Abstract
The neurohormone leptin regulates energy homeostasis. Circulating levels of leptin secreted by adipose tissue acts on hypothalamic neurons in the brain leading to decreased appetite and increased energy expenditure. Although leptin signaling in the central nervous system (CNS) is fundamental to its ability to regulate the body’s metabolic balance, leptin also has a variety of effects in many peripheral tissues including the heart, the liver, and the sympathetic nervous system. Leptin stimulation of the hypothalamus can stimulate glucose uptake via the sympathetic nervous system in heart, muscle, and brown adipose tissue. Leptin receptors (Ob-Rb) are also expressed by peripheral sympathetic neurons, but their functional role is not clear. In this study, we found that leptin stimulates axonal growth of both adult and neonatal sympathetic neurons in vitro. Leptin stimulates acute activation of the transcription factor STAT3 via phosphorylation of tyrosine 705. STAT3 phosphorylation is required for leptin-stimulated sympathetic axon outgrowth. Thus, circulating levels of leptin may enhance sympathetic nerve innervation of peripheral tissues.
Keywords: Leptin, axon outgrowth, obesity, cardiac arrhythmia, STAT3
Introduction
The neurohormone leptin controls energy homeostasis by relaying signals from adipose cells to the brain. Genetic studies have shown that removing leptin, or its receptor, from the central nervous system results in animals that are morbidly obese [1–3]. Not surprisingly, the majority of work in this field has been focused on understanding leptin’s central effects [4–7]. However, leptin is secreted into the circulation and leptin receptors (Ob-Rb) are found in many peripheral targets including heart, liver, muscle, lungs, adrenals, lymph nodes, and the sympathetic nervous system [8–12]. It is well known that central neurons stimulated by leptin activate the sympathetic nervous system [13–18]. In addition, postganglionic sympathetic neurons express Ob-Rb and therefore can respond directly to changing leptin levels [19, 20]. However, the effect of direct stimulation of sympathetic neurons by leptin has not been characterized.
Many studies show that obesity exacerbates cardiovascular disease states. Recently, we found that obese rats had increased axon growth and developed sympathetic hyperinnervation of the heart compared to rats fed normal chow [21]. A potential mediator could be leptin, which is elevated during the obese state. Interestingly, Ob-Rb is a member of the cytokine receptor class I super family and is most closely related to gp130 receptor subunit [22]. We recently reported that gp130 cytokines are required for maximal axon outgrowth in sympathetic neurons [23]. Due to leptin’s closely related homology to gp130 cytokines, it is possible they share similar mechanisms as well. Leptin binding to Ob-Rb activates Jak2—STAT3 and MAPK signaling pathways [24] [25]. However, STAT3 activation is required for leptin’s central effects regulating body weight and energy homeostasis [26, 27] [28]. Indeed, the chronic inflammatory state associated with obesity and cancer is characterized by increased circulating cytokines and activated STAT3 levels [29]. Phosphorylation of STAT3 is required for sympathetic axon outgrowth [23], but leptin activation of STAT3 has not been tested in this paradigm.
In the present study, we tested the hypothesis that leptin stimulation of sympathetic neurons regulates axon growth. We found that leptin increased sympathetic axon growth in adult and neonatal explants. This effect required phosphorylation of STAT3 on tyrosine 705. Thus, similar to related gp130 cytokines, leptin stimulated sympathetic axon outgrowth requires activation of STAT3. Leptin-stimulated increased sympathetic tone to peripheral tissue targets may help explain how circulating leptin levels contribute to obesity related diseases in humans.
Materials and Methods
Materials
Matrigel™ was purchased from BD Biosciences (San Jose, CA). CNTF was from Preprotech (Rocky Hills, NJ). Recombinant mouse leptin was purchased from R & D Systems (Minneapolis, MN). Nerve growth factor (NGF) was purchase from Austral Biologicals (San Ramon, CA). Dispase was purchased from Boehringer Mannheim (Indianapolis, IN). Collagenase type II was purchased from Worthington Biochemicals (Freehold NJ). Nitrocellulose membranes were from Schleicher & Schuell (Dassel, Germany). Protease inhibitor cocktail, phosphatase inhibitor cocktail (#2 & #3), and poly-L-lysine were purchased from Sigma-Aldrich (St. Louis, MO). Bovine Serum Albumin-Fraction V (BSA) was from Thermo Fischer Scientific (Waltham, MA). STATi/Stattic (STAT3 phosphorylation inhibitor) was from Calbiochem (Darmstadt, Germany).
