Abstract
The in vitro macrophage migration inhibition test was used to detect the development of delayed-type hypersensitivity in guinea pigs infected with Salmonella typhimurium. Four different preparations from supernatants of S. typhimurium cultures were used as the antigens in this test. They included the concentrated bacterial antigens, the high-molecular-weight (>50,000) antigens, the ammonium sulfate-precipitated antigens, and the ribonuclease-treated antigens. All four antigen preparations were shown to inhibit the migration of peritoneal macrophages of salmonella-infected (immune) guinea pigs from capillary tubes, in comparison with cells of normal control animals. By use of the high-molecular-weight antigens and the ammonium sulfate-precipitated antigens, the production of the migration inhibition factor(s) was elicited from cultures of lymphocytes obtained from the peripheral blood of immune guinea pigs. The activity of the migration inhibition factor(s) was demonstrated by its ability to inhibit the migration of peritoneal macrophages of normal guinea pigs from capillary tubes. In contrast, normal peritoneal macrophages exposed to products of antigen-stimulated immune lymphocytes did not exhibit an enhanced phagocytic or bactericidal action against virulent S. typhimurium as compared with those of the normal control. The present study indicated that the bacterial antigens responsible for the elicitation of the production of the migration inhibition factor from lymphocytes of immune guinea pigs are inactivated by proteolytic enzymes, but not by ribonuclease, and have molecular weights of >50,000.
Full text
PDF


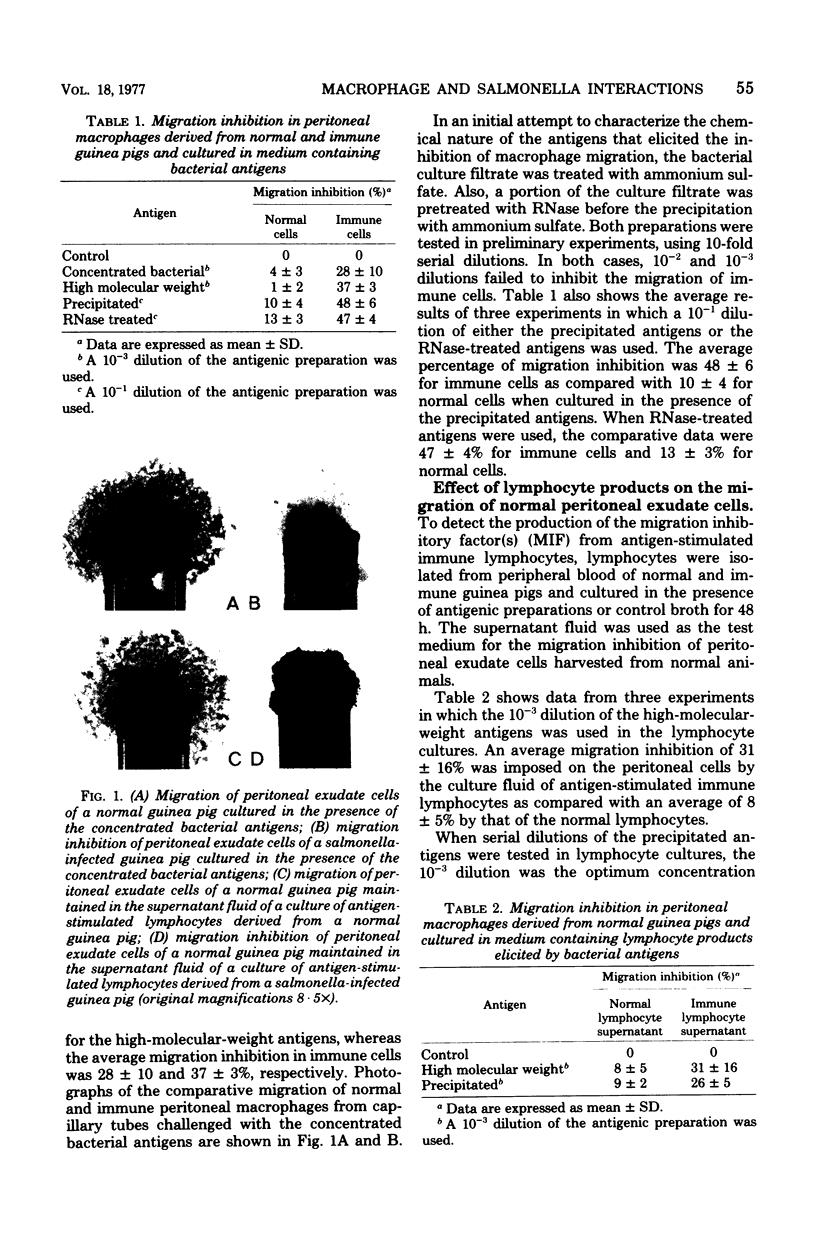
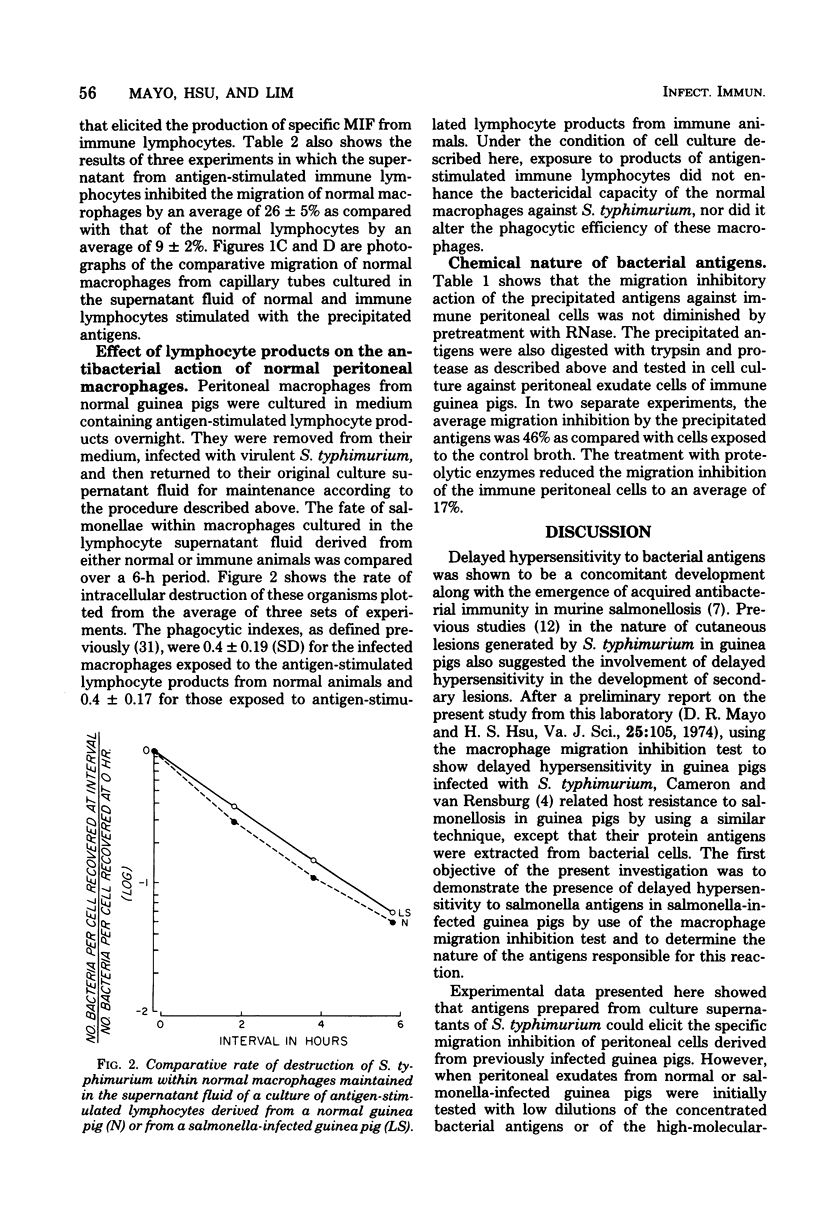



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckerdite S., Mooney C., Weiss J., Franson R., Elsbach P. Early and discrete changes in permeability of Escherichia coli and certain other gram-negative bacteria during killing by granulocytes. J Exp Med. 1974 Aug 1;140(2):396–409. doi: 10.1084/jem.140.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V. Modification of macrophage function. J Reticuloendothel Soc. 1968 Jun;5(3):179–202. [PubMed] [Google Scholar]
