Abstract
The immune system responds to cues in the microenvironment to make acute and chronic adaptations in response to inflammation and injury. Locally produced purine nucleotides and adenosine provide receptor-mediated signaling to all bone-marrow derived cells of the immune system to modulate their responses. This review summarizes recent advances in our understanding of the effects of adenosine signaling through G protein-coupled adenosine receptors on cells of the immune system. Adenosine A2A receptors (A2ARs) have a generally suppressive effect on the activation of immune cells. Moreover, their transcription is strongly induced by signals that activate macrophages or dendritic cells through toll-like receptors, or T cells through T cell receptors. A2AR induction is responsible for producing a gradual dissipation of inflammatory responses. A2AR activation is particularly effective in limiting the activation of invariant NKT (iNKT) cells that play a central role in acute reperfusion injury. A2A agonists have clinical promise for the treatment of vaso-occlusive tissue injury. Blockade of A2A receptors may be useful to enhance immune-mediated killing of cancer cells. A2BR expression also is transcriptionally regulated by hypoxia, cytokines, and oxygen radicals. Acute A2BR activation attenuates the production of proinflammatory cytokines from macrophages, but sustained activation facilitates macrophage and dendritic cell remodeling and the production of acute phase proteins and angiogenic factors that may participate in evoking insulin resistance and tissue fibrosis. A2BR activation also influences macrophage and neutrophil function by influencing expression of the anti-inflammatory netrin receptor, UNC5B. The therapeutic significance of adenosine-mediated effects on the immune system is discussed.
Keywords: Leukocytes, Lymphocytes, platelets, dendritic cells, macrophages, invariant NKT cells
I. Introduction
Both innate and adaptive immunity are strongly influenced by purinergic signaling. Innate immunity is the most ancient system that protects multicellular hosts from infections and comprises of immune cells that are activated in response to either to pathogen-associated molecular patterns (PAMPs) or sterile host tissue injury resulting in inflammation in response to damage-associated molecular patterns (DAMPs; Pelegrin, 2008). The adaptive immune system evolved subsequent to the innate system and utilizes antigen presenting macrophages and DCs, MHC molecules, and TCRs to recognize specific pathogenic antigens or host autoantigens. All cells of the immune system express multiple purinergic receptors, and these receptors play a major role in their regulation. The reader is directed to previous reviews for background information about adenosine signaling in the immune system (Hasko et al., 2007; Kumar & Sharma, 2009). This review focuses on recent findings that have shed new light on the role that purinergic signaling plays in regulating both innate and adaptive immune responses. Of particular interest are recent discoveries demonstrating that adenosine receptor transcripts can be rapidly upregulated in response to local cues such as activation of excitatory receptors or tissue hypoxia. It has also become evident that the extra-cellular metabolism of adenine nucleotides by ectoenzymes such as CD39 and CD73 is a major source of adenosine, based on proinflammatory responses in mice upon deletion of these enzymes.
A diagram of the suppressive effects of A2AR on adaptive and innate immunity is shown in Fig. 1. Conventional T cells are part of the adaptive immune system. Selective activation of highly variable T cell receptors results in the expansion of these cells and the release of cytokines such as INF-γ. A minor subset of T cells known as invariant NKT (iNKT) cells express invariant T cells receptors. In addition to responding to various pathogens, iNKT cells are activated by injury to host tissues and contribute to sterile inflammation. Since NKT cells possess T cell receptors than can be rapidly activated by innate signals either from pathogens or danger signals produced by the injured host, they bridge innate and adaptive immunity. Both systems are strongly influenced by inducible A2AR signaling, as well as other purinergic receptors. Suppression of the innate immune response due to adenosine signaling can be beneficial to limit tissue inflammation and injury. However, too much immunosuppression by adenosine can blunt the ability of the immune system to control infections (Hasko et al., 2008). Activation of adaptive immune responses can be beneficial, for example, by enhancing immune surveillance of tumors (Jin et al., 2010), or harmful, for example, by reducing immune sensitization to persistent viral infections (Alam et al., 2009). We discuss how recent developments may be useful to the goal of exploiting adenosine signaling for therapeutic uses such as treatment of reperfusion injury, chronic inflammatory diseases, and tumor killing.
Figure 1.
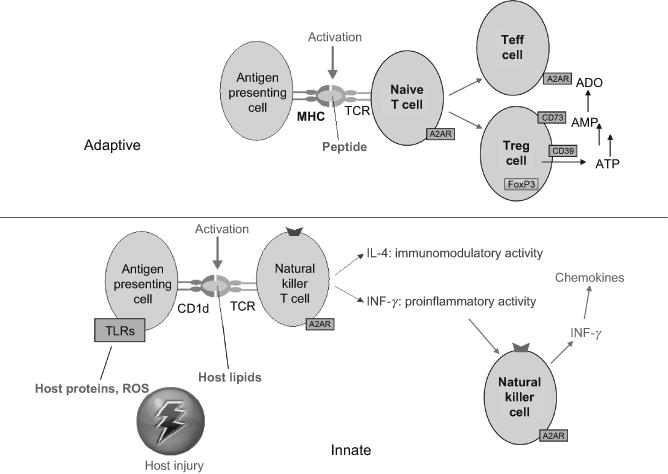
Comparison of A2AR effects on T cells and iNKT cells. The top panel illustrates that the adaptive immune response to peptide antigens are processed by antigen presenting cells and presented on major histocompatibility complex (MHC) molecules to variable T cell receptors. Upon TCR activation, naive T cells expand and generate T effector (Teff) cells, T regulatory (Treg) cells, or other types of daughter T cells. A2AR activation on naive T cells during antigen presentation enhances the production of Treg cells and produces persistent anergy of Teff cells. Activation of A2ARs on Teff cells during TCR activation suppresses their expansion and cytokine production. Among lymphocytes, only Treg cells express ectonucleotidases CD39 and CD73 that generate adenosine from the extracellular metabolism of adenosine nucleotides. The bottom panel illustrates the innate response of NKT cells. Glycolipid antigens can be derived from pathogens but also are thought to be derived from glycolipids derived from necrotic host cells and are presented by the MHC-like antigen presenting molecule, CD1d, to invariant TCRs on NKT cells. NKT cells usually express TCRs and NK cell markers such as NK1.1. Upon activation of their TCR, INKT cells rapidly produce large quantities of several cytokines including IFN-γ and IL-4. NK cells are transactivated by cytokines released form NKT cells and produce additional IFN-γ which stimulates the production of IFN-γ inducible chemokines that recruit additional leukocytes into the inflamed tissues. A2ARs are induced upon TCR activation of NKT and NK cells, and A2AR signaling strongly suppresses cytokine production by these cells.
II. Immune Responses to Adenosine Receptor Signaling
Activation of the immune system elicits immune cell-mediated killing of pathogens and the release of proinflammatory cytokines. The rapid induction of proinflammatory mediators by the immune system is accompanied by the initiation of transcriptional programs that limit inflammation. These include production of TGF-β, IL-10, vascular endothelial growth factor (VEGF), insulin-like growth factor-1, HO-1, and netrin-1. Adenosine and the A2A and A2B receptors are included among anti-inflammatory factors that are produced or induced during inflammation. A1R signaling also is important in immune regulation, but it acts primarily by influencing the sympathetic nervous system. Prejunctional A1 receptors inhibit the release of the sympathetic cotransmitters norepinephrine and ATP. All primary and secondary immune organs receive sympathetic innervations from sympathetic postganglionic neurons (Nance & Sanders, 2007). Innate immune cells express both α- and β-adrenergic receptor subtypes, while T and B lymphocytes express anti-inflammatory β2 adrenergic receptors exclusively. The A3 receptor has been implicated in influencing neutrophil chemotaxis (Chen et al., 2006) and mast cell degranulation (Feoktistov et al., 2003), and may contribute to inhibiting reperfusion injury (Ge et al., 2010), but in general, the role of the A3 receptor in immune regulation remains enigmatic (Gessi et al., 2008).