Animals
Pregnant adult Sprague–Dawley rats were obtained from Charles River Laboratories (Wilmington, MA). Animals were anesthetized using isoflurane and decapitated prior to harvest of ganglia. All procedures were approved by the OHSU Institutional Animal Care and Use Committee and comply with the Guide for the Care and Use of Laboratory Animals published by the National Academies Press (8th edition).
Western Blotting and Antibodies
Total STAT3 (#9132) and phospho-STAT3 (Tyr705) (#9131) antibodies were from Cell Signaling Technology (Danvers, MA). Species-specific secondary antibodies conjugated to horseradish peroxidase were from Pierce (Rockford, IL). Neonatal sympathetic neurons (postnatal day 1–4) were dissociated and cultured in serum free medium as described previously [23]. Cells were treated with 200 ng/ml leptin for the times specified, and lysates subjected to western blotting as described previously [23]. Bands were captured using the ChemiDoc™ XRS+ system and image lab software (Bio-Rad Laboratories, Hercules, CA). Immunoreactive bands were quantified using Image Lab Software 4.0.1 from Bio-Rad Laboratories.
Sympathetic axon outgrowth from SCG explants
Both SCGs were removed from neonatal (postnatal days 1–4) or adult rats, halved, and explanted into a 12-well tissue culture plate and covered with 35 μL Matrigel (BD Biosciences, San Jose, CA). Serum-free DMEM/F12 media (Life Technologies, Grand Island, NY) + 1% penicillin/ streptomycin was layered over the solidified Matrigel, and wells were treated with 2ng/mL NGF. Culture plates were placed in a humidified incubator of 95%O2/5%CO2 at 37°C. Axon outgrowth was visualized with phase contrast microscopy, and axon length was measured in the images using Nikon Elements AR 3.0 software (Melville, NY). Initial images for “time-zero” were taken 24-hrs after plating. At time-zero, explants were either treated with vehicle or leptin (Sigma, 100 ng/mL), and additional images were taken 24-hrs later (Time 24-hr). For the Stattic experiments neonatal ganglia were used and Stattic (20μm) was added to all explants for 6 hrs after the 24 hr pictures were taken. The rate of axon outgrowth (μm/hr) was calculated from the difference in axon growth length before and after treatment, and then dividing this value over the duration of the treatment (24hr for leptin or 6hr for Stattic). Multiple sites of growth (3–6) from were taken for each explant and averaged. Each condition had 4 replicates and these experiments were performed 4 times. For adult explants, SCGs were bisected and axon growth was measured in two wells for each animal (N=3). The values from each well were averaged to determine the mean rate of axon outgrowth for each animal.
Statistics
Student’s T test was used for a comparison between two groups. Statistics were carried out using Prism 5.0 (GraphPad Software, La Jolla, CA).
Results
Leptin enhances sympathetic outgrowth
To test the hypothesis that leptin stimulates sympathetic axon outgrowth, we measured axon outgrowth from explanted SCGs from adult rats (n=3) treated with either vehicle or leptin. At time-zero, axon length was similar between SCGs treated with vehicle (205±30 μm) or leptin (253±12 μm). After the 24-hr treatment period, axon length was longer in leptin (701±90 μm) compared to vehicle (484±30 μm), and the rate of axon outgrowth was 39% higher in SCG treated with leptin compared to vehicle-treated SCG (Figure 1A–C, p<0.05).
Figure 1.
Assessment of axon outgrowth in SCG explants treated with leptin. (A) In adult rats (n=3), leptin (100 ng/ml; striped bar) accelerated axon outgrowth in SCG explants compared to vehicle (white bar). (B–C) Photos at Time 24-hr in SCG explants treated with vehicle (B) or leptin (C). Data are mean±SEM, *p<0.05. Three animals were assayed in duplicate (N=3).
Leptin stimulates phosphorylation of STAT3 in sympathetic neurons
To investigate the signaling pathway activated by leptin in sympathetic neurons, we examined levels of STAT3 phosphorylation using SDS-PAGE. We treated neonatal sympathetic neurons with leptin (200ng/ml) over a 30 minute time course using ciliary neurotrophic factor (CNTF) as a positive control (Figure 2A). Leptin stimulated phosphorylation of STAT3 on Y705 at all time points tested. Leptin stimulated a maximum 163.72% increase in the ratio of phospho-STAT3 to total STAT3 at 30 min and we used this time point to test the STAT3 inhibitor Stattic’s ability to block leptin-stimulated STAT3 phosphorylation. We pretreated sympathetic neurons with Stattic and then stimulated the neurons with leptin for 30 minutes. Stattic pretreatment blocks basal and leptin-stimulated STAT3 phosphorylation (Figure 2B).