- Cameron C. M., Van Rensburg J. J. Inhibition of macrophage migration in Salmonella immunity. Onderstepoort J Vet Res. 1975 Mar;42(1):15–24. [PubMed] [Google Scholar]
- Campbell P. A. Immunocompetent cells in resistance to bacterial infections. Bacteriol Rev. 1976 Jun;40(2):284–313. doi: 10.1128/br.40.2.284-313.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. Delayed hypersensitivity and arthus reactivity in relation to host resistance in salmonella-infected mice. J Immunol. 1968 Nov;101(5):830–845. [PubMed] [Google Scholar]
- Collins F. M. Vaccines and cell-mediated immunity. Bacteriol Rev. 1974 Dec;38(4):371–402. doi: 10.1128/br.38.4.371-402.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowles R. E., Fajardo I. M., Leibowitch J. L., David J. R. The enhancement of macrophage bacteriostasis by products of activated lymphocytes. J Exp Med. 1973 Oct 1;138(4):952–964. doi: 10.1084/jem.138.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSU H. S. IN VITRO STUDIES ON THE INTERACTIONS BETWEEN MACROPHAGES OF RABBITS AND TUBERCLE BACILLI. II. CELLULAR AND HUMORAL ASPECTS OF ACQUIRED RESISTANCE. Am Rev Respir Dis. 1965 Apr;91:499–509. doi: 10.1164/arrd.1965.91.4.499. [DOI] [PubMed] [Google Scholar]
- Hsu H. S., Mayo D. R. Interactions between macrophages of guinea pigs and salmonellae. 3. Bactericidal action and cytophilic antibodies of macrophages of infected guinea pigs. Infect Immun. 1973 Aug;8(2):165–172. doi: 10.1128/iai.8.2.165-172.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H. S., Piper V. M. Acquired resistance to and comparative virulence of Salmonella typhimurium demonstrated by cutaneous lesions in guinea pigs. J Reticuloendothel Soc. 1972 Apr;11(4):343–357. [PubMed] [Google Scholar]
- Hsu H. S., Radcliffe A. S. Interactions between macrophages of guinea pigs and Salmonellae. I. Fate of Salmonella typhimurium within macrophages of normal guinea pigs. J Bacteriol. 1968 Jul;96(1):191–197. doi: 10.1128/jb.96.1.191-197.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H. S. The fate of Mycobacterium tuberculosis within macrophages of guinea pigs. Am Rev Respir Dis. 1971 May;103(5):607–611. doi: 10.1164/arrd.1971.103.5.607. [DOI] [PubMed] [Google Scholar]