A. Platelets
Platelets are activated during sterile inflammation that occurs in response to tissue trauma or ischemia reperfusion injury (IRI). Substantial platelet activation is associated with sickle cell disease that has been extensively studied as a model of simultaneous IRI in multiple tissues. Intravital microscopy analyses in mice with sickle cell disease indicate that sickle RBCs interact primarily with adherent platelets and leukocytes in postcapillary and collecting venules leading to vascular obstruction (Turhan et al., 2002). ATP and ADP released from activated or damaged cells activate platelets via two G protein-coupled ADP receptors (P2Y1 and P2Y12) and via ATP through the ligand-gated P2X1 receptor (Oury et al., 2006).
It is now appreciated that the metabolic flux of adenine nucleotides and adenosine in the extracellular space regulates platelet activation due to counterbalancing signaling through P2 and adenosine receptors (Iyu et al., 2010). Activation of A2A receptors on platelets causes an increase in cyclic AMP accumulation and a decrease in platelet aggregation (Cooper et al., 1995; Table I). In A2A receptor-knockout mice, platelet aggregation is increased, proving the importance of this receptor subtype in limiting platelet activation (Ledent et al., 1997). Platelet activation is not only important for regulation platelet aggregation and secretion but also because it stimulates the production of platelet heteroaggregates with other leukocytes including monocytes, eosinophils, and neutrophils (Polanowska-Grabowska et al., 2010). Blockade of P-selectin-mediated platelet–leukocyte aggregation is beneficial in the animal models of vascular injury (Merhi et al., 1999). Hence, platelet A2AR activation may contribute to reduced sterile inflammation by direct effects on singlet platelets and platelet–leukocyte heteroaggregates. Although it was thought that the only adenosine receptor on platelets was the A2AR, Yang et al. (2010) recently showed that systemic inflammation induces the expression of A2BRs on platelets, and activation of these receptors inhibits the expression of the P2Y1 receptor and ADP-induced platelet aggregation.
Table I. Summary of the Effects of A2AR and A2BR Signaling on Some Cells of the Immune System.
| A2A | A2B | Other | |
|---|---|---|---|
| Platelets | ↑ Cyclic AMP ↓ Aggregation ↓ Secretion ↑ Leukocyte heteroaggregates |
↓ P2Y1 expression ↓ ADP-induced aggregation |
ADP and ATP receptors |
| Neutrophils | ↑ Cyclic AMP ↓ Oxidative burst ↓ α4/β1 integrin(VLA-4) |
ATP release, pannexin channels | |
| Macrophages | ↑ M1 to M2-like switch ↓ TNF-α, IL-12 ↑ VEGF, IL-10 Induced by HO-1 Induced by endotoxin |
↓ TNF, IL-12 ↑ VEGF, IL-10 ↑ IL-6 Induced by HIF Induced by IFN-γ Induced by diabetes Controls UNC5B expression |
M1 inflammatory M2 angiogenic |
| T cells | ↑ Cyclic AMP ↓ IFN-γ production ↓ CD-69 ↓ Proliferation, IL-2 ↑ Anergy Induced by TCR activation ↑ Treg production ↑ Treg function |
CD73 and CD39 (Tregs only) |
|
| iNKT cells | ↑ Cyclic AMP ↓ IFN-γ production ↓ TNF-α Induced by TCR activation |
Activated by lipidantigens Coactivated by TIM-1 Coactivates NK cells |
B. Neutrophils
Tissue trauma or IRI results in an inflammatory cascade that ultimately results in neutrophil infiltration into tissues (Lappas et al., 2006; McDonald et al., 2010). In the absence of infection, neutrophil accumulation in tissues can be very destructive. Platelet activation is associated with increased platelet adhesion to microvascular endothelium (Brittain et al., 1993), and formation of platelet heteroaggregates with erythrocytes (Inwald et al., 2000) and leukocytes including neutrophils, monocytes, and eosinophils. Oxidative burst in activated neutrophils and elevated expression of α4/β1 integrin (VLA-4, CD49d/CD29) are decreased as a result of A2AR activation (Fredholm et al., 1996; Revan et al., 1996; Sullivan et al., 2001, 2004b).
Neutrophils release ATP through pannexin-1 hemichannels in response to inflammatory mediators (Chen et al., 2010). Released ATP is necessary for maintaining neutrophil activation, but metabolism of ATP to adenosine inhibits neutrophil activation and adhesion to endothelial cells by direct effects on neutrophils (Sullivan et al., 2001) as well as indirect effects that reduced cytokine-mediated expression of P-selectin and ICAM-1 on endothelial cells (Okusa et al., 2000). Neutrophils are guided to sites of tissue injury by chemokines and formal peptides released from necrotic cells (McDonald et al., 2010). Thus, purinergic signaling is one of the several mechanisms required for regulation of neutrophil trafficking during inflammation. A2BRs also indirectly influence neutrophil trafficking by effects on tissue production of cytokines that are chemotactic to neutrophils such as KC. For example, A2BR activation plays a role in mediating lung inflammation after ischemia–reperfusion by stimulating neutrophil chemotaxis (Anvari et al., 2010).
C. Macrophages and DCs
Macrophages are broadly classified into inflammatory M1 (NOS2+) and angiogenic M2 (arginase+). Toll-like receptor (TLR) 2, 4, 7, and 9 agonists, together with A2AR agonists, switch macrophages from an M1 to an M2-like phenotype. This switch involves induction of A2ARs by TLR agonists, diminished TNF-α and IL-12 production, and enhanced production of VEGF and IL-10 (Grinberg et al., 2009). LPS suppresses PLCβ1 and β2 expression in macrophages in vitro and in several tissues in vivo. Signaling through TLRs suppresses PLC-β2 and this switches M1 macrophages into an M2-like state (Grinberg et al., 2009). Recognition of apoptotic cells also polarizes macrophages toward the anti-inflammatory M2-like phenotype by a process involving macrophage production of sphingosine-1-phosphate and VEGF and the induction of the A2AR (Weis et al., 2009). These responses are mediated in part by the transcription factor HO-1. These findings suggest that HO-1, which is induced by apoptotic cell-derived S1P, is involved in macrophage polarization toward an M2 phenotype that includes A2AR induction (Weis et al., 2009).
The release of proinflammatory cytokines such as TNF-α and IL-12 can be inhibited by either A2AR or A2BR activation. A2BR receptors are induced in response to arterial injury or by IFN-γ. Stimulation of A2BRs inhibits the IFN-γ-induced expression of MHC class II genes, nitric oxide synthase, and proinflammatory cytokines (Xaus et al., 1999).
In addition to binding adenosine, the A2BR has also been reported to bind another anti-inflammatory signaling molecule, netrin-1 (Corset et al., 2000). Netrin-1 mediates its functions through stimulation of the deleted in colorectal cancer (DCC) family receptors DCC and neogenin, and the UNC5 family receptors UNC5A, UNC5B, UNC5C, and UNC5D (Barallobre et al., 2005). Netrin-1 can act as chemoattractant or chemorepellent. The DCC family of receptors mediates attraction to netrin-1, whereas the UNC5 family of receptors forms a netrin-1-dependent complex with DCC and mediates repulsion (Hong et al., 1999). In addition to its function in neuronal development, netrin-1 expressed outside the nervous system inhibits migration of leukocytes in vitro and in vivo and attenuates inflammation-mediated tissue injury. The netrin-1 receptor UNC5B is highly expressed on human monocytes, granulocytes, and lymphocytes, and netrin-1 acting through UNC5B receptor inhibits migration of monocytes (Wang et al., 2009) in vitro. Activation of the A2BR, originally proposed to contribute to netrin effects on axons, is not required for axon outgrowth or Xenopus spinal axon attraction to netrin-1. Thus, DCC plays a central role in netrin signaling of axon growth and guidance independent of A2BR activation (Stein et al., 2001). Administration of recombinant netrin-1 before or after renal IRI reduced kidney injury, apoptosis, monocyte and neutrophil infiltration, and cytokine and chemokine production (Tadagavadi et al., 2010). Analysis of different netrin-1 receptors on leukocytes showed very high expression of UNC5B but little or no expression of UNC5A, UNC5C, UNC5D, neogenin, or DCC. These findings suggest that the A2BR may in fact not be the netrin-1 receptor. Rather, A2BR activation may influence the expression of the netrin receptor, UNC5B, on macrophages and other leukocytes. Neutralization of UNC5B receptor reduced netrin-1-mediated protection against renal IRI, and it increased monocyte and neutrophil infiltration, as well as serum and renal cytokine and chemokine production, with increased kidney injury. These studies suggest that netrin-1 acts through UNC5B receptors that are regulated by A2BR signaling to reduce inflammation.