Figure 2.
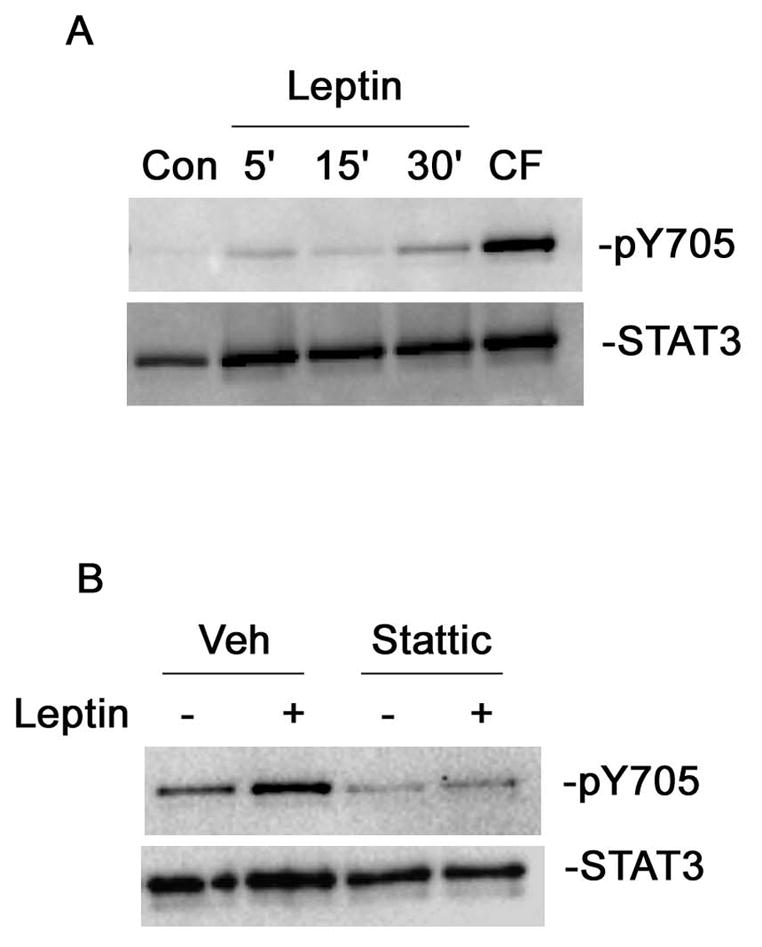
Stattic blocks leptin-stimulated STAT3 phosphorylation. (A) Dissociated sympathetic neurons were stimulated with leptin (200ng/ml) for the indicated time points or CNTF (100ng/ml) for 5min. Phospho-STAT3 (pY705) and total STAT3 levels were measured via western blotting. (B) Dissociated sympathetic neurons were pre-treated with vehicle or Stattic (20μm) for 10min prior to stimulation with leptin (200ng/ml) for 30min. Phospho-STAT3 (pY705) and total STAT3 levels were measured as in A. This is a representative experiment that was repeated at least 3 times.
STAT3 phosphorylation is required for sympathetic axon outgrowth
To test the role of phosphorylated STAT3 in sympathetic axon outgrowth, we measured axon outgrowth of neonatal SCG explants. We compared untreated (n=4, 37.48±2.30 μm) to leptin treated (n=4, 48.13±1.42 μm) explants. After the 24-hr treatment period, leptin stimulation increased growth rates 28.41% compared to untreated explants (Figure 3A, p<0.01). After 24 hrs of leptin treatment, Stattic was added to all explants for 6 hrs to test the requirement of STAT3 phosphorylation in leptin-stimulated axon outgrowth. Stattic blocked both untreated (−94.61±1.68%) and leptin-stimulated (−101.8±5.92%) axon outgrowth equally (Figure 3B, p=0.2888).
Figure 3.
