolization mixture was applied, and to block it branches, 0.5 mL of embolization mixture was applied. After embolization, the catheter, guide wire and sheath were withdrew, the proximal end of arteries was ligated, and incision was sutured.
Figure 1.
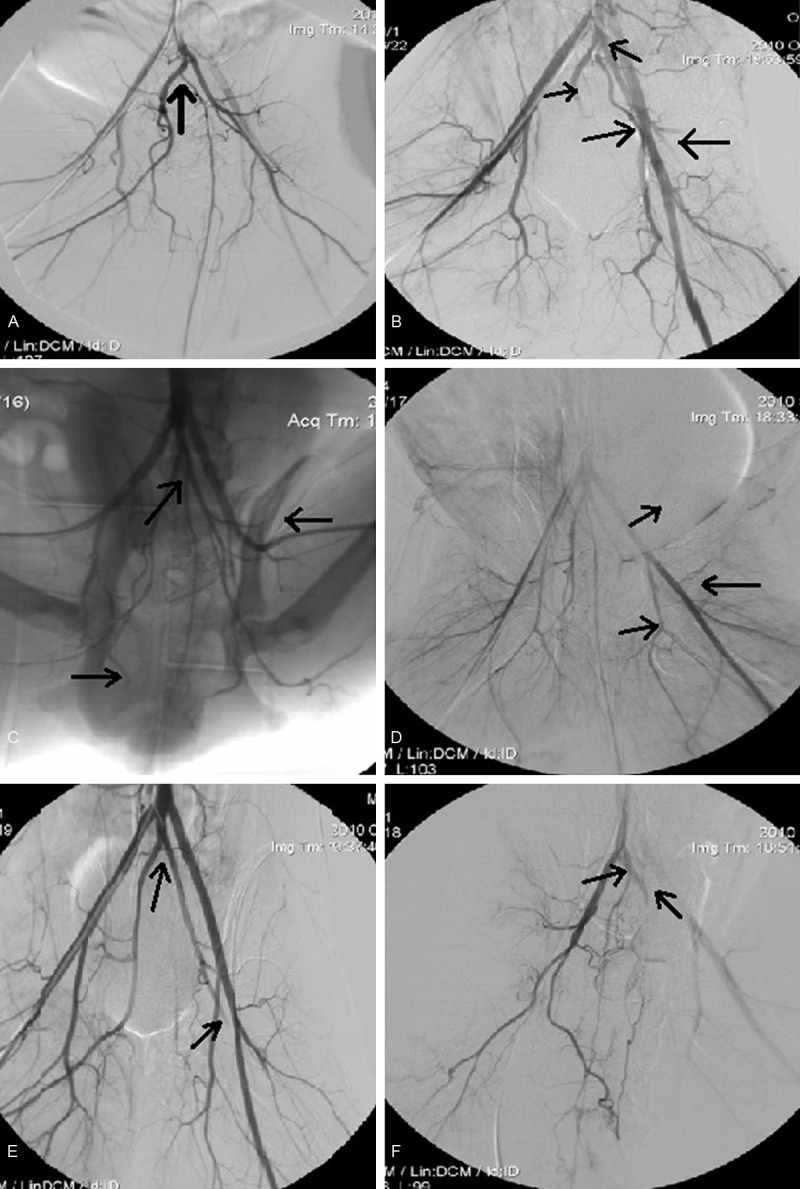
The radioimaging of internal iliac and the median sacral artery of every group. A. Group A before embolization, the median sacral artery originates from the right internal iliac artery. B. Group A embolization to the trunk of bilateral internal iliac and the median sacral artery, the median sacral artery originates from abdominal aorta artery. C. Group B embolization to the first branch of bilateral internal iliac and the median sacral artery, the median sacral artery originates from the left internal iliac artery. D. Group C embolization to the trunk of bilateral internal iliac artery, the median sacral artery originates from abdominal aorta artery. E. Group D embolization to the trunk of unilateral internal iliac and the median sacral artery, the median sacral artery originates from abdominal aorta artery. F. Group E embolization to the trunk of unilateral internal iliac artery, the median sacral artery originates from the left internal iliac artery.