D. T Cells
Incubation of purified C57BL/6 murine CD4(+) T lymphocytes with anti-CD3 mAb serves as a model of TCR-mediated activation and results in increased IFN-γ production and cell surface expression of activation markers, CD25 and CD69. Signaling through the TCR causes a rapid fivefold increase in A2AR mRNA, which is correlated with a significant increase in the efficacy of A2AR-mediated cAMP accumulation in these cells (Lappas et al., 2005). A2AR stimulation not only inhibits the generation of adaptive effector T cells but also promotes the induction of adaptive regulatory T cells. In vitro, antigen recognition in the setting of A2AR engagement induces T-cell anergy, even in the presence of costimulation (Zarek et al., 2008). T cells initially stimulated in the presence of an A2AR agonist fail to proliferate and produce IL-2 and IFN-γ when rechallenged in the absence of A2AR stimulation.
A2AR stimulation inhibits interleukin-6 expression while enhancing the production of TGF-β. TGF-β favors the production of anti-inflammatory T regulatory cells, while IL-6, in conjunction with TGF-β, favors the production of inflammatory Th17 inflammatory cells. Consequently, treating mice with A2AR agonists not only inhibits Th1 and Th17 effector cell generation but also promotes the generation of Foxp3(+) T regulatory cells. Overall, the effect of A2AR activation on T cells is to promote long-term T-cell anergy and the generation of adaptive T regulatory cells.
A2ARs also regulate the function of T regulatory cells. Although the transfer of T regulatory cells (CD45RB(low)) blocks colitis induced by pathogenic CD45RB(high) Th cells, CD45RB(low) cells from A2AR-deficient mice do not prevent colitis (Naganuma et al., 2006). A2AR agonists suppress the production of proinflammatory cytokines by CD45RB(high) and CD45RB(low) T cells in association with a loss of mRNA stability. In contrast, anti-inflammatory cytokines, including IL-10 and TGF-β, are minimally affected. Oral administration of the A2AR agonist ATL313 attenuated colitis in mice receiving CD45RB(high) Th cells. These data suggest that A2AR activation controls T-cell-mediated colitis by suppressing the expression of proinflammatory cytokines while sparing anti-inflammatory activity mediated by IL-10 and TGF-β.
A2BR stimulation has not been reported to have strong direct effects on T-cell function. However, activation of A2BRs may indirectly promote the development of tissue rejection by inhibiting CD4+/CD25+/Foxp3+ regulatory T-cell infiltration (Zhao et al., 2010).
E. NKT Cells
A2A agonists have also been found to reduce injury following ischemia or trauma in liver (Alchera et al., 2008; Ben-Ari et al., 2005; Cao et al., 2009; Day et al., 2004, 2005b; Harada et al., 2000), kidney (Day et al., 2003, 2005a; Okusa et al., 1999, 2001), skin (Peirce et al., 2001), lung (Gazoni et al., 2008; Rivo et al., 2007; Sharma et al., 2010), heart (Patel et al., 2009; Rork et al., 2008; Xi et al., 2009; Yang et al., 2006b), intestine (Di Paola et al., 2010), and spinal cord (Cassada et al., 2002; Li et al., 2006; Reece et al., 2008). The cellular targets of A2ARs initially were not clear. As noted above, platelets, neutrophils, and macrophages express A2ARs that, respectively, inhibit oxidative burst and adhesion molecule expression (Sullivan et al., 2004a) and cytokine production (Murphree et al., 2005). We introduced loxp sites flanking the first A2AR gene, adora2a, and crossed these mice to LysMCre mice. All lines were made congenic to C57BL/6J using marker-assisted selection. The resultant LysMCre × A2ARf/fmice selectively lack A2ARs in neutrophils and macrophages. Nevertheless, A2AR activation was still highly effective at reducing injury in response to liver or lung IRI (Reutershan et al., 2007). Adoptive transfer of CD4+ (but not CD8+ T cells) to Rag1−/− mice reconstituted injury from IRI (Zhai et al., 2006). The A2A agonist, ATL146e, inhibited this injury if the transferred cells had A2ARs, but not if they lacked A2ARs (Yang et al., 2006b). This result is striking because Rag1−/− mice reconstituted with A2AR−/− CD4+ T cells have a normal complement of A2ARs in all cells except the reconstituted T cells. The results indicate that despite the widespread distribution of A2ARs on platelets and leukocytes, A2A agonists reduce IRI primarily by their effects on T cells.
In 2005, Shimamura et al. found that liver reperfusion injury was associated with an expansion and activation of CD1d-restricted NKT cells (Shimamura et al. (2005)). Subsequently, we found that depletion of NKT and NK cells with PK136, an antibody that binds to NK1.1 found only on NKT and NK cells, or an anti-CD1d antibody produces protection from liver IRI that is equivalent to and not additive to protection by ATL146e (Lappas et al., 2006). These studies indicate that the adenosine-sensitive T cells that mediate IRI are iNKT cells. The putative endogenous ligands that are responsible for activating iNKT following IRI have not been identified, but recent studies suggest that tissue injury may result in the formation of one or more galactose-containing glycolipids that can activate the invariant TCR (Darmoise et al., 2010). In addition, iNKT cell activation may be facilitated by the binding of phosphatidylserine on the surface of apoptotic cells to T cell Ig-like mucin-like-1 (TIM-1) receptors on NKT cells (Lee et al., 2010). Hepatic preconditioning produced by preactivating NKT cells protects the liver from IRI via an IL-13 response and induction of A2ARs (Cao et al., 2009).
As sickle cell disease is characterized by persistent multiorgan microvascular IRI, we examined the role of iNKT cells in sickle cell disease. Deletion or blockade of iNKT cell activation was found to greatly attenuate pulmonary vaso-occlusive pathophysiology in sickle cell mice. In addition, sickle cell patients were found to have increased numbers of activated iNKT cells in their blood (Wallace et al., 2009). These findings suggest that iNKT cells orchestrate a leukocyte inflammatory cascade that triggers vaso-occlusive episodes. A2AR agonists produce substantial protection to mouse lungs in sickle cell disease, primarily by targeting A2A receptors that are induced on iNKT cells and NK cells (Wallace & Linden, 2010).
III. Disease Relevance of Adenosine to Immune Signaling
A. Diabetes
Inflammation in diabetes may be triggered in part by elevated concentrations of free fatty acids that increase CD11c+ macrophage accumulation and activation in adipose tissue (Nguyen et al., 2007). Insulin resistance due to a high-fat diet causes macrophage accumulation in adipose tissue and M2-like remodeling (Shaul et al., 2010). Endothelial dysfunction is also a hallmark of diabetes because inflammatory mediators activate receptors and transcription factors such as nuclear factor-κB, TLRs, c-Jun amino terminal kinase, and the receptor for advanced glycation end products, which cause systemic endothelial dysfunction (Goldberg, 2009). Signaling through the A2BR also contributes to insulin resistance by altering the production of IL-6 and other cytokines. IL-6 is produced primarily by macrophages and adipocytes and drives the production of CRP.