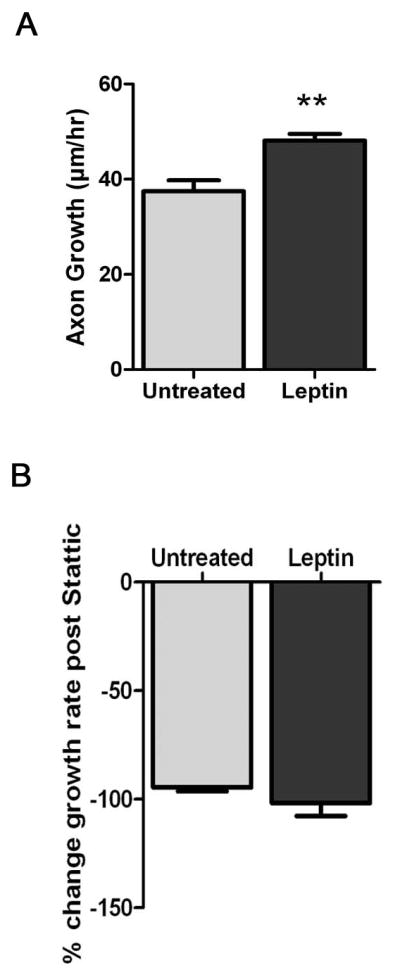
Leptin stimulates sympathetic axon growth via STAT3. (A) Paired SCG explants were treated with leptin for 24 hours and axon growth rates were calculated. Leptin treated explants (n=4) had faster growth rates than untreated controls (n=4). Data are mean±SEM, **p<0.01. (B) The same explants shown in A were treated with Stattic to block STAT3 phosphorylation. The percent change in growth was similar in both untreated and leptin treated explants after 6 hours. This is a representative experiment that was repeated 4 times (N=4).
Discussion
In 2008, over 1.4 billion people suffered from obesity-related health issues worldwide (World Health Organization, web reference, 2013). A key regulator of energy homeostasis is the neurohormone leptin. Plasma levels of leptin signal the state of fat stores to the brain and energy regulation is adjusted accordingly. Sympathetic nerve activation is one of the major tools the brain uses to control metabolic states of peripheral tissues. Thus, leptin effects on the nervous system have been investigated thoroughly in the brain, but fewer studies have examined peripheral effects of leptin. In this study, we show that direct stimulation of post-ganglionic sympathetic neurons with leptin promotes axon outgrowth. In addition, we provide evidence that STAT3 phosphorylation is required for leptin effects on sympathetic outgrowth. Thus, our work identifies a novel mechanism by which circulating leptin regulates sympathetic input to peripheral targets in an obesity paradigm.
The leptin receptor belongs to the class 1 cytokine receptor family [30, 31]. Although there are multiple forms of the leptin receptor, the long form (ObRb) is the best characterized and is the only form that activates STAT3 [32, 33]. The 3 main signaling pathways triggered by leptin are STAT3, MAPKs, and PI3K pathways. Different arms of leptin signaling regulate specific facets of energy homeostasis. Leptin activation of MAPKs has been implicated in combating stress, proliferation, and protection after ischemia reperfusion [34–37]. STAT3 and PI3K pathways are required for central control of feeding behavior [38, 39]. In central neurons, the Ob-Rb, STAT3 and MAPK pathways are required for leptin stimulated axon outgrowth [40]. Recently, we showed cytokine-activated STAT3 mediates sympathetic axon outgrowth in an injury paradigm [23]. Numerous studies implicate STAT3 activation in axon regeneration and outgrowth [23, 41–43]. Since leptin shares homology with the IL-6 cytokine family, and activates STAT3, it is not surprising that it can enhance sympathetic axon outgrowth.
Although obesity is associated with a number of metabolic syndromes, abnormal weight maintenance is clearly linked to cardiovascular disease states. Abnormal leptin expression can alter β3-adreneric receptor expression in cardiac myocytes [44] and induce ventricular hypertrophy resulting in higher mortality in mice [45]. Recent studies have shown that animals fed a high fat diet have an increased risk of ventricular arrhythmias and sudden cardiac death [21,46, 47]. Sympathetic hyperinnervation in the heart has been linked to arrhythmias in animals and humans [48, 49]. Interestingly, development of arrhythmias is seen even in the absence of obesity [47], and a high fat diet without overt obesity results in cardiac sympathetic hyperinnervation [21]. A high fat diet increases serum levels of many growth factors including EGF, IGF-1, Insulin, MCP-1, IFN-γ, and leptin [50]. In addition, adipose tissue is capable of secreting the classic sympathetic growth factor, NGF [51]. Thus, increased fat intake changes serum levels of multiple nerve growth promoting compounds, but leptin-stimulated sympathetic nerve sprouting may provide an additional mechanism for hyperinnervation of peripheral tissues.