Observational parameters
(1) General observation: Survival and hind legs standing situation were observed in dogs after embolization. If dogs die, then grossing is performed. (2) Grossing: Doges were anesthetized with 3% sodium pentobarbital three days after embolism. Straight incision was cut on abdomen, and curved incision was cut on hips, general changes of the rectum, bladder, hip muscles and the sciatic nerve were observed; a 20 mL syringe was used to drain and measure bladder urine. (3) Histological study: After grossing, the bladder, rectum, hip muscles and the sciatic nerve were fixed in 4% formalin, embedded in paraffin, sectioned with 5 μm thickness. Pathological changes were observed under the microscope to determine the size, diameter of the embolization blood vessel. Number of the smooth muscle fiber layers in the embolized vessel wall were used to determine the level of vasculature: the medial layer in the wall of arterioles is composed of 1-2 layers of smooth muscle fibers, while in small arteries, it is composed of 3-9 layers of smooth muscle fibers.
Results
DSA observation
Dogs have external iliac artery and internal iliac artery, but no general iliac artery. Bilateral external iliac arteries starting directly from the abdominal aorta, which continues downward after external iliac artery bifurcation and internaliliac artery and middle sacral artery (caudal artery) were separated in the end. There are variations in terms of the starting of median sacral artery, eight of sixteen dogs had middle sacral artery start at the end of the abdominal aorta after the bilateral external iliac artery bifurcation, three starting at the right internal iliac artery, and five starting at the left internal iliac artery (Figure 1).
General situation
Total of seven dogs died within two days after embolization, including three in group A, three in group C, one in group D; the rest of the dogs survived until completion of the experiment. In group B, dogs started drinking water two days after embolization, eating little; one dog resumes normal bowel movements, two had no bowel movements after embolization; three dogs could stand two days after embolization, and could walk three days after embolization. In Group D, three surviving dogs started drinking water two days after embolization; one had no bowel movement after embolization, one had diarrhea two days after embolization and one resumed normal bowel movements; the dogs could barely stand two days after embolization. In Group E, two dogs began drinking water one day after embolization, one beginning two days after embolization; one had diarrhea two days after embolization, two resumed normal bowel movements; one could stand and walk one day after embolization, the rest two dogs started standing and walking two days after embolization. In all groups, dogs were walking unsteadily within three days after embolization, showing left (puncture side) limb weakness.
Grossing
The dead dogs in group A, C and D showed rectal blackening and necrosis, the length of the necrotized rectum is 2.5~8.0 cm; the bladder is also darkened with spotty bleeding and edema, bladder is full with urine volume ranging 223~602 mL, an average of 270.6 mL. There are no obvious abnormalities in sciatic nerve and hip muscles (Figure 2).
Figure 2.
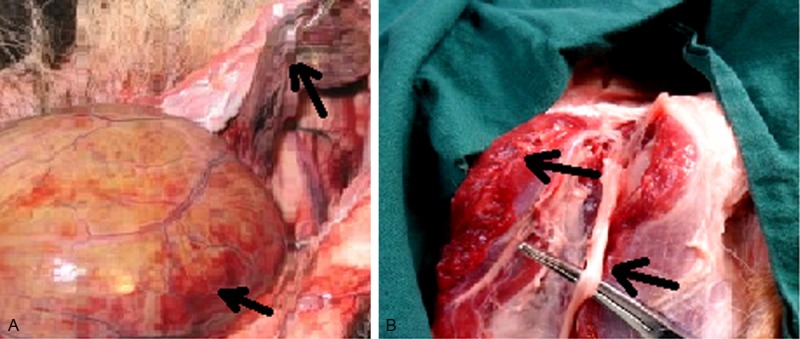
The general change 30 hours after embolization in group A. A. Rectum and bladder. B. The sciatic nerve and hip muscles.
The survived dogs in groups B, D and E showed no obvious abnormalities in the rectum three days after embolization. There was only mild sporadic edema in the bladder without necrosis; the bladder urine volume is 30~221 mL in group B with average 129.3 mL; the bladder urine volume is 14~602 mL in group D with average 264.3 mL; the bladder urine volume is 4~175 mL in group E with average 69 mL. Except one dog is group D had sciatic nerve congestion and edema, the rest showed no abnormalities. No significant changes in the hip muscles were observed (Figure 3).
Figure 3.
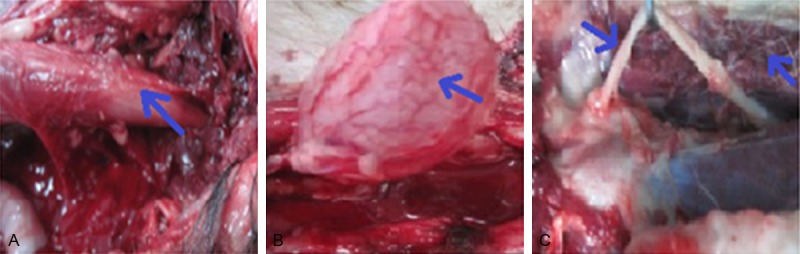
The general changes three days after embolization in group B. A. Rectum. B. Bladder. C. The sciatic nerve and muscles.