Several studies have linked adenosine receptor blockade with reversal of insulin resistance. Challis and coworkers reported that adenosine receptor antagonists (Challis et al., 1984) or degradation of adenosine with adenosine deaminase (Budohoski et al., 1984) reverse insulin resistance in skeletal muscle isolated from diabetic animals. The orally active antagonist, adenosine receptor antagonist BW-1433, was found to persistently reverse insulin resistance in obese Zucker rats (Crist et al., 1998, 2001; Xu et al., 1998). In mice rendered insulin resistant due to a high-fat diet, ADORA2B gene deletion was reported to reduce body fat, reduce liver glycogen, increase energy expenditure, and increase lean body mass (Treadway et al., 2006). It is notable that statins stimulate the induction of CD73 and have been shown to cause insulin resistance. Statins also enhance ischemia-mediated vasodilation in people, and this is blocked by caffeine, consistent with an effect to enhance adenosine production (Meijer et al., 2010). Enhanced adenosine production, by activating A2BRs, may contribute to the effect of statins to provoke insulin resistance.
Diabetes triggers induction of A2BR mRNA in macrophages and endothelial cells, resulting in increased IL-6 production in response to A2BR activation (Figler et al., 2011). Deletion of the mouse A2BR resulted acutely in a proinflammatory phenotype manifested as mild vascular inflammation at baseline and exacerbation of cytokine production in response to endotoxin (Yang et al., 2006a). Thus, in some settings, signaling by the A2BR reduces inflammation. However, persistant activation of A2BRs increased IL-6 plasma levels in mice, and by several types of isolated cells (Linden, 2006), including macrophages (Ryzhov et al., 2008b) and dendritic cells (Novitskiy et al., 2008; Ryzhov et al., 2008b). IL-6 is directly involved in stimulating the production of transcription factors that enhance CRP production (Young et al., 2008). Analyses of the cloned human A2BR promoter identified a functional binding site for hypoxia-inducible factor (Kong et al., 2006) and identified TNF-α and the oxidative stress-promoting enzyme NAD(P)H oxidase as additional regulators of A2BR gene expression (Kolachala et al., 2005). Since elevated TNF-α and oxidative stress are associated with diabetes (Castoldi et al., 2007; Gokulakrishnan et al., 2009), it is reasonable to speculate that these factors contribute to induction of A2BR mRNA in diabetics. Hence, A2BR-facilitated production of IL-6 and other adipokines by macrophages that accumulate in adipose tissue of obese animals and people may contribute to insulin resistance associated with type II diabetes (Figler et al., 2011). Chronic activation of A2BRs has been implicated in other pathological processes, such as pulmonary fibrosis (Sun et al., 2006).
B. Cancer
Both agonists and antagonists of adenosine receptors have been evaluated in mouse models of cancer, and in some cases have direct effects on tumor cells that sometimes express various adenosine receptor subtypes (Fishman et al., 2009; Merighi et al., 2007). Another approach has been to target adenosine receptors in immunocompetent hosts for blockade as a means of enhancing immune killing of tumors. Most tumors are thought to produce some degree of immune activation that might be exploited to facilitate tumor rejection. For example, in bladder cancer, activation of the immune system by the immune adjuvant bacillus Calmette–Guerin (BCG) has been shown to significantly reduce tumor progression (Demkow et al., 2008). Sequential activation of NKT cells and NK cells provides effective innate immunotherapy of cancer (Smyth et al., 2005). As discussed above, signaling through A2Aand A2B receptors generally has a strong negative effect on T cell responses. Activation of the A2AR on T effector cells can reduce by 98% INF-γ release (Lappas et al., 2005). A2AR activation on CD1d-restricted NKT cells reduces the production of INF-γ, TNF-α, and IL-2 in response to glycolipid antigens (Lappas et al., 2006). Treating mice with synthetic A2A agonists inhibits Th1 and Th17 effector cell generation and promotes the generation of Foxp3+ regulatory T cells (Zarek et al., 2008). Given the suppressive effects of A2ARs on T cells and other leukocytes, A2AR blockade or deletion has been investigated to enhance immune killing of tumors. These studies have met with some success in immunocompetent mouse models with syngeneic tumors (Lukashev et al., 2007; Ohta and Sitkovsky, 2011; Ohta et al., 2006). Ohta et al. (2006) showed that solid tumors produce high concentrations of adenosine and demonstrated that genetic deletion of the A2AR resulted in rejection of established immunogenic lung tumors in ∼60% of mice with no rejection observed in control WT mice. Caffeine, a weak nonselective adenosine receptor antagonist, also significantly increased tumor rejection.
In addition to conventional Foxp3+ T regulatory cells, adaptive regulatory T cells (Tr1) are induced in the periphery upon encountering cognate antigens. In cancer, their frequency is increased; however, Tr1-mediated suppression mechanisms have only recently begun to be studied. Both ectonucleotidases (CD39/CD73) and cyclooxygenase 2 (COX-2) are involved in Tr1-mediated suppression. The concomitant inhibition of prostaglandin E2 and adenosine receptors via their common intracellular cyclic AMP pathway has been suggested as an additional approach for improving results of immune therapies for cancer (Mandapathil et al., 2010).
In addition to their effects on the function of T cells, A2AR and A2BR blockade may have indirect effects on tumor angiogenesis. In addition to effects of A2B signaling on macrophages and DCs, both A2B and A3 receptors have been shown to facilitate the release of angiogenic factors from mast cells (Feoktistov et al., 2003). A2BR blockade impairs production of IL-8 in a mouse melanoma model (Merighi et al., 2009). In a Lewis lung carcinoma isograft model, deletion of the host A2BR lowered tumor levels of VEGF and attenuated tumor growth (Ryzhov et al., 2008a). Since A2AR activation strongly suppresses the production of IFN-γ by both NKT and NK cells, blockade of these receptors increases the production of IFN-γ-inducible chemokines. CXC chemokines are important in controlling leukocyte trafficking, enhancing innate and adaptive immunity, and regulating angiogenesis. CXC chemokines behave as both potent promoters of Th1-dependent cell-mediated immunity and inhibitors of angiogenesis. These chemokines bind to a specific receptor known as CXCR3. This receptor has been found on Th1 T cells, B cells, NK cells, and endothelial cells. The CXCR3 ligands represent the major chemoattractants for the recruitment of Th1 cells during cell-mediated immunity. Recently, CXCR3 has been found to exist in two alternatively spliced mRNAs (CXCR3A and CXCR3B). CXCR3B is expressed on endothelial cells and mediates the angiostatic effects of CXCR3 ligands, whereas CXCR3A appears to be expressed on T cells, B cells, and NK cells (Struyf et al., 2010). IL-2 is the major agonist for triggering the expression of CXCR3A on these leukocytes. The regulation of the expression of CXCR3B on endothelial cells remains to be fully elucidated. In addition to their role in mediating Th1-mediated immunity, CXCR3 ligands are potent and efficacious cytokines for inhibiting angiogenesis induced by VEGF, bFGF, and ELR+ CXC chemokines. A2AR blockade enhances the production of interferon-inducible CXC chemokines to promote Th1 immunity and inhibit angiogenesis. Studies are ongoing in several laboratories to evaluate effects of A2AR and A2BR blockade on tumor progression.
IV. Conclusion
It is now clear that purineric signaling exerts major regulatory effects on the immune system. A2AR activation produces strong anti-inflammatory effects on multiple cell types. As A2A agonists make their way toward the clinic, it may be possible to exploit their anti-inflammatory effects to inhibit tissue injury in response to acute insults such as tissue transplantation, myocardial infarction, and flares in autoimmune diseases or sickle cell anemia. A2BR signaling is more complex. Although A2BR activation seems to produce some of the acute antiinflammatory effects on macrophages as are produced by A2A agonists, acute A2BR activation may elevate blood glucose, and prolonged A2BR signaling results in tissue reparative programs, such as fibrosis, angiogenesis, and IL-6 production that may be detrimental in some instances. A2BR antagonists are currently in clinical development for the treatment of asthma (due in part to inhibition of mast cell deregulation). It will be of interest to determine if such antagonists prove to be useful for the treatment of chronic inflammatory states such as pulmonary fibrosis, type II diabetes, and others.
