Abstract
In human infants, neonatal imitation and preferences for eyes are both associated with later social and communicative skills, yet the relationship between these abilities remains unexplored. Here we investigated whether neonatal imitation predicts facial viewing patterns in infant rhesus macaques. We first assessed infant macaques for lipsmacking (a core affiliative gesture) and tongue protrusion imitation in the first week of life. When infants were 10–28 days old, we presented them with an animated macaque avatar displaying a still face followed by lipsmacking or tongue protrusion movements. Using eye tracking technology, we found that macaque infants generally looked equally at the eyes and mouth during gesture presentation, but only lipsmacking-imitators showed significantly more looking at the eyes of the neutral still face. These results suggest that neonatal imitation performance may be an early measure of social attention biases and might potentially facilitate the identification of infants at risk for atypical social development.
Social interactions are inherently complex: many important non-verbal cues can occur in rapid succession. Failure to attend to such cues can cause an inability to extract meaning from the social situation and lead to a lack of appropriate responses (Klin, Warren, Schultz, Volkmar & Cohen, 2002). For human infants, this challenge to attend and respond appropriately to social cues begins early in life: mothers commonly engage in intimate nonverbal communication with their infants, which includes body contact, mutual gaze, and facial expressions (Stern, 1985; Trevarthen, 1974; Trevarthen, 1980; Tronick, 1989). Even newborns have the ability to respond to these cues, for example by mirroring mouth opening or tongue protrusion movements of their interaction partners, a phenomenon termed neonatal imitation in the scientific literature (Meltzoff & Moore, 1977). These early social interactions are thought to promote social bonding between mother and infant, support intersubjective interactions, and provide opportunities for infants to learn about social communication norms (Trevarthen, 1998; Nagy, 2006; but see LeVine et al., 1994 and Gaskins, 2006, for examples of cultural variations in mother-infant communication patterns).
Although there is now considerable evidence that the quality of face-to-face interactions between mothers and infants in the first weeks after birth can significantly affect cognitive and social-emotional development (Feldman & Greenbaum, 1997; Feldman, 2007), much less is known about how neonatal imitation in particular may be associated with longitudinal developmental outcomes (Suddendorf, Oostenbroek, Nielsen & Slaughter, 2013). Previous studies have shown that about 50% of infants perform poorly when formally tested for neonatal imitation (Heimann, Nelson & Schaller, 1989), and that infants who do not imitate at 2–3 days of age exhibit increased gaze aversion during play interactions at 3 months old (Heimann, 1989). Making eye contact is one of the most important signals for social interaction and communication (Csibra & Gergely, 2006), and scanning of the eye area can be a predictor of social and communicative abilities. For example, there have been reports of positive associations between eye scanning of static faces at 6 months and both joint attention skills at 12 months (Schietecatte, Roeyers & Warreyn, 2012)as well associal development at 18 months (Wagner, Luyster, Yim, Tager-Flusberg & Nelson, 2013). If individual differences in neonatal imitation predict later social-communicative abilities, they may be particularly useful for identifying infants at risk for developmental disorders such as autism spectrum disorders (ASD), which exhibit impairments in several core social skills including joint attention and verbal and nonverbal communication (Elsabbagh & Johnson, 2010).
In the present study, we measured neonatal imitation and facial viewing patterns in infant rhesus macaques (Macaca mulatta). Shortly after birth and throughout the first months of life, rhesus macaques have superior visual acuity compared to humans (Ordy, Latanick, Samorajski & Massopust, 1964), and even though adult rhesus macaques use direct staring at the eyes as a threat signal, they nonetheless are particularly inclined to explore the eye region of others (Gothard, Erickson & Amaral, 2004;Dahl, Wallraven, Bulthoff & Logothetis, 2009; Nahm, Perret, Amaral & Albright, 1997), even as infants (Mendelson, Haith & Goldman-Rakic, 1982). Furthermore, rhesus macaque mother-infant pairs often gaze at each other’s eyes, intently and intimately, during the first month of infants’ lives (Ferrari, Paukner, Ionica & Suomi, 2009a). These interactions are not followed by aggressive displays, but often lead to the mother grooming the infant, either with her hands or her mouth (Ferrari et al., 2009a). Thus, at least in macaque infants, direct gaze is not associated with aggression but more commonly with affiliation. Macaques also engage in neonatal imitation during mother-infant interactions (Ferrari et al., 2009a) and, similar to human infants, about 50% of macaques perform poorly on neonatal imitation tests (Paukner, Ferrari & Suomi, 2011). Together these factors make rhesus macaques an excellent model for investigating the basic mechanisms early in life that underlie the emergence of social and cognitive skills.
