Abstract
Despite the fact that up to half of all heart failure occurs in patients without evidence of systolic cardiac dysfunction, there are no universally accepted diagnostic markers and no approved therapies for heart failure with preserved ejection fraction (HFpEF). HFpEF, otherwise known as diastolic heart failure, has nearly the same grim prognosis as systolic heart failure, and diastolic heart failure is increasing in incidence and prevalence. Major trials have shown that many of the treatments that are salutary in systolic heart failure have no beneficial effects in diastolic heart failure, suggesting different underlying mechanisms for these two disorders. Even criteria for diagnosis of HFpEF are still debated, and there is still no gold standard marker to detect diastolic dysfunction. Here, we will review some promising new insights into the pathogenesis of diastolic dysfunction that may lead to new diagnostic and therapeutic tools.
Keywords: heart failure, diastolic dysfunction, hypertension, diabetes, oxidative stress
INTRODUCTION
Heart failure is a major and growing public health problem in the United States affecting ~5 million patients in this country. Up to half of the 550,000 patients newly diagnosed with heart failure in each year have diastolic heart failure or heart failure with preserved ejection fraction (HFpEF). The disorder is the primary reason for 12 to 15 million office visits and 6.5 million hospital days each year.(1) HFpEF is increasing in prevalence and incidence. Both systolic and diastolic heart failure has a similar grim prognoses,(2) and there are no approved therapies for diastolic heart failure.
What is diastolic dysfunction or diastolic heart failure?
Diastolic heart failure is a diagnosis of exclusion applied when a patient has heart failure symptoms, no evidence of other causes, and diastolic dysfunction. The disorder is thought to arise from impaired cardiac relaxation. While a considerable number of patients may have demonstrated diastolic dysfunction, a much smaller number suffer from heart failure. The determinants of progression from asymptomatic dysfunction to overt heart failure are unknown, and many people remain without clinical symptoms of heart failure. Another area of investigation is the role of diastolic dysfunction in right heart failure syndromes.
Symptoms
The major signs and symptoms of diastolic heart failure are lung congestion accompanied with breathlessness, coughing, tachypnea, dyspnea on exertion, or paroxysmal nocturnal dyspnea. Dyspnea on exertion is the sensation of difficult or uncomfortable breathing after a level of activity. Paroxysmal nocturnal dyspnea is a sensation of shortness of breath that awakens the patient, often after one or two hours of sleep and is usually relieved in the upright position.
Diagnosis
The incidence of diastolic dysfunction is increasing, affecting fifteen percent of patients less than 50 years old and 50% of patients older than 70. Furthermore, there appears to be a gender bias towards women with approximately 75% patients with diastolic dysfunction being women. There are no specific blood markers of diastolic dysfunction, but there are some useful diagnostic tests.
Echocardiography
The fundamental requirements of the diagnosis are heart failure with a normal left ventricular ejection fraction (i.e. >50%). Suggestive of the diagnosis is the presence of left ventricular diastolic dysfunction. While the gold standard for diastolic dysfunction is thought to be derived from ventricular pressure volume lops, this is generally an impractical measure. Commonly, echocardiography can be used to evaluate the characteristics of diastolic left ventricular relaxation, filling, and distensibility. Echocardiography has been used to assess the dimensional changes and abnormal diastolic function by E/A ratio (i.e. early to late left ventricular blood filling velocity as measured by Doppler flow across the mitral value in diastole). In early, mild diastolic dysfunction, impaired relaxation results in an inversion of the normal E/A ratio, increased mitral flow deceleration time, and increased isovolumic relaxation time, the time interval from closure of the aortic valve to onset of left ventricular filling. In a second stage of diastolic dysfunction thought to be more severe, the pseudo-normal stage, the E/A ratio normalizes to E>A. Finally, patients can show a restrictive filling pattern with the E/A ratio >2. Aside from blood flow velocity across the mitral valve during diastole, direct assessment of mitral annular displacement can be used as a marker of diastolic function. Diastolic dysfunction is accompanied by significant reductions in tissue mitral annulus early longitudinal (E′) velocities and the ratio of early annulus to late annulus (E′/A′) velocities. Also, the ratio of early diastolic filling velocity to the early diastolic mitral annulus velocity (E/E′) has been reported to have the highest correlation with invasive hemodynamic measures of diastolic dysfunction and can predict LV filling pressures (E/E′ >15 suggests increased filling pressures).(3) Color Doppler echocardiography can be used to estimate the rapidity of movement of a wave of blood across the mitral valve, and slow flow velocity is an indication for diastolic dysfunction.
