Abstract
Introduction
Previous studies have identified hyperlipidemia as a potential risk factor for dementia and Alzheimer’s disease. However, studies on cholesterol measured in late-life and cognitive function have been inconsistent. Few studies have explored nonlinear relationships or considered interactions with other biomarker measures.
Methods
A cross-sectional sample of 1,889 participants from four rural counties in the People’s Republic of China was included in this analysis. Serum total cholesterol, high-density lipoprotein, triglycerides, and homocysteine levels were measured in fasting blood samples. A composite cognitive score was derived based on nine standardized cognitive test scores. Analysis of covariance models were used to investigate the association between biomarker measures and the composite cognitive scores.
Results
There was a significant interaction between the homocysteine quartile group and the cholesterol quartile group on cognitive scores (P=0.0478). In participants with normal homocysteine levels, an inverse U-shaped relationship between total cholesterol level and cognitive score was found, indicating that both low and high cholesterol levels were associated with lower cognitive scores. In participants with high homocysteine levels, no significant association between cholesterol and cognition was found.
Conclusion
The relationship between cholesterol levels and cognitive function depends upon homocysteine levels, suggesting an interactive role between cholesterol and homocysteine on cognitive function in the elderly population. Additional research is required to confirm our findings in other populations, and to explore potential mechanisms underlying the lipid–homocysteine interaction.
Keywords: cholesterol, homocysteine, cognitive function
Introduction
With the increasing public health challenge of dementia and cognitive impairment throughout the world, the identification of a modifiable risk factor would be critical in the prevention of dementia.1 Previous studies have indicated that hyperlipidemia may be a risk factor for dementia and Alzheimer’s disease (AD).2–4 However, although earlier results suggested a link between high midlife cholesterol levels and increased dementia risk,5–8 later studies found no association between midlife cholesterol and subsequent dementia.9–12 In addition, studies on late-life cholesterol levels conflicted with some studies reporting that low late-life cholesterol was associated with increased dementia risk, although others found no association.2,13,14
One potential explanation for the inconsistencies in the relationship between cholesterol and cognitive function is that it may be due to a confounding treatment effect. Most previous studies were from developed countries in which individuals with high cholesterol levels had been aggressively treated with lipid-lowering medications, some of which have been shown to be potentially protective of dementia risk.3 Thus, cholesterol levels, particularly those measured in late-life, may reflect a combination of the detrimental effect of high cholesterol and the protective effect of lipid-lowering medications, making it more difficult to untangle the two opposite effects in such populations. Another potential explanation for the conflicting results on cholesterol and cognition is the failure to control for other relevant risk factors. One recent meta-analysis based on cohort studies found that higher levels of homocysteine were associated with increased dementia risk,15 but no studies to date have considered potential interactions between cholesterol and homocysteine on cognitive function. We hypothesize that a clear relationship between cholesterol and cognitive function may be observed in populations in which few individuals take cholesterol-lowering medications and in which other biomarkers are measured. In this article, we report results from a cross-sectional study of cholesterol, homocysteine, and cognitive function in a rural, elderly Chinese cohort.
Methods
Study population
Participants for this study were recruited on two occasions. Between December 2003 and May 2005, 2,000 individuals aged 65 years and over from four counties in the People’s Republic of China were enrolled. Two counties were from Sichuan province in southwestern People’s Republic of China and the other two counties were from Shandong province in eastern People’s Republic of China. Cognitive assessment was conducted at baseline, and at 2.5 years and 7 years after baseline. Of the 2,000 participants enrolled during 2003–2005, 1,248 were reevaluated during 2010–2012.