Abbreviations
- DAMPs
damage-associated molecular patterns
- DCC
deleted in colorectal cancer
- DCs
dendritic cells
- ECs
endothelial cells
- HIF-1
hypoxia-inducible factor-α
- HO-1
heme oxygenase-1
- IL
interleukin
- iNKT
invariant NKT
- IRI
ischemia reperfusion injury
- MHC
major histocompatibility complex
- TCR
T cell receptor
- TGF-β
transforming growth factor-β
- TIM-1
T cell Ig-like mucin-like-1
- VEGF
vascular endothelial growth factor
Footnotes
Disclosure Statement: The author is a paid consultant to Clinical Data, Inc., which has A2A agonists and A2B antagonists in clinical development.
References
- Alam MS, Kurtz CC, Wilson JM, Burnette BR, Wiznerowicz EB, Ross WG, Figler RA, Linden J, Crowe SE, Ernst PB. A2A adenosine receptor (AR) activation inhibits pro-inflammatory cytokine production by human CD4+ helper T cells and regulates Helicobacter-induced gastritis and bacterial persistence. Mucosal Immunology. 2009;2:232–242. doi: 10.1038/mi.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alchera E, Tacchini L, Imarisio C, Dal Ponte C, De Ponti C, Gammella E, Cairo G, Albano E, Carini R. Adenosine-dependent activation of hypoxia-inducible factor-1 induces late preconditioning in liver cells. Hepatology. 2008;48:230–239. doi: 10.1002/hep.22249. [DOI] [PubMed] [Google Scholar]
- Anvari F, Sharma AK, Fernandez LG, Hranjec T, Ravid K, Kron IL, Laubach VE. Tissue-derived proinflammatory effect of adenosine A2B receptor in lung ischemia-reperfusion injury. The Journal of Thoracic and Cardiovascular Surgery. 2010;140:871–877. doi: 10.1016/j.jtcvs.2010.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barallobre MJ, Pascual M, Del Rio JA, Soriano E. The Netrin family of guidance factors: Emphasis on Netrin-1 signalling. Brain Research. Brain Research Reviews. 2005;49:22–47. doi: 10.1016/j.brainresrev.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Z, Pappo O, Sulkes J, Cheporko Y, Vidne BA, Hochhauser E. Effect of adenosine A2A receptor agonist (CGS) on ischemia/reperfusion injury in isolated rat liver. Apoptosis. 2005;10:955–962. doi: 10.1007/s10495-005-0440-3. [DOI] [PubMed] [Google Scholar]
- Brittain HA, Eckman JR, Swerlick RA, Howard RJ, Wick TM. Thrombospondin from activated platelets promotes sickle erythrocyte adherence to human microvascular endothelium under physiologic flow: A potential role for platelet activation in sickle cell vaso-occlusion. Blood. 1993;81:2137–2143. [PubMed] [Google Scholar]
- Budohoski L, Challiss RA, Cooney GJ, McManus B, Newsholme EA. Reversal of dietary-induced insulin resistance in muscle of the rat by adenosine deaminase and an adenosine-receptor antagonist. The Biochemical Journal. 1984;224:327–330. doi: 10.1042/bj2240327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Yuan Y, Jeyabalan G, Du Q, Tsung A, Geller DA, Billiar TR. Preactivation of NKT cells with alpha-GalCer protects against hepatic ischemia-reperfusion injury in mouse by a mechanism involving IL-13 and adenosine A2A receptor. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2009;297:G249–G258. doi: 10.1152/ajpgi.00041.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassada DC, Tribble CG, Young JS, Gangemi JJ, Gohari AR, Butler PD, Rieger JM, Kron IL, Linden J. Adenosine A2A analogue improves neurologic outcome after spinal cord trauma in the rabbit. The Journal of Trauma. 2002;53:225–229. doi: 10.1097/00005373-200208000-00005. discussion 229–231. [DOI] [PubMed] [Google Scholar]
- Castoldi G, Galimberti S, Riva C, Papagna R, Querci F, Casati M, Zerbini G, Caccianiga G, Ferrarese C, Baldoni M, Valsecchi MG, Stella A. Association between serum values of C-reactive protein and cytokine production in whole blood of patients with type 2 diabetes. Clinical Science (London) 2007;113:103–108. doi: 10.1042/CS20060338. [DOI] [PubMed] [Google Scholar]
- Challis RA, Budohoski L, McManus B, Newsholme EA. Effects of an adenosine-receptor antagonist on insulin-resistance in soleus muscle from obese Zucker rats. The Biochemical Journal. 1984;221:915–917. doi: 10.1042/bj2210915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yao Y, Sumi Y, Li A, To UK, Elkhal A, Inoue Y, Woehrle T, Zhang Q, Hauser C, Junger WG. Purinergic signaling: A fundamental mechanism in neutrophil activation. Science Signaling. 2010;3:ra45. doi: 10.1126/scisignal.2000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JA, Hill SJ, Alexander SP, Rubin PC, Horn EH. Adenosine receptor-induced cyclic AMP generation and inhibition of 5-hydroxytryptamine release in human platelets. British Journal of Clinical Pharmacology. 1995;40:43–50. doi: 10.1111/j.1365-2125.1995.tb04533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corset V, Nguyen-Ba-Charvet KT, Forcet C, Moyse E, Chedotal A, Mehlen P. Netrin-1-mediated axon outgrowth and cAMP production requires interaction with adenosine A2b receptor. Nature. 2000;407:747–750. doi: 10.1038/35037600. [DOI] [PubMed] [Google Scholar]
- Crist GH, Xu B, Berkich DA, LaNoue KF. Effects of adenosine receptor antagonism on protein tyrosine phosphatase in rat skeletal muscle. The International Journal of Biochemistry & Cell Biology. 2001;33:817–830. doi: 10.1016/s1357-2725(01)00051-6. [DOI] [PubMed] [Google Scholar]
- Crist GH, Xu B, Lanoue KF, Lang CH. Tissue-specific effects of in vivo adenosine receptor blockade on glucose uptake in Zucker rats. The FASEB Journal. 1998;12:1301–1308. doi: 10.1096/fasebj.12.13.1301. [DOI] [PubMed] [Google Scholar]
- Darmoise A, Teneberg S, Bouzonville L, Brady RO, Beck M, Kaufmann SH, Winau F. Lysosomal alpha-galactosidase controls the generation of self lipid antigens for natural killer T cells. Immunity. 2010;33:216–228. doi: 10.1016/j.immuni.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day YJ, Huang L, McDuffie MJ, Rosin DL, Ye H, Chen JF, Schwarzschild MA, Fink JS, Linden J, Okusa MD. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. Journal of Clinical Investigation. 2003;112:883–891. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day YJ, Huang L, Ye H, Linden J, Okusa MD. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: Role of macrophages. American Journal of Physiology. Renal Physiology. 2005a;288:F722–F731. doi: 10.1152/ajprenal.00378.2004. [DOI] [PubMed] [Google Scholar]
- Day YJ, Li Y, Rieger JM, Ramos SI, Okusa MD, Linden J. A2A adenosine receptors on bone marrow-derived cells protect liver from ischemia-reperfusion injury. Journal of Immunology. 2005b;174:5040–5046. doi: 10.4049/jimmunol.174.8.5040. [DOI] [PubMed] [Google Scholar]
- Day YJ, Marshall MA, Huang L, McDuffie MJ, Okusa MD, Linden J. Protection from ischemic liver injury by activation of A2A adenosine receptors during reperfusion: Inhibition of chemokine induction. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2004;286:G285–G293. doi: 10.1152/ajpgi.00348.2003. [DOI] [PubMed] [Google Scholar]
- Demkow T, Alter A, Wiechno P. Intravesical bacillus Calmette-Guerin therapy for T1 superficial bladder cancer. Urologia Internationalis. 2008;80:74–79. doi: 10.1159/000111734. [DOI] [PubMed] [Google Scholar]
- Di Paola R, Melani A, Esposito E, Mazzon E, Paterniti I, Bramanti P, Pedata F, Cuzzocrea S. Adenosine A2A receptor-selective stimulation reduces signaling pathways involved in the development of intestine ischemia and reperfusion injury. Shock. 2010;33:541–551. doi: 10.1097/SHK.0b013e3181c997dd. [DOI] [PubMed] [Google Scholar]