Conclusions
Leptin signaling in CNS is central to its ability to regulate the body’s energy stores, but leptin has a variety of effects in many peripheral tissues including the heart and the sympathetic nervous system. In this study, we demonstrate that leptin stimulates axon outgrowth in adult and neonatal sympathetic neurons. Phosphorylation of STAT3 on Y705 is required for leptin-stimulated axon growth. Circulating levels of leptin may explain sympathetic hyperinnervation of the heart and increased risk of arrhythmias. Given the predominance of obesity today, these findings clearly warrant further study.
Highlights.
Leptin stimulates increased axon growth in sympathetic explants
STAT3 phosphorylation is required for leptin-stimulated axon outgrowth
This work links a high fat diet to increased risk of cardiac arrhythmias
Acknowledgments
This work was supported by generous support from the National Institute of Health (HL068231) (MJP and BAH) and from the American Heart Association (AHA 7500041) (BHM).
Abbreviations
- STAT3
Signal transducer and activator of transcription 3
- Ob-Rb
Long form Leptin Receptor
- MAPK
Mitogen-activated protein kinase
- SCG
superior cervical ganglion
- JAK2
Janus kinase-2
- PI3K
Phosphoinositide 3-kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michael J. Pellegrino, Email: pellegri@ohsu.edu.
Belinda H. McCully, Email: houghtob@ohsu.edu.
Beth A. Habecker, Email: habecker@ohsu.edu.
References
- 1.Montague CT, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387(6636):903–8. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 2.Clement K, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392(6674):398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 4.Levin BE, Dunn-Meynell AA. Reduced central leptin sensitivity in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2002;283(4):R941–8. doi: 10.1152/ajpregu.00245.2002. [DOI] [PubMed] [Google Scholar]
- 5.Li B, et al. Leptin acts in the forebrain to differentially influence baroreflex control of lumbar, renal, and splanchnic sympathetic nerve activity and heart rate. Hypertension. 2013;61(4):812–9. doi: 10.1161/HYPERTENSIONAHA.111.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campfield LA, et al. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269(5223):546–9. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 7.Stephens TW, et al. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature. 1995;377(6549):530–2. doi: 10.1038/377530a0. [DOI] [PubMed] [Google Scholar]
- 8.Hoggard N, et al. Localization of leptin receptor mRNA splice variants in murine peripheral tissues by RT-PCR and in situ hybridization. Biochem Biophys Res Commun. 1997;232(2):383–7. doi: 10.1006/bbrc.1997.6245. [DOI] [PubMed] [Google Scholar]
- 9.Chen SC, et al. Splice variants of the OB receptor gene are differentially expressed in brain and peripheral tissues of mice. J Recept Signal Transduct Res. 1999;19(1–4):245–66. doi: 10.3109/10799899909036649. [DOI] [PubMed] [Google Scholar]
- 10.Kellerer M, et al. Leptin activates PI-3 kinase in C2C12 myotubes via janus kinase-2 (JAK-2) and insulin receptor substrate-2 (IRS-2) dependent pathways. Diabetologia. 1997;40(11):1358–62. doi: 10.1007/s001250050832. [DOI] [PubMed] [Google Scholar]
- 11.Szanto I, Kahn CR. Selective interaction between leptin and insulin signaling pathways in a hepatic cell line. Proc Natl Acad Sci U S A. 2000;97(5):2355–60. doi: 10.1073/pnas.050580497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fei H, et al. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci U S A. 1997;94(13):7001–5. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mark AL, et al. Leptin signaling in the nucleus tractus solitarii increases sympathetic nerve activity to the kidney. Hypertension. 2009;53(2):375–80. doi: 10.1161/HYPERTENSIONAHA.108.124255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahmouni K, et al. Intracellular mechanisms involved in leptin regulation of sympathetic outflow. Hypertension. 2003;41(3 Pt 2):763–7. doi: 10.1161/01.HYP.0000048342.54392.40. [DOI] [PubMed] [Google Scholar]