A second enrollment was conducted between 2010 and 2012, approximately 7 years after the baseline study was conducted. Individuals from the four original study sites who had since turned 65 years old, and who were not enrolled in the original cohort, were invited to participate in the study. Eight-hundred-and-eight individuals were enrolled during the second enrollment wave; thus 2,056 participants underwent cognitive assessment during 2010–2012. This article reports the analysis of the data obtained during the 2010–2012 evaluation. The study was approved by the Indiana University (IU) Institutional Review Board and the Institute for Environmental Health and Related Safety, Chinese Center for Disease Control and Prevention. Details of the study were described previously.16
Biomarker measures
Blood samples were collected during the 2010–2012 evaluation. Of 2,056 individuals evaluated with the cognitive assessment, 1,889 (91.9%) consented to blood sample collection. Fasting blood samples were collected in the morning using 10 mL purple top (containing ethylenediaminetetraacetic acid) Vacutainer® tubes manufactured in the United States. Serum homocysteine, triglycerides, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) were measured using Roche Diagnostic kits and a Hitachi Automatic Biochemistry Analyzer 9700. Total cholesterol levels were calculated using the sum of the LDL and HDL plus one-fifth of triglyceride levels. APOE genotype was determined by the multiplex tetra-primer amplification refractory mutation system polymerase chain reactions.17
Cognitive assessment
Cognitive assessment was conducted in face-to-face interviews using nine cognitive tests: the Community Screening Instrument for Dementia (CSID); the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) ten-word list learning; word list recall;18 IU Story Recall;19 Animal Fluency test;20 Boston Naming Test;21 Stick Design; Delayed Stick Design;22 and the IU Token test.23,24 The CSID was developed as a screening tool for dementia in populations with various cultural backgrounds and literacy levels. Details of the instrument have been published elsewhere.25 The CSID cognitive assessment items measure the following functions in a short interview with the study participants: memory; abstract thinking; judgment; and other disturbances of higher cortical function (aphasia, apraxia, agnosia, and constructional difficulty). The CSID has demonstrated both good 2-week test–retest reliability and interrater reliability, in addition to good validity in detecting dementia in various populations.25,26 CSID has 30 items and scores range from 0–30.
The CERAD Word List Learning test is one of the measures from the CERAD neuropsychological assessment battery, which was designed to assess cognitive skills in the elderly.18 It consists of a ten-item, three-trial word list in which free recall is taken after each learning trial and after a brief delay (approximately 5 minutes). The score is the total number of words recalled across the three learning trials (range: 0–30) and at delay (range: 0–10). The IU Story Recall task19 was created by the research team to be suitable to the Chinese culture and the rural population. The examiner reads the story out loud to the subject, who then attempts to recall the story verbatim. The story has 14 units of information that are gist scored (range: 0–14), meaning a recalled answer is deemed correct if the main point is recalled, in contrast to verbatim. The story was tested in 1,500 elderly Chinese adults in a previous pilot study and was found to be acceptable to the villagers; it produced a normally distributed range of scores.27 The Animal Fluency test is a measure of executive function in which a subject names as many animals as possible in 60 seconds.20 The IU Token test is a brief measure of language comprehension and working memory (12 items; range: 0–24).23 The validity of the CSID, CERAD Word List Learning and recall, and the Animal Fluency test have been previously established in the Chinese population and elsewhere.28 In this analysis, a standardized score for each cognitive test was created by subtracting the sample mean and dividing it by the sample standard deviation (SD). A composite cognitive Z-score was derived using the average of standardized scores of the nine cognitive tests.
Other information
Other information collected during the interview included age, sex, whether the participant attended school and years of schooling, marital status, household composition, alcohol consumption and smoking history, as well as a history of cancer, Parkinson’s disease, diabetes, hypertension, stroke, heart attack, head injury, and bone fracture. For each reported medical condition, participants were also asked whether they were taking medications for the condition. None of the participants reported hyperlipidemia, and none reported taking medication for this condition. Participants’ height, weight, and blood pressure (measured twice) were measured during the interview. Body mass index (BMI) was derived from height and weight measurements. The average of the two blood pressure measures were used in our analyses.
Statistical analysis
Univariate analysis of covariance (ANCOVA) models using the composite cognitive Z-score as the dependent variable and each participant’s characteristics as the independent variables were used to select potential covariates associated with cognitive score. To detect potential nonlinear relationships between biomarker measures and cognitive scores, we divided biomarker values into quartile groups and used quartile groups in both univariate and multivariate ANCOVA models. We also examined interactions among significant markers in multivariate models and conducted a stratified analysis when significant interactions were detected. In addition, we explored potential interactions between APOEe4 carrier status and each of the biomarkers.
Results
Participants (n=2,055) completed the cognitive assessment during the 2010–2012 evaluation. This analysis included 1,889 participants with both biomarker measures and cognitive test scores. In Table 1, we present data on the patients’ demographic characteristics and on the medical history of the participants. The mean age (SD) of the participants in the analysis was 73.5 years (5.9 years), with 53.9% being female. The average number of years (SD) of education was low at 2.4 years (3.0 years), and the average BMI (SD) was 23.1 (3.8), which was within the normal weight range.
Table 1.