- Feoktistov I, Ryzhov S, Goldstein AE, Biaggioni I. Mast cell-mediated stimulation of angiogenesis: Cooperative interaction between A2B and A3 adenosine receptors. Circulation Research. 2003;92:485–492. doi: 10.1161/01.RES.0000061572.10929.2D. [DOI] [PubMed] [Google Scholar]
- Figler RA, Wang G, Srinivasan S, Jung DY, Zhiyou Z, Pankow JS, Ravid K, Fredholm B, Hedrick CC, Rich SS, Kim JK, LaNoue KF, Linden J. Links between insulin resistance, adenosine A2B receptors and inflammatory markers in mice and humans. Diabetes. 2011;60:1–11. doi: 10.2337/db10-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman P, Bar-Yehuda S, Synowitz M, Powell JD, Klotz KN, Gessi S, Borea PA. Adenosine receptors and cancer. Handbook of Experimental Pharmacology. 2009;193:399–441. doi: 10.1007/978-3-540-89615-9_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Zhang Y, van der Ploeg I. Adenosine A2A receptors mediate the inhibitory effect of adenosine on formyl-Met-Leu-Phe-stimulated respiratory burst in neutrophil leucocytes. Naunyn Schmiedebergs Archives of Pharmacology. 1996;354:262–267. doi: 10.1007/BF00171056. [DOI] [PubMed] [Google Scholar]
- Gazoni LM, Laubach VE, Mulloy DP, Bellizzi A, Unger EB, Linden J, Ellman PI, Lisle TC, Kron IL. Additive protection against lung ischemia-reperfusion injury by adenosine A2A receptor activation before procurement and during reperfusion. The Journal of Thoracic and Cardiovascular Surgery. 2008;135:156–165. doi: 10.1016/j.jtcvs.2007.08.041. [DOI] [PubMed] [Google Scholar]
- Ge ZD, van der Hoeven D, Maas JE, Wan TC, Auchampach JA. A(3) adenosine receptor activation during reperfusion reduces infarct size through actions on bone marrow-derived cells. Journal of Molecular and Cellular Cardiology. 2010;49:280–286. doi: 10.1016/j.yjmcc.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessi S, Merighi S, Varani K, Leung E, Mac Lennan S, Borea PA. The A3 adenosine receptor: An enigmatic player in cell biology. Pharmacology & Therapeutics. 2008;117:123–140. doi: 10.1016/j.pharmthera.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Gokulakrishnan K, Mohanavalli KT, Monickaraj F, Mohan V, Balasubramanyam M. Subclinical inflammation/oxidation as revealed by altered gene expression profiles in subjects with impaired glucose tolerance and Type 2 diabetes patients. Molecular and Cellular Biochemistry. 2009;324:173–181. doi: 10.1007/s11010-008-9996-x. [DOI] [PubMed] [Google Scholar]
- Goldberg RB. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. The Journal of Clinical Endocrinology and Metabolism. 2009;94:3171–3182. doi: 10.1210/jc.2008-2534. [DOI] [PubMed] [Google Scholar]
- Grinberg S, Hasko G, Wu D, Leibovich SJ. Suppression of PLCbeta2 by endotoxin plays a role in the adenosine A(2A) receptor-mediated switch of macrophages from an inflammatory to an angiogenic phenotype. The American Journal of Pathology. 2009;175:2439–2453. doi: 10.2353/ajpath.2009.090290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N, Okajima K, Murakami K, Usune S, Sato C, Ohshima K, Katsuragi T. Adenosine and selective A(2A) receptor agonists reduce ischemia/reperfusion injury of rat liver mainly by inhibiting leukocyte activation. The Journal of Pharmacology and Experimental Therapeutics. 2000;294:1034–1042. [PubMed] [Google Scholar]
- Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: Therapeutic aspects for inflammatory and immune diseases. Nature Reviews. Drug Discovery. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasko G, Pacher P, Deitch EA, Vizi ES. Shaping of monocyte and macrophage function by adenosine receptors. Pharmacology & Therapeutics. 2007;113:264–275. doi: 10.1016/j.pharmthera.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K, Hinck L, Nishiyama M, Poo MM, Tessier-Lavigne M, Stein E. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell. 1999;97:927–941. doi: 10.1016/s0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- Inwald DP, Kirkham FJ, Peters MJ, Lane R, Wade A, Evans JP, Klein NJ. Platelet and leucocyte activation in childhood sickle cell disease: Association with nocturnal hypoxaemia. British Journal Haematology. 2000;111:474–481. doi: 10.1046/j.1365-2141.2000.02353.x. [DOI] [PubMed] [Google Scholar]
- Iyu D, Glenn JR, White AE, Fox SC, Heptinstall S. Adenosine derived from ADP can contribute to inhibition of platelet aggregation in the presence of a P2Y12 antagonist. Arteriosclerosis, Thrombosis and Vascular Biology. 2010 doi: 10.1161/ATVBAHA.110.219501. [DOI] [PubMed] [Google Scholar]
- Jin D, Fan J, Wang L, Thompson LF, Liu A, Daniel BJ, Shin T, Curiel TJ, Zhang B. CD73 on tumor cells impairs antitumor T-cell responses: A novel mechanism of tumor-induced immune suppression. Cancer Research. 2010;70:2245–2255. doi: 10.1158/0008-5472.CAN-09-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolachala V, Asamoah V, Wang L, Obertone TS, Ziegler TR, Merlin D, Sitaraman SV. TNF-alpha upregulates adenosine 2b (A2b) receptor expression and signaling in intestinal epithelial cells: A basis for A2bR overexpression in colitis. Cellular and Molecular Life Sciences. 2005;62:2647–2657. doi: 10.1007/s00018-005-5328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP. HIF-dependent induction of adenosine A2B receptor in hypoxia. The FASEB Journal. 2006;20:2242–2250. doi: 10.1096/fj.06-6419com. [DOI] [PubMed] [Google Scholar]
- Kumar V, Sharma A. Adenosine: An endogenous modulator of innate immune system with therapeutic potential. European Journal of Pharmacology. 2009;616:7–15. doi: 10.1016/j.ejphar.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. The Journal of Experimental Medicine. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. Journal of Immunology. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, Costentin J, Heath JK, Vassart G, Parmentier M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- Lee HH, Meyer EH, Goya S, Pichavant M, Kim HY, Bu X, Umetsu SE, Jones JC, Savage PB, Iwakura Y, Casasnovas JM, Kaplan G, Freeman GJ, DeKruyff RH, Umetsu DT. Apoptotic cells activate NKT cells through T cell Ig-like mucin-like-1 resulting in airway hyperreactivity. Journal of Immunology. 2010;185:5225–5235. doi: 10.4049/jimmunol.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Oskouian RJ, Day YJ, Rieger JM, Liu L, Kern JA, Linden J. Mouse spinal cord compression injury is reduced by either activation of the adenosine A2A receptor on bone marrow-derived cells or deletion of the A2A receptor on non-bone marrow-derived cells. Neuroscience. 2006;141:2029–2039. doi: 10.1016/j.neuroscience.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Linden J. New insights into the regulation of inflammation by adenosine. Journal of Clinical Investigation. 2006;116:1835–1837. doi: 10.1172/JCI29125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukashev D, Sitkovsky M, Ohta A. From “Hellstrom Paradox” to antiadenosinergic cancer immunotherapy. Purinergic Signalling. 2007;3:129–134. doi: 10.1007/s11302-006-9044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandapathil M, Szczepanski MJ, Szajnik M, Ren J, Jackson EK, Johnson JT, Gorelik E, Lang S, Whiteside TL. Adenosine and prostaglandin E2 cooperate in the suppression of immune responses mediated by adaptive regulatory T cells. The Journal of Biological Chemistry. 2010;285:27571–27580. doi: 10.1074/jbc.M110.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- Meijer P, Wouters CW, van den Broek PH, de Rooij M, Scheffer GJ, Smits P, Rongen GA. Upregulation of ecto-5′-nucleotidase by rosuvastatin increases the vasodilator response to ischemia. Hypertension. 2010;56:722–727. doi: 10.1161/HYPERTENSIONAHA.110.155689. [DOI] [PubMed] [Google Scholar]