- 15.Satoh N, et al. Sympathetic activation of leptin via the ventromedial hypothalamus: leptin-induced increase in catecholamine secretion. Diabetes. 1999;48(9):1787–93. doi: 10.2337/diabetes.48.9.1787. [DOI] [PubMed] [Google Scholar]
- 16.Mark AL, et al. Loss of leptin actions in obesity: two concepts with cardiovascular implications. Clin Exp Hypertens. 2004;26(7–8):629–36. doi: 10.1081/ceh-200031948. [DOI] [PubMed] [Google Scholar]
- 17.Haynes WG, et al. Sympathetic and cardiorenal actions of leptin. Hypertension. 1997;30(3 Pt 2):619–23. doi: 10.1161/01.hyp.30.3.619. [DOI] [PubMed] [Google Scholar]
- 18.Haynes WG. Interaction between leptin and sympathetic nervous system in hypertension. Curr Hypertens Rep. 2000;2(3):311–8. doi: 10.1007/s11906-000-0015-1. [DOI] [PubMed] [Google Scholar]
- 19.Czaja K, et al. Leptin receptors, NPY, and tyrosine hydroxylase in autonomic neurons supplying fat depots in a pig. Biochem Biophys Res Commun. 2002;293(3):1138–44. doi: 10.1016/S0006-291X(02)00335-2. [DOI] [PubMed] [Google Scholar]
- 20.Miller SM, et al. Leptin receptor immunoreactivity in sympathetic prevertebral ganglion neurons of mouse and rat. Neurosci Lett. 1999;265(2):75–8. doi: 10.1016/s0304-3940(99)00215-3. [DOI] [PubMed] [Google Scholar]
- 21.McCully BH, et al. Sympathetic cardiac hyperinnervation and atrial autonomic imbalance in diet-induced obesity promote cardiac arrhythmias. Am J Physiol Heart Circ Physiol. 2013;305(10):H1530–7. doi: 10.1152/ajpheart.00196.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tartaglia LA, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83(7):1263–71. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 23.Pellegrino MJ, Habecker BA. STAT3 integrates cytokine and neurotrophin signals to promote sympathetic axon regeneration. Mol Cell Neurosci. 2013;56:272–82. doi: 10.1016/j.mcn.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kloek C, et al. Regulation of Jak kinases by intracellular leptin receptor sequences. J Biol Chem. 2002;277(44):41547–55. doi: 10.1074/jbc.M205148200. [DOI] [PubMed] [Google Scholar]
- 25.Ihle JN. The Stat family in cytokine signaling. Curr Opin Cell Biol. 2001;13(2):211–7. doi: 10.1016/s0955-0674(00)00199-x. [DOI] [PubMed] [Google Scholar]
- 26.Bates SH, Myers MG. The role of leptin-->STAT3 signaling in neuroendocrine function: an integrative perspective. J Mol Med (Berl) 2004;82(1):12–20. doi: 10.1007/s00109-003-0494-z. [DOI] [PubMed] [Google Scholar]
- 27.Bates SH, et al. LRb-STAT3 signaling is required for the neuroendocrine regulation of energy expenditure by leptin. Diabetes. 2004;53(12):3067–73. doi: 10.2337/diabetes.53.12.3067. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, et al. Time-dependent transcriptional profiles of genes of the hypothalamic-pituitary-gonadal axis in medaka (Oryzias latipes) exposed to fadrozole and 17beta-trenbolone. Environ Toxicol Chem. 2008;27(12):2504–11. doi: 10.1897/08-082.1. [DOI] [PubMed] [Google Scholar]
- 29.Wunderlich CM, Hovelmeyer N, Wunderlich FT. Mechanisms of chronic JAK-STAT3-SOCS3 signaling in obesity. JAKSTAT. 2013;2(2):e23878. doi: 10.4161/jkst.23878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baumann H, et al. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci U S A. 1996;93(16):8374–8. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bates SH, Myers MG., Jr The role of leptin receptor signaling in feeding and neuroendocrine function. Trends Endocrinol Metab. 2003;14(10):447–52. doi: 10.1016/j.tem.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Ghilardi N, et al. Defective STAT signaling by the leptin receptor in diabetic mice. Proc Natl Acad Sci U S A. 1996;93(13):6231–5. doi: 10.1073/pnas.93.13.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bjorbaek C, et al. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem. 1997;272(51):32686–95. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- 34.Smith CC, et al. Leptin, the obesity-associated hormone, exhibits direct cardioprotective effects. Br J Pharmacol. 2006;149(1):5–13. doi: 10.1038/sj.bjp.0706834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajapurohitam V, et al. The obesity-associated peptide leptin induces hypertrophy in neonatal rat ventricular myocytes. Circ Res. 2003;93(4):277–9. doi: 10.1161/01.RES.0000089255.37804.72. [DOI] [PubMed] [Google Scholar]