Echocardiographic speckle tracking
Developing methods for diagnosis of diastolic dysfunction include assessing left ventricular relaxation directly. Speckle-tracking echocardiography is a new method that evaluates myocardial deformation. In this technique distinct echocardiography patterns, speckles, are followed to assess wall motion. Speckle analysis can be used to assess radial, longitudinal, and circumferential displacement and strain. Decreased strain rate in diastole is consistent with diastolic dysfunction.
Cardiac magnetic resonance (CMR) imaging
Recently, CMR imaging has been used to evaluate diastolic dysfunction. This technology provides the excellent spatial resolution, visualization of mitral valve inflow velocity, and pulmonary veins blood flows. With myocardial tagging (a pulse sequence that amounts to marking specific locations on the myocardium that can be followed in time), diastolic myocardial strain rate can be calculated. Delay and prolonged strain rates are related to relaxation impairment.
Mechanism and therapeutic approaches
Research is shedding new light on the mechanism of diastolic dysfunction, and these new insights might lead to improved diagnostics and specific therapies. American Heart Association/American College of Cardiology guidelines recommend treatment of hypertension, maintenance of sinus rhythm, prevention of tachycardia, venous pressure reduction, and prevention of myocardial ischemia.(4)
Epidemiological risk factors for diastolic dysfunction include age, hypertension, atrial fibrillation, and diabetes mellitus. Of note, diastolic dysfunction is observed in about 40% of patients with diabetes.(5) These risk factors have considerable overlap with atherosclerosis, which suggested that these two conditions might have similar pathology. This idea was reinforced by the observation that β-blockers, angiotensin converting enzyme inhibitors (ACE inhibitors), angiotensin receptor blockers (ARBs), and aldosterone antagonists, all salutary in systolic heart failure, have no beneficial effect in diastolic heart failure. These observations suggested that systolic and diastolic heart failure represent fundamentally different pathologies.
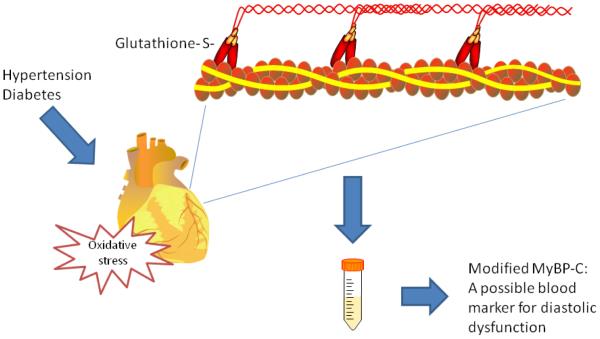
To begin to address the underlying pathology, we developed two unique mouse models of isolated diastolic dysfunction by inducing hypertension or glucose intolerance in the mice. Just like in blood vessels, nitric oxide made by nitric oxide synthase (NOS) is thought to contribute to cardiac relaxation. Hypertension-induced diastolic dysfunction was accompanied by cardiac tetrahydrobiopterin (BH4; a co-factor in NOS) depletion, NOS dysfunction, a depression in myofilament cross-bridge kinetics, and S-glutathionylation of cardiac myosin binding protein C (MyBP-C).(6, 7) BH4 supplementation was able to ameliorate diastolic dysfunction by preventing glutathionylation of MyBP-C and by reversing changes of myofilament properties that occurred during diastolic dysfunction. MyBP-C glutathionylation correlated with the presence of diastolic dysfunction. Our results suggest that by depressing S-glutathionylation of MyBP-C, BH4 ameliorates diastolic dysfunction by reversing a decrease in cross-bridge turnover kinetics. These data provide evidence for modulation of cardiac relaxation by post-translational modification of myofilament proteins. In the same model, we found that ranolazine, a drug used now for angina, was able to ameliorate diastolic dysfunction. This effect was a result of ranolazine acting directly on the myofilaments.(6) Recently, we have demonstrated a similar pathology occurs in a mouse model of type II diabetes mellitus, and a mitochondria-target anti-oxidant is useful in reversing diastolic dysfunction.(8) In this same study, glucose control alone was ineffective in reversing diastolic dysfunction. In preliminary studies, we have found that modified MyBP-C can be measured in blood and is elevated in patients with diastolic dysfunction (Fig 1).