Demographic characteristics of the study population (N=1,889)
| Participant characteristics | Mean or N | SD or % |
|---|---|---|
| Age (years) | 73.45 | 5.93 |
| Female (%) | 1,017 | 53.9 |
| Years of education | 2.39 | 3.00 |
| Body mass index (kg/m2) | 23.08 | 3.77 |
| Systolic blood pressure | 152.21 | 27.87 |
| Diastolic blood pressure | 86.18 | 12.83 |
| Consume alcohol (%) | 725 | 38.4 |
| Smoker (%) | 750 | 39.7 |
| APOEe4 carriers | 299 | 15.9 |
| History of (%) | ||
| Parkinson’s | 10 | 0.5 |
| Diabetes | 78 | 4.1 |
| Hypertension | 1,316 | 69.7 |
| Stroke | 72 | 3.8 |
| Heart disease | 277 | 14.7 |
| Arthritis | 223 | 11.8 |
| Thyroid disease | 12 | 0.6 |
| Kidney disease | 34 | 1.8 |
| Liver disease | 19 | 1.0 |
| Cancer | 21 | 1.1 |
| Head injury | 71 | 3.8 |
| Fracture | 167 | 8.9 |
Abbreviations: N, number of participants; SD, standard deviation.
In Table 2, we present summary data on the cognitive tests in calculating the composite Z-score. In Table 3, we present the summary data of biomarker measures. Approximately one-half of the participants (the first [Q1] and second [Q2] quartile groups) had homocysteine levels in the normal range (<14 μmol/L), and three-quarters of the participants (the first three quartile groups, Q1–Q3) had cholesterol levels in the normal range (<200 mg/dL). Of all biomarkers considered in univariate models, total cholesterol, LDL, and homocysteine levels were significantly related to composite cognitive scores (P<0.0001). HDL, triglyceride, glucose, or APOE status were not significantly associated with cognitive function. Since LDL and total cholesterol were highly correlated, we concentrated on describing the results using cholesterol quartile groups in the final multivariate models. Results for LDL were similar. In the multivariate model adjusting for age, sex, years of education, BMI, smoking, and history of heart disease, we found a significant interaction between the homocysteine quartile group and the cholesterol quartile group (P=0.0478), indicating that the association between cholesterol levels and cognitive scores depends on homocysteine levels.
Table 2.
Summary of cognitive functions measured in the study population (N=1,889)
| Cognitive functions | Summary measures
|
||||
|---|---|---|---|---|---|
| Mean | SD | Min | Median | Max | |
| CSID test | 25.84 | 3.54 | 6.00 | 27.00 | 30.00 |
| IU Story Recall test | 5.06 | 3.13 | 0.00 | 5.00 | 14.00 |
| Animal Fluency test | 13.36 | 4.93 | 0.00 | 13.00 | 32.00 |
| CERAD Word Listing Learning test | 13.24 | 4.46 | 0.00 | 13.00 | 29.00 |
| CERAD Word Listing Recall test | 4.45 | 2.30 | 0.00 | 5.00 | 10.00 |
| IU Token test | 16.66 | 5.26 | 0.00 | 18.00 | 24.00 |
| Boston Naming test | 13.73 | 3.54 | 0.00 | 14.00 | 20.00 |
| Stick Design test | 10.40 | 2.54 | 0.00 | 12.00 | 12.00 |
| Delayed Stick Design test | 5.01 | 2.69 | 0.00 | 5.00 | 12.00 |
Abbreviations: N, number of participants; SD, standard deviation; Min, minimum; Max, maximum; CSID, Community Screening Instrument for Dementia; IU, Indiana University; CERAD, the Consortium to Establish a Registry for Alzheimer’s Disease.
Table 3.
Summary of biomarker measures (N=1,889)
| Biomarker | Mean | SD | Median | Interquartile range |
|---|---|---|---|---|
| Total cholesterol level (mg/dL) | 158.9 | 47.1 | 154.3 | (125.3, 186.7) |
| HDL (mg/dL) | 49.3 | 17.7 | 46.0 | (37.1, 58) |
| LDL (mg/dL) | 91.1 | 35.2 | 88.9 | (65.0, 112.1) |
| Triglycerides (mg/dL) | 40.6 | 32.1 | 32.9 | (22.8, 48.3) |
| Homocysteine (μmol/L) | 17.2 | 8.4 | 15.3 | (11.8, 20.1) |
| Glucose (mg/dL) | 85.9 | 30.3 | 79.3 | (69.7, 92.1) |
| Cholesterol quartile groups | ||||
| Q1 (≤125.26 mg/dL) | 104.3 | 16.0 | 106.7 | |
| Q2 (125.26, 154.25 mg/dL) | 140.1 | 8.4 | 139.6 | |
| Q3 (154.25, 186.73 mg/dL) | 170.0 | 9.5 | 170.1 | |
| Q4 (>186.73 mg/dL) | 222.4 | 30.4 | 214.9 | |
| Homocysteine quartile groups | ||||
| Q1 (≤11.81 μmol/L) | 9.4 | 1.9 | 9.8 | |
| Q2 (11.81, 15.25 μmol/L) | 13.5 | 0.9 | 13.4 | |
| Q3 (15.25, 20.12 μmol/L) | 17.5 | 1.4 | 17.4 | |
| Q4 (>20.12 μmol/L) | 28.5 | 8.5 | 25.9 | |
Abbreviations: N, number of participants; SD, standard deviation; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Q, quartile group.