- JENKIN C. R., ROWLEY D., AUZINS I. THE BASIS FOR IMMUNITY TO MOUSE TYPHOID. I. THE CARRIER STATE. Aust J Exp Biol Med Sci. 1964 Apr;42:215–228. doi: 10.1038/icb.1964.23. [DOI] [PubMed] [Google Scholar]
- Jones T., Youmans G. P. The in vitro inhibition of growth of intracellular Listeria monocytogenes by lymphocyte products. Cell Immunol. 1973 Dec;9(3):353–362. doi: 10.1016/0008-8749(73)90050-6. [DOI] [PubMed] [Google Scholar]
- MITSUHASHI S., SATO I., TANAKA T. Experimental salmonellosis. Intracellular growth of Salmonella enteritidis ingested in mononuclear phagocytes of mice, and cellular basis of immunity. J Bacteriol. 1961 Jun;81:863–868. doi: 10.1128/jb.81.6.863-868.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marecki N. M., Hsu H. S., Mayo D. R. Cellular and humoral aspects of host resistance in murine salmonellosis. Br J Exp Pathol. 1975 Jun;56(3):231–243. [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Karnovsky M. L., David J. R. Alterations of macrophage functions by mediators from lymphocytes. J Exp Med. 1971 Jun 1;133(6):1356–1376. doi: 10.1084/jem.133.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornellas E. P., Roantree R. J., Steward J. P. The specificity and importance of humoral antibody in the protection of mice against intraperitoneal challenge with complement-sensitive and complement-resistant Salmonella. J Infect Dis. 1970 Feb;121(2):113–123. doi: 10.1093/infdis/121.2.113. [DOI] [PubMed] [Google Scholar]
- Osebold J. W., Pearson L. D., Medin N. I. Relationship of antimicrobial cellular immunity to delayed hypersensitivity in Listeriosis. Infect Immun. 1974 Feb;9(2):354–362. doi: 10.1128/iai.9.2.354-362.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Demonstration in tissue culture of lymphocyte-mediated immunity to tuberculosis. Infect Immun. 1970 Jun;1(6):600–603. doi: 10.1128/iai.1.6.600-603.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWLEY D., TURNER K. J., JENKIN C. R. THE BASIS FOR IMMUNITY TO MOUSE TYPHOID. 3. CELL-BOUND ANTIBODY. Aust J Exp Biol Med Sci. 1964 Apr;42:237–248. doi: 10.1038/icb.1964.25. [DOI] [PubMed] [Google Scholar]
- Rhodes M. W., Hsu H. S. Effect of kanamycin on the fate of Salmonella enteritidis within cultured macrophages of guinea pigs. J Reticuloendothel Soc. 1974 Jan;15(1):1–12. [PubMed] [Google Scholar]