For the current study, macaque infants were first assessed for neonatal imitation of lipsmacking and tongue protrusion movements in the first week of life. Lipsmacking is a core affiliative gesture in macaques and is typically displayed by macaque mothers in the first month after giving birth. Lipsmacking is always directed at infants in combination with mutual gaze and resembles the ritualized “motherese” between human mothers and infants (Ferrari et al., 2009a). Tongue protrusions are not commonly performed by macaques and do not carry any particular communicative meaning; however, macaque infants also imitate tongue protrusion movements (Ferrari, Visalberghi, Paukner, Fogassi, Ruggiero& Suomi, 2006). Thus, in the present study we examined infants’ ability to imitate both social-communicative and non-communicative gestures in relation to their facial viewing patterns. To analyze how imitators and non-imitators process faces, we created an animated monkey avatar, which, due to its dynamic display, offers increased realism compared to photographs of facial expressions. It also provides advantages over conventional video playback such as control over additional factors (e.g., duration and speed of head and eye movements) that might otherwise influence gaze behavior. The test showed the avatar displaying a neutral still face followed by either lipsmacking or tongue protrusion movements. Using eye tracking technology, we presented each gesture to infants 3–4 times between ages 1–28 days and measured the number of fixations and duration of fixations (both measures of general interest or exploration) to the overall face, but also more specifically to the eye and mouth areas of the avatar (Figure 1; supplemental movies S1 and S2). Based on Heimann et al.’s (1989) finding that neonatal imitation performance predicts gaze aversion in human infants, we hypothesized that infant macaques who imitate mouth movements in the first week of life would show increased scanning of the eye region (rather than the mouth region) when 10–28 days old. We further aimed to examine whether the type of movement infants imitated (lipsmacking or tongue protrusion) made them particularly sensitive to the eye region when that movement (rather than a still face) was displayed.
Figure 1.
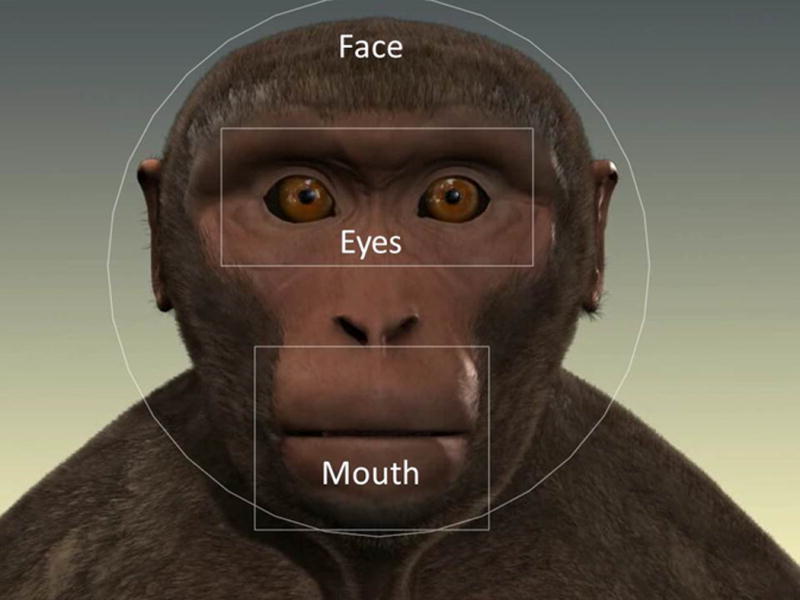
Illustration of Face, Eye and Mouth Areas Of Interest (AOIs) on monkey avatar.
Materials and Methods
The following procedures were approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Animal Care and Use Committee (ASP#08-043 and ASP#11-043). The study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals and complied with the Animal Welfare Act.