- Merhi Y, Provost P, Chauvet P, Theoret JF, Phillips ML, Latour JG. Selectin blockade reduces neutrophil interaction with platelets at the site of deep arterial injury by angioplasty in pigs. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19:372–377. doi: 10.1161/01.atv.19.2.372. [DOI] [PubMed] [Google Scholar]
- Merighi S, Benini A, Mirandola P, Gessi S, Varani K, Simioni C, Leung E, Maclennan S, Baraldi PG, Borea PA. Caffeine inhibits adenosine-induced accumulation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and interleukin-8 expression in hypoxic human colon cancer cells. Molecular Pharmacology. 2007;72:395–406. doi: 10.1124/mol.106.032920. [DOI] [PubMed] [Google Scholar]
- Merighi S, Simioni C, Gessi S, Varani K, Mirandola P, Tabrizi MA, Baraldi PG, Borea PA. A(2B) and A(3) adenosine receptors modulate vascular endothelial growth factor and interleukin-8 expression in human melanoma cells treated with etoposide and doxorubicin. Neoplasia. 2009;11:1064–1073. doi: 10.1593/neo.09768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphree LJ, Sullivan GW, Marshall MA, Linden J. Lipopolysaccharide rapidly modifies adenosine receptor transcripts in murine and human macrophages: Role of NF-kappaB in A(2A) adenosine receptor induction. The Biochemical Journal. 2005;391:575–580. doi: 10.1042/BJ20050888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB. Cutting edge: Critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. Journal of Immunology. 2006;177:2765–2769. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain, Behavior, and Immunity. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. The Journal of Biological Chemistry. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- Novitskiy SV, Ryzhov S, Zaynagetdinov R, Goldstein AE, Huang Y, Tikhomirov OY, Blackburn MR, Biaggioni I, Carbone DP, Feoktistov I, Dikov MM. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood. 2008;112:1822–1831. doi: 10.1182/blood-2008-02-136325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, Chen JF, Jackson EK, Apasov S, Abrams S, Sitkovsky M. A2A adenosine receptor protects tumors from antitumor T cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta A, Sitkovsky M. Methylxanthines, inflammation, and cancer: Fundamental mechanisms. Handbook of Experimental Pharmacology. 2011;200:469–481. doi: 10.1007/978-3-642-13443-2_19. [DOI] [PubMed] [Google Scholar]
- Okusa MD, Linden J, Huang L, Rieger JM, Macdonald TL, Huynh LP. A(2A) adenosine receptor-mediated inhibition of renal injury and neutrophil adhesion. American Journal of Physiology. Renal Physiology. 2000;279:F809–F818. doi: 10.1152/ajprenal.2000.279.5.F809. [DOI] [PubMed] [Google Scholar]
- Okusa MD, Linden J, Huang L, Rosin DL, Smith DF, Sullivan G. Enhanced protection from renal ischemia-reperfusion [correction of ischemia:reperfusion] injury with A(2A)-adenosine receptor activation and PDE 4 inhibition. Kidney International. 2001;59:2114–2125. doi: 10.1046/j.1523-1755.2001.00726.x. [DOI] [PubMed] [Google Scholar]
- Okusa MD, Linden J, Macdonald T, Huang L. Selective A2A adenosine receptor activation reduces ischemia-reperfusion injury in rat kidney. The American Journal of Physiology. 1999;277:F404–F412. doi: 10.1152/ajprenal.1999.277.3.F404. [DOI] [PubMed] [Google Scholar]
- Oury C, Toth-Zsamboki E, Vermylen J, Hoylaerts MF. The platelet ATP and ADP receptors. Current Pharmaceutical Design. 2006;12:859–875. doi: 10.2174/138161206776056029. [DOI] [PubMed] [Google Scholar]
- Patel RA, Glover DK, Broisat A, Kabul HK, Ruiz M, Goodman NC, Kramer CM, Meerdink DJ, Linden J, Beller GA. Reduction in myocardial infarct size at 48 hours after brief intravenous infusion of ATL-146e, a highly selective adenosine A2A receptor agonist. American Journal of Physiology. Heart and Circulatory Physiology. 2009;297:H637–H642. doi: 10.1152/ajpheart.00705.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce SM, Skalak TC, Rieger JM, Macdonald TL, Linden J. Selective A (2A) adenosine receptor activation reduces skin pressure ulcer formation and inflammation. American Journal of Physiology. Heart and Circulatory Physiology. 2001;281:H67–H74. doi: 10.1152/ajpheart.2001.281.1.H67. [DOI] [PubMed] [Google Scholar]
- Pelegrin P. Targeting interleukin-1 signaling in chronic inflammation: Focus on P2X(7) receptor and Pannexin-1. Drug News & Perspectives. 2008;21:424–433. doi: 10.1358/dnp.2008.21.8.1265800. [DOI] [PubMed] [Google Scholar]
- Polanowska-Grabowska R, Wallace K, Field JJ, Chen L, Marshall MA, Figler R, Gear AR, Linden J. P-selectin-mediated platelet-neutrophil aggregate formation activates neutrophils in mouse and human sickle cell disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30:2392–2399. doi: 10.1161/ATVBAHA.110.211615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece TB, Tribble CG, Okonkwo DO, Davis JD, Maxey TS, Gazoni LM, Linden J, Kron IL, Kern JA. Early adenosine receptor activation ameliorates spinal cord reperfusion injury. Journal of Cardiovascular Medicine (Hagerstown) 2008;9:363–367. doi: 10.2459/JCM.0b013e3282eee836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutershan J, Cagnina RE, Chang D, Linden J, Ley K. Therapeutic antiinflammatory effects of myeloid cell adenosine receptor A2a stimulation in lipopolysaccharide-induced lung injury. Journal of Immunology. 2007;179:1254–1263. doi: 10.4049/jimmunol.179.2.1254. [DOI] [PubMed] [Google Scholar]
- Revan S, Montesinos MC, Naime D, Landau S, Cronstein BN. Adenosine A2 receptor occupancy regulates stimulated neutrophil function via activation of a serine/threonine protein phosphatase. The Journal of Biological Chemistry. 1996;271:17114–17118. doi: 10.1074/jbc.271.29.17114. [DOI] [PubMed] [Google Scholar]
- Rivo J, Zeira E, Galun E, Einav S, Linden J, Matot I. Attenuation of reperfusion lung injury and apoptosis by A2A adenosine receptor activation is associated with modulation of Bcl-2 and Bax expression and activation of extracellular signal-regulated kinases. Shock. 2007;27:266–273. doi: 10.1097/01.shk.0000235137.13152.44. [DOI] [PubMed] [Google Scholar]
- Rork TH, Wallace KL, Kennedy DP, Marshall MA, Lankford AR, Linden J. Adenosine A2A receptor activation reduces infarct size in the isolated, perfused mouse heart by inhibiting resident cardiac mast cell degranulation. American Journal of Physiology. Heart and Circulatory Physiology. 2008;295:H1825–H1833. doi: 10.1152/ajpheart.495.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryzhov S, Novitskiy SV, Zaynagetdinov R, Goldstein AE, Carbone DP, Biaggioni I, Dikov MM, Feoktistov I. Host A(2B) adenosine receptors promote carcinoma growth. Neoplasia. 2008a;10:987–995. doi: 10.1593/neo.08478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryzhov S, Zaynagetdinov R, Goldstein AE, Novitskiy SV, Blackburn MR, Biaggioni I, Feoktistov I. Effect of A2B adenosine receptor gene ablation on adenosine-dependent regulation of proinflammatory cytokines. The Journal of Pharmacology and Experimental Therapeutics. 2008b;324:694–700. doi: 10.1124/jpet.107.131540. [DOI] [PubMed] [Google Scholar]