In Table 4, we present ANCOVA models for the relationship between cholesterol levels and cognitive scores stratified by homocysteine quartile groups, while adjusting for other covariates. An inverse U-shaped relationship between cholesterol level and cognitive scores was observed in the group with the lowest homocysteine level. Using the highest cholesterol group as the reference, participants in the lowest cholesterol group had significantly lower cognitive scores (P=0.0166), and those in the third cholesterol quartile group (Q3) had significantly higher cognitive scores (P=0.0148) than those in the reference group. It is worth noting that participants in the Q3 group with cholesterol levels ranging from 154.6–186.7 mg/dL had the highest cognitive scores.
Table 4.
Results of ANCOVA models with composite cognitive scores as the outcome variable, cholesterol Qs as independent variables stratified by homocysteinea
| Homocysteine | Cholesterol | Total cohort (N=1,889)
|
Sub-sample (N=1,700)b
|
||||
|---|---|---|---|---|---|---|---|
| Estimation | SE | P-value | Estimation | SE | P-value | ||
| Q1 (≤11.81 μmol/L) | Q1 (≤125.26 mg/dL) | −0.18 | 0.08 | 0.0208* | −0.19 | 0.07 | 0.0063* |
| Q2 (125.26, 154.25 mg/dL) | −0.08 | 0.08 | 0.3078 | −0.03 | 0.07 | 0.6506 | |
| Q3 (154.25, 186.73 mg/dL) | 0.20 | 0.08 | 0.0146* | 0.15 | 0.07 | 0.0342 | |
| Q4 (>186.73 mg/dL) | Reference | – | – | Reference | – | – | |
| Q2 (11.81, 15.25 μmol/L) | Q1 (≤125.26 mg/dL) | −0.16 | 0.08 | 0.0355* | −0.10 | 0.07 | 0.1342 |
| Q2 (125.26, 154.25 mg/dL) | −0.15 | 0.08 | 0.0506 | −0.07 | 0.07 | 0.2960 | |
| Q3 (154.25, 186.73 mg/dL) | 0.01 | 0.08 | 0.8820 | 0.06 | 0.07 | 0.3653 | |
| Q4 (>186.73 mg/dL) | Reference | – | – | Reference | – | – | |
| Q3 (15.25, 20.12 μmol/L) | Q1 (≤125.26 mg/dL) | −0.22 | 0.08 | 0.0063* | −0.23 | 0.07 | 0.0017* |
| Q2 (125.26, 154.25 mg/dL) | −0.06 | 0.07 | 0.4055 | −0.13 | 0.07 | 0.0490 | |
| Q3 (154.25, 186.73 mg/dL) | 0.00 | 0.07 | 0.9613 | −0.05 | 0.07 | 0.4076 | |
| Q4 (>186.73 mg/dL) | Reference | – | – | Reference | – | – | |
| Q4 (>20.12 μmol/L) | Q1 (≤125.26 mg/dL) | −0.06 | 0.08 | 0.4372 | −0.05 | 0.07 | 0.4335 |
| Q2 (125.26, 154.25 mg/dL) | 0.06 | 0.09 | 0.5061 | 0.00 | 0.07 | 0.9747 | |
| Q3 (154.25, 186.73 mg/dL) | −0.05 | 0.08 | 0.5209 | −0.02 | 0.06 | 0.7252 | |
| Q4 (>186.73 mg/dL) | Reference | – | – | Reference | – | – | |
| Normal homocysteine (≤14 μmol/L) | Q1 (≤125.26 mg/dL) | −0.17 | 0.06 | 0.0050 | −0.14 | 0.05 | 0.0103 |
| Q2 (125.26, 154.25 mg/dL) | −0.09 | 0.06 | 0.1219 | −0.02 | 0.05 | 0.6965 | |
| Q3 (154.25, 186.73 mg/dL) | 0.12 | 0.06 | 0.0411 | 0.12 | 0.05 | 0.0220 | |
| Q4 (>186.73 mg/dL) | Reference | – | – | Reference | – | – | |
Notes:
All models were adjusted for age, sex, years of education, smoking status, body mass index, and history of heart disease.