Subjects
Subjects were 37 infant rhesus macaques (Macaca mulatta), 13 females and 24 males. All infants were separated from their mothers on the day they were born (typically by 8am), and were reared in a nursery facility for ongoing, unrelated research studies. Infants were individually housed in incubators (51 cm × 38 cm × 43 cm) for the first two weeks of life and in metal cages thereafter. Both housing arrangements contained an inanimate surrogate mother covered with fleece fabric as well as loose pieces of fleece fabric and various toys (see Shannon, Champoux & Suomi, 1998, for further details on rearing practices). For the first month of life, infants could see and hear, but not physically contact, other infants of similar age. Human caretakers were present for 13h each day and interacted with infants every 2h for feeding and cleaning purposes. Two additional infants were tested but excluded from the analysis due to insufficient data.
Procedure
Imitation assessment
Neonatal imitation tests followed previously published procedures (Paukner et al., 2011), and are outlined in detail in the supplementary materials. Briefly, infants were tested three times per day on up to four separate days within the first week of life. During the baseline period, a human experimenter presented an emotionally neutral still face (or still disk), followed by one of three different stimulus periods: Lipsmacking, Tongue Protrusion, and Disk Control. Total frequencies of responses were recorded from videotaped sessions by an experimenter blind to the experimental condition, and were averaged across all testing days. Infant were classified as lipsmacking-imitators if (i) they showed higher rates of lipsmacking responses during the stimulus period than the baseline period in the Lipsmacking condition, and (ii) the difference in lipsmacking rates between baseline and stimulus was higher in the Lipsmacking condition than in the Disk Control condition. The same criteria were applied for tongue protrusion imitation, i.e. tongue protrusion-imitators displayed more tongue protrusions during the stimulus period than the baseline period of the Tongue Protrusion condition, and this increase was larger in the Tongue Protrusion condition than in the Disk Control condition.
Eye tracking data
Eye movements were recorded via corneal reflection using a Tobii T60XL eye tracker, a remote 24” monitor with integrated eye tracking technology and a sampling rate of 60 Hertz. Tobii Studio software (Tobii Technology, Sweden) was used to collect and summarize the data. Since rhesus infants’ interpupilar distance is smaller than standard human interpupilar distance, data from only one eye were available during calibration and data collection. Two different video stimuli were used, a lipsmacking video and a tongue protrusion video. Both videos were created using Maya and Zbrush software and showed an animated adult rhesus macaque (head and shoulders) looking at infants. Screen and video resolution were set to 1024 × 768 pixels, and both videos were without sound and 40 seconds in duration. For the first 20 seconds, the rhesus macaque displayed an emotionally neutral still face (eye blinks and small head movements were included to maintain an animated impression). For the second 20 seconds, the animated macaque lipsmacked at infants in the lipsmacking video, or showed repeated tongue protrusions in the tongue protrusion video (see supplementary materials for illustration of video clips).
Eye tracking data were collected when infants were between ages 10 to 28 days old. At the beginning of each session, one experimenter held each infant wrapped in soft fleece fabric at a distance of approximately 62cm from the screen. Each infant was calibrated using a 5-point calibration procedure to Tobii Studio’s pre-set locations; individual calibration points that were judged to be unreliable were repeated until an acceptable calibration was obtained. For twelve infants, the same calibration was used throughout the study; all other infants were newly calibrated for each test session. Following calibration, one randomly selected video was presented. Infants were tested once a day for a total of 3–4 times for each video (averages: 3.4 for Lipsmacking and 3.2 for Tongue Protrusion).
Data Analysis
Several Areas of Interest (AOIs) were created for analysis: Screen, Face, Eye, and Mouth AOI (see Figure 1). The screen AOI comprised the entire screen and was 1024 × 768 pixel; the Face AOI was 698 × 707 pixel. Eye and Mouth AOIs were 374 × 165 pixel and 281 × 220 pixel respectively. Tobii Studio with Tobii Fixation Filter (default settings) was used to extract data in two forms: total fixation duration and number of fixations for each AOI. We found no significant differences across test days (all p>0.1), therefore data were averaged within each individual and across test days prior to analysis. Since the still face period at the start of each trial was identical for Lipsmacking and Tongue Protrusion, data were averaged from both videos to create a single Still Face period. We therefore analyzed three periods: Still Face, Lipsmacking, and Tongue Protrusion. To further examine preferences for eye and mouth areas, we created an Eye-Mouth-Index (EMI) using Eyes / (Eyes + Mouth) in order to compare duration of fixations to both areas concurrently. A value of 0.5 indicates equal looking at both Eye and Mouth, values closer to 1 indicate increased looking at the eyes, and values closer to 0 indicate increased looking at the mouth (Merin et al., 2007).