Figure 1. A possible mechanism and marker for diastolic dysfunction.
Hypertension and diabetes lead to cardiac oxidation and S-glutathionylation of cardiac myosin binding protein-C (MyBP-C), a cardiac contractile protein. This leads to impaired relaxation, and modified MyBP-C in the blood may represent a biomarker for diastolic dysfunction.
Despite these promising observations, it appears that age associated diastolic dysfunction may be a distinct pathology involving myocardial fibrosis. In a senescence-accelerated mouse model, diastolic dysfunction was accompanied by fibrosis that arose in conjunction with an increase in pro-fibrotic cytokines.(9) This model suggests that there may be more than one form of diastolic dysfunction and that age-associated dysfunction would have distinctly different biological markers and treatments than hypertension or diabetes-associated diastolic dysfunction.
Summary
In summary, diastolic heart failure occurs in approximately half of all heart failure cases. This type of heart failure is caused by a failure of the myocardium to relax properly. Cardiac diastolic dysfunction and subsequent heart failure appear to be distinct pathological entities from systolic heart failure. Diastolic dysfunction is accompanied by cardiac oxidation and oxidative modification of a cardiac contractile protein, MyBP-C (Figure 1). Oxidation of this protein appears to result in increased sensitivity to calcium and delayed and incomplete relaxation. Treatments that inhibit oxidation such as BH4 and mitochondria-target antioxidants can treat diastolic dysfunction caused by hypertension or diabetes mellitus. Levels of modified MyBP-C may represent a new blood test for the presence of diastolic dysfunction and a marker of therapy. These observations suggest that physicians may be able to diagnose diastolic heart failure more accurately and dispense specific therapies in the future.
Acknowledgments
Grants and support: National Institutes of Health grants P01 HL058000, R01 HL1024025, R01 HL106592, Veterans Administration Merit Award, and R41 HL112355 to SCD.
Abbreviation
- BH4
tetrahydrobiopterin
- CMR
cardiac magnetic resonance
- DM
diabetes mellitus
- HFpEF
heart failure with preserved ejection fraction
- MyBP-C
cardiac myosin binding protein C
- NOS
nitric oxide synthase
Footnotes
Disclosures: SCD is the inventor on patent applications: 1) 11/895,883 Methods and Compositions for Treating Diastolic Dysfunction, 2) 13/503,812 Methods of Diagnosing Diastolic Dysfunction, 3) 13/397,622 Methods for Treating Diastolic Dysfunction and Related Conditions, 4) 13/658,943 Method of Improving Diastolic Dysfunction, 5) 13/841,843 Myosin Binding Protein-C for Use in Methods Relating to Diastolic Heart Failure, and 6) 61/728,302 Mitochondrial Antioxidants and Diabetes.
References
- 1.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 3.Jeong EM, Monasky MM, Gu L, et al. Tetrahydrobiopterin improves diastolic dysfunction by reversing changes in myofilament properties. J Mol Cell Cardiol. 2013;56:44–54. doi: 10.1016/j.yjmcc.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1–e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 6.Lovelock JD, Monasky MM, Jeong EM, et al. Ranolazine improves cardiac diastolic dysfunction through modulation of myofilament calcium sensitivity. Circ Res. 2012;110:841–50. doi: 10.1161/CIRCRESAHA.111.258251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silberman GA, Fan TH, Liu H, et al. Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction. Circulation. 2010;121:519–28. doi: 10.1161/CIRCULATIONAHA.109.883777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung J, Jeong EM, Go Y, et al. Mitochondria-Targeted Antioxidant Ameliorates Diet-Induced Diabetes and Diastolic Dysfunction (abstr) J Am Coll Cardiol. 2013;61:E597. [Google Scholar]
- 9.Reed AL, Tanaka A, Sorescu D, et al. Diastolic dysfunction is associated with cardiac fibrosis in the senescence-accelerated mouse. Am J Physiol Heart Circ Physiol. 2011;301:H824–H831. doi: 10.1152/ajpheart.00407.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]