- Sharma AK, Laubach VE, Ramos SI, Zhao Y, Stukenborg G, Linden J, Kron IL, Yang Z. Adenosine A2A receptor activation on CD4+ T lymphocytes and neutrophils attenuates lung ischemia-reperfusion injury. The Journal of Thoracic and Cardiovascular Surgery. 2010;139:474–482. doi: 10.1016/j.jtcvs.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet–induced obesity in mice. Diabetes. 2010;59:1171–1181. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura K, Kawamura H, Nagura T, Kato T, Naito T, Kameyama H, Hatakeyama K, Abo T. Association of NKT cells and granulocytes with liver injury after reperfusion of the portal vein. Cellular Immunology. 2005;234:31–38. doi: 10.1016/j.cellimm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Wallace ME, Nutt SL, Yagita H, Godfrey DI, Hayakawa Y. Sequential activation of NKT cells and NK cells provides effective innate immunotherapy of cancer. The Journal of Experimental Medicine. 2005;201:1973–1985. doi: 10.1084/jem.20042280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein E, Zou Y, Poo M, Tessier-Lavigne M. Binding of DCC by netrin-1 to mediate axon guidance independent of adenosine A2B receptor activation. Science. 2001;291:1976–1982. doi: 10.1126/science.1059391. [DOI] [PubMed] [Google Scholar]
- Struyf S, Salogni L, Burdick MD, Vandercappellen J, Gouwy M, Noppen S, Proost P, Opdenakker G, Parmentier M, Gerard C, Sozzani S, Strieter RM, Van Damme J. Angiostatic and chemotactic activities of the CXC chemokine CXCL4L1 (platelet factor-4 variant) are mediated by CXCR3. Blood. 2010;117:480–488. doi: 10.1182/blood-2009-11-253591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GW, Fang G, Linden J, Scheld WM. A2A adenosine receptor activation improves survival in mouse models of endotoxemia and sepsis. The Journal of Infectious Diseases. 2004a;189:1897–1904. doi: 10.1086/386311. [DOI] [PubMed] [Google Scholar]
- Sullivan GW, Lee DD, Ross WG, DiVietro JA, Lappas CM, Lawrence MB, Linden J. Activation of A2A adenosine receptors inhibits expression of alpha 4/beta 1 integrin (very late antigen-4) on stimulated human neutrophils. Journal of Leukocyte Biology. 2004b;75:127–134. doi: 10.1189/jlb.0603300. [DOI] [PubMed] [Google Scholar]
- Sullivan GW, Rieger JM, Scheld WM, Macdonald TL, Linden J. Cyclic AMP-dependent inhibition of human neutrophil oxidative activity by substituted 2-propynylcyclohexyl adenosine A(2A) receptor agonists. British Journal of Pharmacology. 2001;132:1017–1026. doi: 10.1038/sj.bjp.0703893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CX, Zhong H, Mohsenin A, Morschl E, Chunn JL, Molina JG, Belardinelli L, Zeng D, Blackburn MR. Role of A2B adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. Journal of Clinical Investigation. 2006;116:2173–2182. doi: 10.1172/JCI27303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadagavadi RK, Wang W, Ramesh G. Netrin-1 regulates Th1/Th2/Th17 cytokine production and inflammation through UNC5B receptor and protects kidney against ischemia-reperfusion injury. Journal of Immunology. 2010;185:3750–3758. doi: 10.4049/jimmunol.1000435. [DOI] [PubMed] [Google Scholar]
- Treadway JL, Sacca R, Jones BK. Adenosine A(2B) receptor knock-out mice display an improved metabolic phenotype. Diabetologia. 2006;49:44–45. [Google Scholar]
- Turhan A, Weiss LA, Mohandas N, Coller BS, Frenette PS. Primary role for adherent leukocytes in sickle cell vascular occlusion: A new paradigm. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3047–3051. doi: 10.1073/pnas.052522799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace KL, Linden J. Adenosine A2A receptors induced on iNKT and NK cells reduce pulmonary inflammation and injury in mice with sickle cell disease. Blood. 2010;116:5010–5020. doi: 10.1182/blood-2010-06-290643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace KL, Marshall MA, Ramos SI, Lannigan JA, Field JJ, Strieter RM, Linden J. NKT cells mediate pulmonary inflammation and dysfunction in murine sickle cell disease through production of IFN-gamma and CXCR3 chemokines. Blood. 2009;114:667–676. doi: 10.1182/blood-2009-02-205492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Reeves WB, Pays L, Mehlen P, Ramesh G. Netrin-1 overexpression protects kidney from ischemia reperfusion injury by suppressing apoptosis. The American Journal of Pathology. 2009;175:1010–1018. doi: 10.2353/ajpath.2009.090224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis N, Weigert A, von Knethen A, Brune B. Heme oxygenase-1 contributes to an alternative macrophage activation profile induced by apoptotic cell supernatants. Molecular Biology of the Cell. 2009;20:1280–1288. doi: 10.1091/mbc.E08-10-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xaus J, Mirabet M, Lloberas J, Soler C, Lluis C, Franco R, Celada A. IFN-gamma up-regulates the A2B adenosine receptor expression in macrophages: A mechanism of macrophage deactivation. Journal of Immunology. 1999;162:3607–3614. [PubMed] [Google Scholar]
- Xi J, McIntosh R, Shen X, Lee S, Chanoit G, Criswell H, Zvara DA, Xu Z. Adenosine A2A and A2B receptors work in concert to induce a strong protection against reperfusion injury in rat hearts. Journal of Molecular and Cellular Cardiology. 2009;47:684–690. doi: 10.1016/j.yjmcc.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Berkich DA, Crist GH, LaNoue KF. A1 adenosine receptor antagonism improves glucose tolerance in Zucker rats. The American Journal of Physiology. 1998;274:E271–E279. doi: 10.1152/ajpendo.1998.274.2.E271. [DOI] [PubMed] [Google Scholar]
- Yang D, Chen H, Koupenova M, Carroll SH, Eliades A, Freedman JE, Toselli P, Ravid K. A new role for the A2b adenosine receptor in regulating platelet function. Journal of Thrombosis and Haemostasis. 2010;8:817–827. doi: 10.1111/j.1538-7836.2010.03769.x. [DOI] [PubMed] [Google Scholar]
- Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, St Hilaire C, Seldin DC, Toselli P, Lamperti E, Schreiber BM, Gavras H, Wagner DD, Ravid K. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. Journal of Clinical Investigation. 2006a;116:1913–1923. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Day YJ, Toufektsian MC, Xu Y, Ramos SI, Marshall MA, French BA, Linden J. Myocardial infarct-sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation. 2006b;114:2056–2064. doi: 10.1161/CIRCULATIONAHA.106.649244. [DOI] [PubMed] [Google Scholar]
- Young DP, Kushner I, Samols D. Binding of C/EBPbeta to the C-reactive protein (CRP) promoter in Hep3B cells is associated with transcription of CRP mRNA. Journal of Immunology. 2008;181:2420–2427. doi: 10.4049/jimmunol.181.4.2420. [DOI] [PubMed] [Google Scholar]
- Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, Drake CG, Powell JD. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Y, Shen XD, Hancock WW, Gao F, Qiao B, Lassman C, Belperio JA, Strieter RM, Busuttil RW, Kupiec-Weglinski JW. CXCR3+CD4+ T cells mediate innate immune function in the pathophysiology of liver ischemia/reperfusion injury. Journal of Immunology. 2006;176:6313–6322. doi: 10.4049/jimmunol.176.10.6313. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Lapar DJ, Steidle J, Emaminia A, Kron IL, Ailawadi G, Linden J, Lau CL. Adenosine signaling via the adenosine 2B receptor is involved in bronchiolitis obliterans development. The Journal of Heart and Lung Transplantation. 2010;29:1405–1414. doi: 10.1016/j.healun.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