Results
Following imitation assessments, 16 infants were classified as lipsmacking-imitators and 21 infants as lipsmacking-non-imitators, and 15 infants were classified as tongue protrusion-imitators and 22 infants as tongue protrusion-non-imitators. We first analyzed all data using lipsmacking-imitator and tongue protrusion-imitator as between-subject factors; however, tongue protrusion-imitator failed to show any significant main effects or interactions, and it was therefore removed from the analyses. Where violations of sphericity were indicated, we applied Huynh-Feldt adjustments. Significant main effects and interactions were followed-up with post-hoc tests including Bonferroni corrections; only significant effects are reported here.
Looking at the screen
Using total fixation duration at the screen AOI, a 3 × 2 repeated measures ANOVA with Period (Still Face, Lipsmacking, Tongue Protrusion) as a within-subject factor and Lipsmacking-imitator status (imitator, non-imitator) as a between-subject factor showed no main effects and no interactions (all P>0.05). There were no differences in total looking time at the three periods (means: Still Face=9.89 (SD=3.53), Lipsmacking=10.32 (SD=3.97), Tongue Protrusion=9.4 (SD=3.80), indicating that infants were equally attentive in all periods.
Looking at faces
A 3 (Period) × 2 (Lipsmacking-imitator status) repeated measures ANOVA on the total duration of fixations on each Face AOI showed a main effect of Period (F(2, 70)=5.00, P=0.014, η2p=0.125). Infants looked significantly less at faces in Tongue Protrusion (M=6.90, SD=3.61) than Still Face (M=7.98 sec, SD=2.88, P=0.007) and Lipsmacking (M=8.13, SD=3.78, P=0.024). Similar results were obtained for frequency of fixations: we found a main effect for Period (F(2, 70)=6.031, P=0.007, η2p=0.147), indicating that infants made significantly fewer fixations on the face during Tongue Protrusion (M=23.95, SD=10.76) than Still Face (M=29.43, SD=10.00, P=0.001) and Lipsmacking (M=27.86, SD=10.76, P=0.051). Thus, infants overall looked less at the face during Tongue Protrusion compared to both Still Face and Lipsmacking, suggesting that socially-relevant characteristics appear to affect general interest in a display.
Preferences for eyes and mouth
For durations of fixations, a 3 (Period) × 2 (Lipsmacking-imitator status) repeated measures ANOVA of EMI showed a main effect for Period (F(2, 70)=6.422, η2p=0.155, P=0.005) as well as an interaction between Period and Lipsmacking-imitator status (F(2, 70)=4.145, η2p =0.106, P=0.027). For lipsmacking-imitators, the Still Face EMI was higher than the Lipsmacking EMI (t(15)=4.33, P=0.001) and Tongue Protrusion EMI (t(15)=5.06, P=0.001), indicating that lipsmacking-imitators looked more at the eyes during Still Face than during Lipsmacking and Tongue Protrusion. Moreover, there was a trend for lipsmacking-imitators to have a higher EMI during Still Face than lipsmacking-non-imitators (t(35)=−1.99, P=0.055; Figure 2A). For frequency of fixations, the same analyses on EMIs indicated a main effect for Period (F(2, 70)=5.36, η2p=0.133, P=0.007) as well as an interaction between Lipsmacking-imitator status and Period (F(2, 70)=3.356, η2p =0.087, P=0.041). Follow-up comparisons showed that the EMI was significantly larger for lipsmacking-imitators compared to lipsmacking-non-imitators in Still Face (t(35)=−2.01, P=0.044; Figure 2B), meaning that lipsmacking-imitators fixated more frequently on the eyes (relative to the mouth) than lipsmacking-non-imitators during Still Face.
Figure 2.