- 36.Shin HJ, et al. Leptin induces hypertrophy via p38 mitogen-activated protein kinase in rat vascular smooth muscle cells. Biochem Biophys Res Commun. 2005;329(1):18–24. doi: 10.1016/j.bbrc.2004.12.195. [DOI] [PubMed] [Google Scholar]
- 37.Hausenloy DJ, Tsang A, Yellon DM. The reperfusion injury salvage kinase pathway: a common target for both ischemic preconditioning and postconditioning. Trends Cardiovasc Med. 2005;15(2):69–75. doi: 10.1016/j.tcm.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Niswender KD, et al. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413(6858):794–5. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- 39.Rahmouni K. Brain effects of leptin: what intracellular mechanism? Curr Diab Rep. 2003;3(6):427–9. doi: 10.1007/s11892-003-0001-5. [DOI] [PubMed] [Google Scholar]
- 40.Bouret SG, et al. Distinct roles for specific leptin receptor signals in the development of hypothalamic feeding circuits. J Neurosci. 2012;32(4):1244–52. doi: 10.1523/JNEUROSCI.2277-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selvaraj BT, et al. Local axonal function of STAT3 rescues axon degeneration in the pmn model of motoneuron disease. J Cell Biol. 2012;199(3):437–51. doi: 10.1083/jcb.201203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee N, et al. STAT3 phosphorylation in injured axons before sensory and motor neuron nuclei: potential role for STAT3 as a retrograde signaling transcription factor. J Comp Neurol. 2004;474(4):535–45. doi: 10.1002/cne.20140. [DOI] [PubMed] [Google Scholar]
- 43.Bareyre FM, et al. In vivo imaging reveals a phase-specific role of STAT3 during central and peripheral nervous system axon regeneration. Proc Natl Acad Sci U S A. 2011;108(15):6282–7. doi: 10.1073/pnas.1015239108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larson JE, et al. Dependence of beta3-adrenergic signaling on the adipokine leptin in cardiac myocytes. Int J Obes (Lond) 2012;36(6):876–9. doi: 10.1038/ijo.2011.137. [DOI] [PubMed] [Google Scholar]
- 45.Barouch LA, et al. Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circ Res. 2006;98(1):119–24. doi: 10.1161/01.RES.0000199348.10580.1d. [DOI] [PubMed] [Google Scholar]
- 46.Prior LJ, et al. Exposure to a High-Fat Diet During Development Alters Leptin and Ghrelin Sensitivity and Elevates Renal Sympathetic Nerve Activity and Arterial Pressure in Rabbits. Hypertension. 2013 doi: 10.1161/HYPERTENSIONAHA.113.02498. [DOI] [PubMed] [Google Scholar]
- 47.Aubin MC, et al. A high-fat diet increases risk of ventricular arrhythmia in female rats: enhanced arrhythmic risk in the absence of obesity or hyperlipidemia. J Appl Physiol (1985) 2010;108(4):933–40. doi: 10.1152/japplphysiol.01281.2009. [DOI] [PubMed] [Google Scholar]
- 48.Zhou S, et al. Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ Res. 2004;95(1):76–83. doi: 10.1161/01.RES.0000133678.22968.e3. [DOI] [PubMed] [Google Scholar]
- 49.Cao JM, et al. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation. 2000;101(16):1960–9. doi: 10.1161/01.cir.101.16.1960. [DOI] [PubMed] [Google Scholar]
- 50.Park H, et al. A high-fat diet increases angiogenesis, solid tumor growth, and lung metastasis of CT26 colon cancer cells in obesity-resistant BALB/c mice. Mol Carcinog. 2012;51(11):869–80. doi: 10.1002/mc.20856. [DOI] [PubMed] [Google Scholar]
- 51.Peeraully MR, Jenkins JR, Trayhurn P. NGF gene expression and secretion in white adipose tissue: regulation in 3T3-L1 adipocytes by hormones and inflammatory cytokines. Am J Physiol Endocrinol Metab. 2004;287(2):E331–9. doi: 10.1152/ajpendo.00076.2004. [DOI] [PubMed] [Google Scholar]