Excluding participants in the bottom 10% of composite cognitive scores.
Statistically significant values.
Abbreviations: ANCOVA, analysis of covariance; N, number of participants; Q, quartile group; SE, standard error.
For participants in the middle two homocysteine quartile groups (Q2 and Q3) with levels from 11.82 μmol/L to 20.12 μmol/L, those with the lowest cholesterol levels had significantly lower cognitive scores than the highest cholesterol group. For participants in the highest homocysteine group (Q1), however, there was no significant association between cholesterol and cognitive scores. Figure 1 displays predicted mean cognitive Z-scores in the four cholesterol groups stratified by homocysteine quartile groups. A curvilinear relationship between cholesterol and cognitive score can be observed in the first homocysteine quartile group, but not in the other homocysteine groups.
Figure 1.
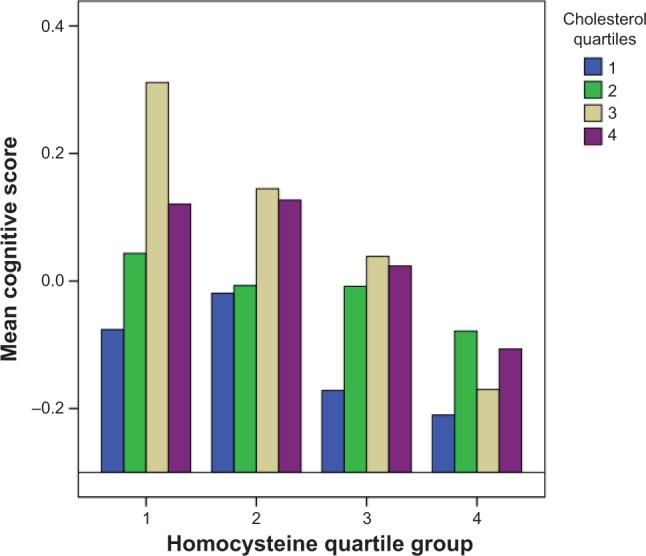
Predicted mean composite cognitive scores by homocysteine and cholesterol quartile groups.
Notes: Homocysteine quartile groups are divided into Q1 (≤11.81 μmol/L); Q2 (11.81, 15.25 μmol/L); Q3 (15.25, 20.12 μmol/L); Q4 (>20.12 μmol/L). Cholesterol quartile groups are divided into Q1 (≤125.26 mg/dL); Q2 (125.26, 154.25 mg/dL); Q3 (154.25, 186.73 mg/dL); Q4 (>186.73 mg/dL).
There were 804 participants with homocysteine in the normal range (≤14 μmol/L).29 A similar inverse U-shape was detected when we fitted another ANCOVA model that was restricted to these participants with normal homocysteine. To ensure that significant associations found in this analysis were not due to poor nutritional status of participants with prevalent dementia or cognitive impairment, we repeated the multivariate ANCOVA models shown in Table 3 by excluding participants in the bottom 10% of the composite cognitive Z-scores. Results were similar to those obtained using the entire sample.
Discussion
In this rural elderly Chinese population, we found that the relationship between cholesterol levels and cognitive function is homocysteine-dependent. In participants with normal homocysteine levels, an inverse U-shaped relationship between total cholesterol levels and cognitive score was found indicating that both low and high cholesterol levels were associated with lower cognitive scores. Our results suggest that as far as cognitive function is concerned, the optimal cholesterol level for individuals with normal homocysteine levels was centered at 170 mg/dL, below the standard treatment cutoff point of 200 mg/dL for hyperlipidemia. In participants with high homocysteine levels, no significant association between cholesterol and cognition was found.
Few previous studies on the association between cholesterol levels and cognitive scores included both cholesterol and homocysteine in identical models, or considered nonlinear trends. No studies to date have explored the interaction between these two vascular risk markers. Of the studies focusing on cholesterol alone, conflicting results have been reported on the relationship between cholesterol, dementia, and cognition.3,4 In studies measuring cholesterol in those aged 65 years or older, high cholesterol levels were reported to be associated with AD30,31 or cognitive impairment.32 However, others found that low cholesterol levels were associated with AD,33 dementia,14,34 or cognitive decline,35 although some studies reported no association between cholesterol levels and dementia risk.12,36 The inconsistency in results was often attributed to the age-related cholesterol decline.9 However, unless it can be shown that the age-related decline in cholesterol differentiates between those with cognitive impairment and those with normal cognition, age-related decline in cholesterol cannot explain the observed discrepancy in results.