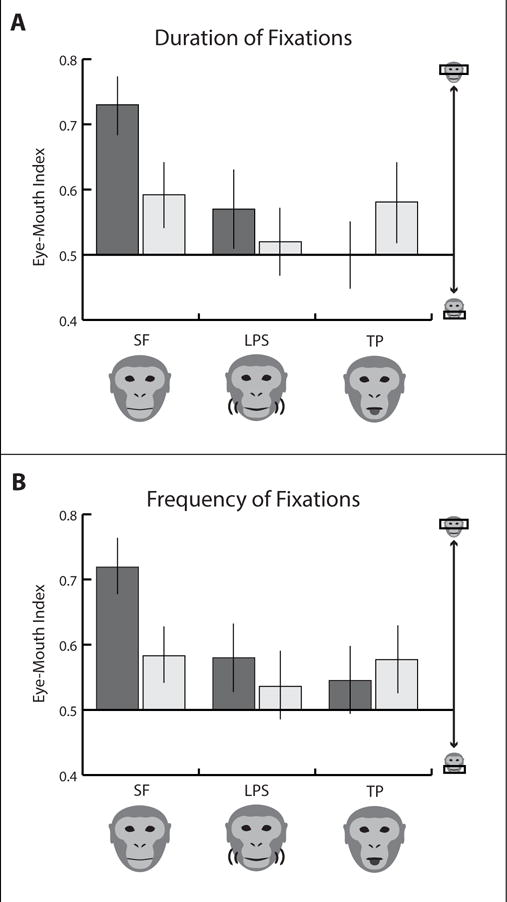
Average duration of fixations and frequency of fixations for lipsmacking-imitators (grey, N=16) and lipsmacking-non-imitators (white, N=21). Phase illustrated on x-axis as SF = still face (combined average from Lipsmacking and Tongue Protrusion videos), LPS = lipsmacking gesture, TP = tongue protrusion gesture. Error bars denote standard error. 2A: Eye-Mouth Index (Eye / Eye + Mouth) for average fixation durations. 2B: Eye-Mouth Index (Eye / Eye + Mouth) for average frequency of fixations.
Discussion
In the present study, we found an association between eye gaze and performance on a social-communicative task: infant macaques who imitated lipsmacking gestures in the first week of life looked longer at the eyes than the mouth of a still face at 10–28 days old. Thus, an individual’s social and communicative abilities, even very early in life, appear related to a visual preference for the eye area in a neutral Still Face.
The Still Face period in the current study should not be confused with the classic Still Face Paradigm typically employed with human infants (SFP; Tronick, Als, Adamson, Wise & Brazelton, 1978). In SFP, the mother first interacts normally with the infant, then becomes unresponsive in a still face phase, and finally resumes normal interactions with the infant. Infants generally show less smiling and increased negative affect during the still face phase (Mesman, van Ijzendoorn & Bakermans-Kranenburg, 2009), which suggests that infants are sensitive to the disruption in social interaction. As a result, infants also show increased looking at the eyes during still face, which may be motivated by information seeking in response to the interruption in social interaction (Merin, Young, Ozonoff & Rogers, 2007). In the present study, macaque infants were presented first with an emotionally neutral avatar followed by either lipsmacking or tongue protrusion, so there was no immediate contrast between an interaction partner who is engaging at first but then unresponsive. Nonetheless, macaque infants showed increased eye scanning during the Still Face period. Thus, eye gaze at a still face could more generally be an indicator of an infant’s motivation to extract social information from others.
Only lipsmacking imitation predicted a preference for the eyes during the still face; tongue protrusion imitation was not associated with differential gaze patterns at faces. Lipsmacking is an emotional communicative gesture, which is commonly used between macaque mothers and infants. Rhesus macaque mothers often place themselves directly in front of the infant, lower their faces to infants’ eye-level, and engage in bouts of head bobbing and exaggerated lipsmacking to which infants respond with matching lipsmacking gestures (Ferrari et al., 2009a). Tongue protrusion on the other hand is not commonly performed by rhesus macaques and does not carry any communicative meaning. Both imitation of lipsmacking gestures and gaze at the eye area may therefore be tied tomacaque infants’general sensitivity to social signals rather than simply being a measure of a specific imitation mechanism. While other developmental factors (such as birth weight, motor maturity of posture, and grasping reflexes; Ferrari et al., 2009b) have failed to show associations with neonatal imitation, a recent study reports that lipsmacking imitation (but not tongue protrusion imitation) in infant macaques is associated with increased looking durations at both social and non-social stimuli during a neonatal imitation task (Simpson, Paukner, Suomi, & Ferrari, 2013). It thus remains an open question whether high general ability (such as e.g., speed of processing or spatial awareness), specific social sensitivity, or other factors could explain interindividual variability on neonatal imitation tasks. Measuring macaque infants’ gaze preferences to different parts of the face in the first week of life during an imitation task could prove particularly useful to clarify this issue.