Histological studies
The dead dogs in group A, C and D group showed rectal and bladder cell disintegration, with a large number of inflammatory cell infiltration and epithelial cell shedding; no obvious abnormalities were seen for sciatic nerve and hip muscles except mild edema (Figure 4).
Figure 4.
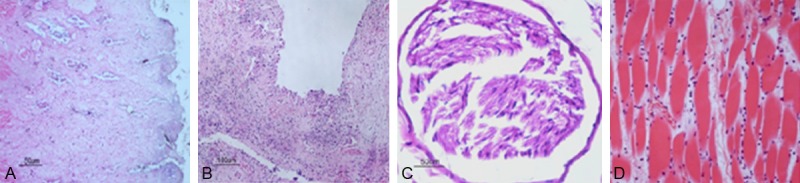
The histological changes of the dead dogs 30 hours after embolization in group A (HE × 200). A. Rectum. B. Bladder. C. Sciatic nerve. D. Hip muscles.
The survived dogs in group B, D and E showed bladder and rectal tissue edema, and mild inflammatory cell infiltration three days after embolization. No obvious abnormalities were seen for sciatic nerve and hip muscles except mild edema. The smallest artery embolized is an arteriole with 50 μm in diameter, but the majority of the embolized arteries are small arteries with 100~200 μm in diameter. The vessels were embolized with gelatin sponge in a reticular pattern, interspersed with large number of red blood cells (Figure 5).
Figure 5.
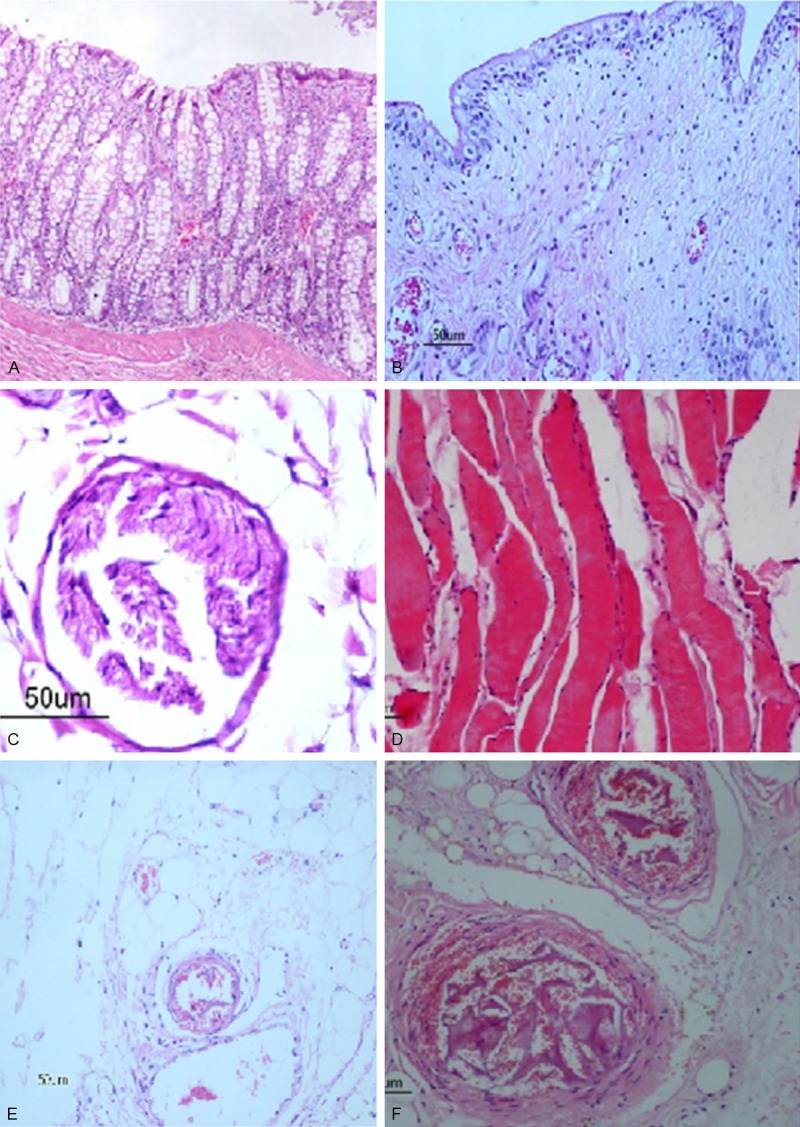
The histological changes of the dogs three days after embolization in group B (He × 200). A. Rectum. B. Bladder. C. Sciatic nerve. D. Hip muscles. E. Arteriole: the smallest embolized artery. F. Small arteries: The major mebolized artery.