Another less often mentioned contributing factor to the inconsistency in results may be the confounding effect of statins and other lipid-lowering medications widely used in the elderly population. Given the potential protective effects of some lipid-lowering medications on cognition,3 analysis without the appropriate adjustment to medications may fail to detect meaningful associations between cholesterol levels and cognitive function. In this Chinese cohort, few participants took regular medications; therefore, the measured cholesterol levels were probably not associated with the confounding effects from lipid medications.
Most previous studies focused on a linear relationship between cholesterol levels and cognitive function, and many did not consider other potential biomarkers. Our results suggest a more complex relationship between cholesterol and cognition that is homocysteine-dependent. In participants with normal homocysteine levels, both low and high cholesterol levels may be detrimental to cognitive health. In addition, our models indicated that optimal cognitive scores are achieved with cholesterol levels centered at 170 mg/dL for participants with normal homocysteine. However, in those with high homocysteine, the effect of high homocysteine on cognition was overwhelming regardless of cholesterol levels.
There are several plausible mechanisms that may underlie a homocysteine-dependent relationship between cholesterol and cognitive function. In animal studies, increased homocysteine in the diet enhanced cortex blood–brain barrier leakage, which could contribute to the deterioration of cognitive function.37 High levels of homocysteine have been associated with increased risk for both cardiovascular disease and all-cause mortality,38,39 but the association between homocysteine and dementia or cognitive decline has not been consistently shown.40 Animal, cellular, and autopsy studies have concluded that cholesterol plays an important role in brain function through synapse formation, membrane organization, amyloid-β production and deposition, and inflammation; thus, it is essential to maintain a certain cholesterol level for normal functioning.3 Under normal homocysteine levels, where the blood–brain barrier is intact, cholesterol levels are maintained to achieve optimal cognitive function. Further, studies on cardiovascular disease risk have found a homocysteine and lipid interaction showing that higher homocysteine levels increased disease risk regardless of lipid levels; however, a low level of plasma cholesterol did not seem to confer protection against the risk associated with increased plasma homocysteine.41
Our study has many strengths. The study cohort was relatively large, allowing for the investigation of potential interactions among biomarker measures. A comprehensive set of cognitive measures was used in our study, ensuring robust results in grouping participants according to their composite cognitive scores. The cohort was unusual because few studies with cognitive and biomarker measures have been conducted in rural elderly Chinese populations.
Finally, this study has important limitations. Our analysis is a cross-sectional study, and thus the observed association could be subject to reverse causation. However, similar results were found after we excluded participants with the worst cognitive scores, which indicates that reverse causation is unlikely. Nevertheless, longitudinal studies will be valuable to confirm our results, and may lead to potential interventions for cognitive impairment because both cholesterol and homocysteine can be effectively controlled by current medications. Another potential limitation is that our study was conducted in a population with a low level of education and with average BMI scores. It remains to be seen whether our results can be confirmed in other populations.
Conclusion
In conclusion, we found that the relationship between cholesterol levels and cognitive function depends on homocysteine levels, which suggests an interactive role between cholesterol and homocysteine in cognitive function in the elderly population. Further research is required to confirm our findings in other populations and to explore potential mechanisms underlying the lipid–homocysteine interaction.