- SATO I., TANAKA T., SAITO K., MITSUHASHI S. Inhibition of Salmonella enteritidis ingested in mononuclear phagocytes from liver and subcutaneous tissue of mice immunized with live vaccine. J Bacteriol. 1962 Jun;83:1306–1312. doi: 10.1128/jb.83.6.1306-1312.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTER E., RAMSEIAR H. CELLULAR REACTIONS IN INFECTION. Adv Immunol. 1964;27:117–173. doi: 10.1016/s0065-2776(08)60707-5. [DOI] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Cellular immunity in vitro. I. Immunologically mediated enhancement of macrophage bactericidal capacity. J Exp Med. 1971 Jun 1;133(6):1377–1389. doi: 10.1084/jem.133.6.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. A., Bigley N. J. Detection of delayed hypersensitivity in mice injected with ribonucleic acid-protein fractions of Salmonella typhimurium. Infect Immun. 1972 Sep;6(3):384–389. doi: 10.1128/iai.6.3.384-389.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. A., Bigley N. J. Ribonucleic acid-protein fractions of virulent Salmonella typhimurium as protective immunogens. Infect Immun. 1972 Sep;6(3):377–383. doi: 10.1128/iai.6.3.377-383.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R., Berry L. J. Cell-mediated resistance induced with immunogenic preparations of Salmonella typhimurium. Infect Immun. 1971 Oct;4(4):381–387. doi: 10.1128/iai.4.4.381-387.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells P. S., Hsu H. S. Interactions Between Macrophages of Guinea Pigs and Salmonellae II. Phagocytosis of Salmonella typhimurium by Macrophages of Normal Guinea Pigs. Infect Immun. 1970 Aug;2(2):145–149. doi: 10.1128/iai.2.2.145-149.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans G. P. Relation between delayed hypersensitivity and immunity in tuberculosis. Am Rev Respir Dis. 1975 Feb;111(2):109–118. doi: 10.1164/arrd.1975.111.2.109. [DOI] [PubMed] [Google Scholar]
- Zwet T. L., Thompson J., Furth R. Effect of glucocorticosteroids on the phagocytosis and intracellular killing by peritoneal macrophages. Infect Immun. 1975 Oct;12(4):699–705. doi: 10.1128/iai.12.4.699-705.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]