Discussion
With the advancement of interventional radiology, selective blood vessel embolization with gelatin sponge particles has become an effective way to prevent and treat bleeding from sacral tumor surgery and pelvic trauma [7-9]. Whether bleeding from sacral tumor or pelvic trauma, internal iliac artery and middle sacral artery are the main blood-supplying blood vessels [10,11], so it is critical to embolize those blood vessels. The diameter of the embolic particle is an important factor affecting the embolization effects and complications: the smaller the embolic particle, the more complete the vascular bed embolized, and the more difficult the formation of collateral circulation [12], while the more likely an ectopic embolism. Some scholars believed that, because the sacral area is rich in blood supply, choosing an absorbable gelatin sponge to embolize a blood vessel before capillary level would not cause serious complications [2-4]. However, we believe that despite the rich blood supply in sacral area, to ensure safety after embolization, the diameter of the gelatin sponge particle and the area of embolization must be strictly limited. William etc. reported that when performing blood vessel embolization with gelatin sponge particles before pelvic tumor surgery, if normal tissue vascular supply cannot be avoided, gelatin sponge particles must have a diameter bigger than 1 mm in order to ensure the safety of embolization [5]. Yang etc. have chosen gelatin sponge particles with a diameter of 200~400 μm to embolize canine bilateral internal iliac and median sacral arteries, and no pelvic organ necrosis was observed after embolization [6]. Therefore, gelatin sponge particles with a diameter of 50~150 μm were chose for embolization in this study.
Our first experiment was to determine the smallest vessel embolized, which was the arteriole with a diameter of about 50 μm; but the majority of embolized arteries are small arteries with diameters of 100-200 μm, possibly because the irregular surface of the gelatin sponge particles caused adhesion to each other when flowing inside blood vessel, and that is why the diameters of embolized vessels are bigger than gelatin sponge particle’s own diameter [13]. The embolic complications are not only correlated to the minimum blood vessel, but also to the level of the proximal vasculature and embolized vessel. Because if the embolized vessel is too short, it won’t be able to effectively prevent the impact of blood flow, and may cause revascularization which results in possible formation of collateral circulation in the embolized segment and downstream blood vessels; in the meantime, short embolized vessel may also lead to rapid communication between the two unembolized ends and result in increase of blood supply to the embolized area. Our study showed that the internal iliac artery is a major blood supplier for the pelvic, and rich in collateral circulation. When bilateral internal iliac arteries were embolized, the shorter median sacral artery which has less collateral circulation cannot establish collateral circulation with peripheral blood vessels rapidly to supply blood for the pelvic, causing pelvic organ necrosis. The dogs in group E had only unilateral internal iliac artery embolization, and since there existed compensatory blood supply from contralateral internal iliac and median sacral artery, dogs survived well three days after embolization, and there were no significant changes in the pelvic organs. Dogs in group Dhad unilateral internal iliac artery embolization plus median sacral artery embolization, one dog had bladder and rectal necrosis, and died within 48 hour after embolization; the remaining three dogs survived well three days after embolization, with mild edema and inflammatory reactions in bladder and rectum.
Blood vessel embolization in dogs in each group would have some impacts in their bladder and rectal function. Bladder function after embolization can be determined by the amount of urine retained. In this study, except one dog in group B, one in group D and two dogs in group E had little bladder urine three days after embolization, the rest five dogs had urine volume over 100 mL, indicating that at least four surviving dogs resumed normal bladder function.During the experiments, we found that most normal dogs would urinate before anesthesia, but there are also dogs that did not urinate, so even though the remaining five dogs had more bladder urine retention, the possibilityof functional recovery could not be ruled out. In our experiment six surviving dogs resumed bowel movement within three days after embolization, three failed to defecate with the same time period. However, since the observation time is only three days, and number of dogs in each group is limited, so the bowel and bladder function recovery time for the rest of the survived dogs survived, and the differences among groups B, D and E could not be determined; In the meantime, bowel movement was also affected by food intake and other conditions, so it has some limitations in assessing rectal function. Dogs in each group after embolization did not show obvious pathological changes in hip muscle tissue. During blood vessel embolization before sacral tumor surgery, Chuang et al. [14] noticed foot drop and foot paralysis and attributed them to embolization of the blood vessels supplying the sciatic nerve. But our experiment did not show any obvious pathological changes in pelvic nerve in each group, except mild edema.