Acknowledgments
This research was supported by grants from the National Institutes of Health: R01 AG019181 and P30 AG10133.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ferri CP, Prince M, Brayne C, et al. Alzheimer’s Disease International Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anstey KJ, Lipnicki DM, Low LF. Cholesterol as a risk factor for dementia and cognitive decline: a systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry. 2008;16(5):343–354. doi: 10.1097/JGP.0b013e31816b72d4. [DOI] [PubMed] [Google Scholar]
- 3.van Vliet P. Cholesterol and late-life cognitive decline. J Alzheimers Dis. 2012;30(Suppl 2):S147–S162. doi: 10.3233/JAD-2011-111028. [DOI] [PubMed] [Google Scholar]
- 4.Purnell C, Gao S, Callahan CM, Hendrie HC. Cardiovascular risk factors and incident Alzheimer disease: a systematic review of the literature. Alzheimer Dis Assoc Disord. 2009;23(1):1–10. doi: 10.1097/WAD.0b013e318187541c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kivipelto M, Helkala EL, Laakso MP, et al. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med. 2002;137(3):149–155. doi: 10.7326/0003-4819-137-3-200208060-00006. [DOI] [PubMed] [Google Scholar]
- 6.Notkola IL, Sulkava R, Pekkanen J, et al. Serum total cholesterol, apolipoprotein E epsilon4 allele, and Alzheimer’s disease. Neuroepidemiology. 1998;17(1):14–20. doi: 10.1159/000026149. [DOI] [PubMed] [Google Scholar]
- 7.Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord. 2009;28(1):75–80. doi: 10.1159/000231980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64(2):277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 9.Mielke MM, Zandi PP, Shao H, et al. The 32-year relationship between cholesterol and dementia from midlife to late life. Neurology. 2010;75(21):1888–1895. doi: 10.1212/WNL.0b013e3181feb2bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalmijn S, Foley D, White L, et al. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men. The Honolulu-Asia aging study. Arterioscler Thromb Vasc Biol. 2000;20(10):2255–2260. doi: 10.1161/01.atv.20.10.2255. [DOI] [PubMed] [Google Scholar]
- 11.Stewart R, White LR, Xue QL, Launer LJ. Twenty-six-year change in total cholesterol levels and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2007;64(1):103–107. doi: 10.1001/archneur.64.1.103. [DOI] [PubMed] [Google Scholar]
- 12.Tan ZS, Seshadri S, Beiser A, et al. Plasma total cholesterol level as a risk factor for Alzheimer disease: the Framingham Study. Arch Intern Med. 2003;163(9):1053–1057. doi: 10.1001/archinte.163.9.1053. [DOI] [PubMed] [Google Scholar]
- 13.Mielke MM, Zandi PP, Sjögren M, et al. High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology. 2005;64(10):1689–1695. doi: 10.1212/01.WNL.0000161870.78572.A5. [DOI] [PubMed] [Google Scholar]
- 14.Reitz C, Tang MX, Luchsinger J, Mayeux R. Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch Neurol. 2004;61(5):705–714. doi: 10.1001/archneur.61.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wald DS, Kasturiratne A, Simmonds M. Serum homocysteine and dementia: meta-analysis of eight cohort studies including 8,669 participants. Alzheimers Dement. 2011;7(4):412–417. doi: 10.1016/j.jalz.2010.08.234. [DOI] [PubMed] [Google Scholar]
- 16.Gao S, Jin Y, Hall KS, et al. Selenium level and cognitive function in rural elderly Chinese. Am J Epidemiol. 2007;165(8):955–965. doi: 10.1093/aje/kwk073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SW, Heo JH, Kim CH, et al. Rapid and direct detection of apolipoprotein E genotypes using whole blood from humans. J Toxicol Environ Health A. 2010;73(21–22):1502–1510. doi: 10.1080/15287394.2010.511573. [DOI] [PubMed] [Google Scholar]
- 18.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 19.Emsley CL, Gao S, Li Y, et al. Trace element levels in drinking water and cognitive function in elderly Chinese. Am J Epidemiol. 2000;151(9):913–920. doi: 10.1093/oxfordjournals.aje.a010295. [DOI] [PubMed] [Google Scholar]
- 20.Isaacs B, Akhtar AJ. The set test: a rapid test of mental function in old people. Age Ageing. 1972;1(4):222–226. doi: 10.1093/ageing/1.4.222. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. 1st ed. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- 22.Baiyewu O, Unverzagt FW, Lane KA, et al. The Stick Design test: a new measure of visuoconstructional ability. J Int Neuropsychol Soc. 2005;11(5):598–605. doi: 10.1017/S135561770505071X. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto K, Evans JD, Johnson KE, Unverzagt FW. Clinical utility of IU Token Test in the diagnosis of dementia. J Int Neuropsychol Soc. 2003;9:316. [Google Scholar]
- 24.Snitz BE, Unverzagt FW, Chang CC, et al. Effects of age, gender, education and race on two tests of language ability in community-based older adults. Int Psychogeriatr. 2009;21(6):1051–1062. doi: 10.1017/S1041610209990214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall KS, Ogunniyi AO, Hendrie HC, et al. A cross-cultural community based study of dementias: methods and performance of the survey instrument: Indianapolis, USA and Ibadan, Nigeria. Int J Methods Psychiatr Res. 1996;6(3):129–142. [Google Scholar]
- 26.Hall KS, Gao S, Emsley CL, Ogunniyi AO, Morgan O, Hendrie HC. Community screening interview for dementia (CSI ‘D’); performance in five disparate study sites. Int J Geriatr Psychiatry. 2000;15(6):521–531. doi: 10.1002/1099-1166(200006)15:6<521::aid-gps182>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 27.Emsley CL, Gao S, Li Y, et al. Trace element levels in drinking water and cognitive function among elderly Chinese. Am J Epidemiol. 2000;151(9):913–920. doi: 10.1093/oxfordjournals.aje.a010295. [DOI] [PubMed] [Google Scholar]
- 28.Prince M, Acosta D, Chiu H, Scazufca M, Varghese M, 10/66 Dementia Research Group Dementia diagnosis in developing countries: a cross-cultural validation study. Lancet. 2003;361(9361):909–917. doi: 10.1016/S0140-6736(03)12772-9. [DOI] [PubMed] [Google Scholar]
- 29.Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346(7):476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 30.Evans RM, Hui S, Perkins A, Lahiri DK, Poirier J, Farlow MR. Cholesterol and APOE genotype interact to influence Alzheimer disease progression. Neurology. 2004;62(10):1869–1871. doi: 10.1212/01.wnl.0000125323.15458.3f. [DOI] [PubMed] [Google Scholar]
- 31.Hall K, Murrell J, Ogunniyi A, et al. Cholesterol, APOE genotype, and Alzheimer disease: an epidemiologic study of Nigerian Yoruba. Neurology. 2006;66(2):223–227. doi: 10.1212/01.wnl.0000194507.39504.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yaffe K, Barrett-Connor E, Lin F, Grady D. Serum lipoprotein levels, statin use, and cognitive function in older women. Arch Neurol. 2002;59(3):378–384. doi: 10.1001/archneur.59.3.378. [DOI] [PubMed] [Google Scholar]
- 33.Kuusisto J, Koivisto K, Mykkänen L, et al. Association between features of the insulin resistance syndrome and Alzheimer’s disease independently of apolipoprotein E4 phenotype: cross sectional population based study. BMJ. 1997;315(7115):1045–1049. doi: 10.1136/bmj.315.7115.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romas SN, Tang MX, Berglund L, Mayeux R. APOE genotype, plasma lipids, lipoproteins, and AD in community elderly. Neurology. 1999;53(3):517–521. doi: 10.1212/wnl.53.3.517. [DOI] [PubMed] [Google Scholar]
- 35.van den Kommer TN, Dik MG, Comijs HC, Fassbender K, Lütjohann D, Jonker C. Total cholesterol and oxysterols: early markers for cognitive decline in elderly? Neurobiol Aging. 2009;30(4):534–545. doi: 10.1016/j.neurobiolaging.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Li G, Shofer JB, Kukull WA, et al. Serum cholesterol and risk of Alzheimer disease: a community-based cohort study. Neurology. 2005;65(7):1045–1050. doi: 10.1212/01.wnl.0000178989.87072.11. [DOI] [PubMed] [Google Scholar]
- 37.Ehrlich D, Pirchl M, Humpel C. Effects of long-term moderate ethanol and cholesterol on cognition, cholinergic neurons, inflammation, and vascular impairment in rats. Neuroscience. 2012;205:154–166. doi: 10.1016/j.neuroscience.2011.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bates CJ, Mansoor MA, Pentieva KD, Hamer M, Mishra GD. Biochemical risk indices, including plasma homocysteine, that prospectively predict mortality in older British people: the National Diet and Nutrition Survey of People Aged 65 Years and Over. Br J Nutr. 2010;104(6):893–899. doi: 10.1017/S0007114510001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hackam DG, Anand SS. Emerging risk factors for atherosclerotic vascular disease: a critical review of the evidence. JAMA. 2003;290(7):932–940. doi: 10.1001/jama.290.7.932. [DOI] [PubMed] [Google Scholar]
- 40.Ho RC, Cheung MW, Fu E, et al. Is high homocysteine level a risk factor for cognitive decline in elderly? A systematic review, meta-analysis, and meta-regression. Am J Geriatr Psychiatry. 2011;19(7):607–617. doi: 10.1097/JGP.0b013e3181f17eed. [DOI] [PubMed] [Google Scholar]
- 41.Daly C, Fitzgerald AP, O’Callaghan P, et al. Homocysteine increases the risk associated with hyperlipidaemia. Eur J Cardiovasc Prev Rehabil. 2009;16(2):150–155. doi: 10.1097/HJR.0b013e32831e1185. [DOI] [PubMed] [Google Scholar]