In summary, we think when choosing gelatin sponge particles of 50~150 μm in diameter to embolize canine internal iliac artery and median sacral artery, at least unilateral internal iliac artery should be retained if the main trunk and proximal vessel are embolized; if bilateral internal iliac arteries are simultaneously embolized with the median sacral artery, the proximal side of the embolized vessel cannot exceed the first branch. Since dog’s arterial diameter is significantly smaller than the same level artery of people, our experiments not only determined the minimum diameter of blood vessel for embolization, but also determined the level of blood vessel. Clinically, based on the complicationinduced in dogs by blood vessel embolization as shown in this study, we can choose appropriate gelatin sponge particles corresponding to the level of vessel to be embolized in human, however its feasibility and accuracy remains to be studied further.
Acknowledgements
It was funded by Anhui Provincial Health Department-funded medical research projects (09B124).
Disclosure of conflict of interest
None.
References
- 1.Ni CF, Xu M, Liu YZ. Animal studies of selective vertebral artery embolization. Chinese Journal of Radiology. 2002;36:657–660. [Google Scholar]
- 2.Broaddus WC, Grady MS, Delashaw JB Jr, Ferguson RD, Jane JA. Preoperative superselective arteriolar embolization: a new approach to enhance respectability of spinal tumors. Neumsurgery. 1990;27:755–9. [PubMed] [Google Scholar]
- 3.Jiang GM, Chen YX, Zhao JW, Huang JZ, Gao TM. Lumbar artery and iliac artery embolization in iliopsoas tumors. Journal of Jiangsu Clinical Medicine. 2001;5:l12–114. [Google Scholar]
- 4.Lin Y, Hu J, Wu C. Preoperative embolization of sacral tumors. Clinical Study of Medical Imaging. 2009;19:894–895. [Google Scholar]
- 5.Hare Ws, Holland CJ. Paresis following internal iliac artery embolization. Radiology. 1983;146:47–51. doi: 10.1148/radiology.146.1.6849068. [DOI] [PubMed] [Google Scholar]
- 6.Yang S, Cheng F, Xu W. Iliac artery embolization collateral circulation was observed after reconstruction experimental study. Spine. 2000;20:431–434. [Google Scholar]
- 7.Gottfried ON, Schmidt MH, Stevens EA. Embolization of sacral tumors. Neurosury Foucs. 2003;15:E4. doi: 10.3171/foc.2003.15.2.4. [DOI] [PubMed] [Google Scholar]
- 8.Zhou T. Modern medical treatment of severe pelvic fractures. Journal of Trauma. 2000;16:453–456. [Google Scholar]
- 9.Wang C, Zhao J, Xu H. Angiographic embolization with gelatin sponge in pelvic fracture bleeding applied in 20 cases. Chinese Journal of Clinical Rehabilitation Tissue Engineering. 2008;12:9723–9725. [Google Scholar]
- 10.Li S, Xu D, Li M. Preoperative embolization in the treatment of tumors of the sacrum value. Chinese Journal of Orthopedics. 2004;l2:977–979. [Google Scholar]
- 11.Lankford A, Senkowski CK. Bilateral external iliac artery dissections after pelvic fraction: case report. J Trauma. 1999;47:784–786. doi: 10.1097/00005373-199910000-00031. [DOI] [PubMed] [Google Scholar]
- 12.Wang YL, Han XW, Gao XM. Sacral tumor embolization before surgery. Application of Clinical Radiology. 2005;24:181–182. [Google Scholar]
- 13.Rösch J, Keller FS, Kozak B, Niles N, Dotter CT. Gelfoam powder embolization of the left gastric artery in treatment of massive small-vessel gastric bleeding. Radiology. 1984;151:365–370. doi: 10.1148/radiology.151.2.6608749. [DOI] [PubMed] [Google Scholar]
- 14.Chuang VP, Soo CS, Wallace S, Benjamin RS. Arterial occlusion: management of giant cell tumor and aneurysmal bone cyst. AJR Am J Roentgenol. 1981;136:1127–30. doi: 10.2214/ajr.136.6.1127. [DOI] [PubMed] [Google Scholar]