In human infants, developmental changes in preferences for eye and mouth areas are affected by whether native or non-native speech is presented (Weikum et al., 2007; Lewkowicz & Hansen-Tift, 2012). This effect has been attributed to the idea that infants become most sensitive to the most commonly encountered stimuli, which for speech perception is observed by 12 months of age (Lewkowicz & Hansen-Tift, 2012). For rhesus macaques, the form and frequency of lipsmacking gestures changes throughout development: infants exhibit slower and more variable mouth movements when producing lipsmacking gestures, but these movements become faster and less variable during the juvenile period (Morrill, Paukner, Ferrari & Ghazanfar, 2012). It is possible that macaque infants become more sensitive to lipsmacking gestures as they grow older. Adult macaques tend to gaze more at the eye area when viewing dynamic facial displays of conspecifics (Nahm et al., 1997), and infants’ viewing patterns of facial gestures may develop in parallel with their ability to produce these gestures themselves. In the present study, lipsmacking-non-imitators as well as imitators looked at eyes and mouth approximately equally during both lipsmacking and tongue protrusion presentations. Failure to differentiate between the communicative gesture and the non-communicative gesture in this respect suggests that the movements in the lower part of the face might have attracted macaque infants’ gaze, and that their scanning patterns did not change based on the communicative meaning of the gesture. Tracing the development of lipsmacking gesture production in relation to scanning of the eye region is therefore a fruitful future research direction, and rhesus macaque infants are an ideal model for separating effects due to general improvements with age and effects of specific visual experiences.
Overall, our study revealed significant associations between neonatal imitation abilities and facial viewing preferences in macaques, with significantly more attention directed at eye areas by neonatal lipsmacking-imitators. Considering related findings with human infants showing that those who fail to imitate facial gestures at 2–3 days of age show increased gaze aversion during a play interaction at 3 months (Heimann, 1989), it appears that the basic mechanisms that underlie the development of typical social and cognitive skills may be shared between humans and macaques, although further studies with both humans and macaques are required to validate this conclusion. For humans, imitation, either executed (Heimann, 1989) or recognized (Young, Merin, Rogers & Ozonoff, 2009), may lead to increases in an individual’s sensitivity to social cues, which in turn could have important implications for infants’ cognitive, social, and emotional development (Feldman, 2007), and especially for populations with diminished social or communication skills. Neonatal imitation assessments may thus have the potential to deliver a convenient early screening tool to identify infants at risk of developing later social and communicative difficulties, and could facilitate the implementations of interventions and treatment plans at a much earlier time point than is currently possible. Future studies of longitudinal developmental outcomes related to neonatal imitation abilities are critical to further our understanding of neonatal imitation and its relation to core social-communicative abilities.
Supplementary Material
Research Highlights.
One-week-old infant macaque monkeys were tested on a neonatal imitation task and were shown avatars of still face, lipsmacking, and tongue protrusion when 10–28 days old.
Macaques who imitated lipsmacking gestures at 1 week old looked significantly more at the eye than the mouth area of the still face when 10–28 days old.
Neonatal imitation and facial viewing patterns may be indicators of social attention biases early in life.
Acknowledgments
This work was supported by the Division of Intramural Research, NICHD, and NICHD P01HD064653. We thank Seth Bower, Sheila Sutti, Jeremy Swan, and Erin Fincher. We also gratefully acknowledge the support of the Autodesk Research Donation Program.
References
- Csibra G, Gergely G. Social learning and social cognition: the case for pedagogy. In: Munakata Y, Johnson MH, editors. Processes of change in brain and cognitive development. Oxford, UK: Oxford University Press; 2006. pp. 249–274. [Google Scholar]
- Dahl CD, Wallraven C, Bulthoff HH, Logothetis NK. Humans and macaques employ similar face-processing strategies. Current Biology. 2009;19:509–513. doi: 10.1016/j.cub.2009.01.061. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Johnson MH. Getting answers from babies about autism. Trends in Cognitive Sciences. 2010;14:81–87. doi: 10.1016/j.tics.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Feldman R. Parent-infant synchrony and the construction of shared timing: physiological precursors, developmental outcomes, and risk conditions. Journal of Child Psychology and Psychiatry. 2007;48:329–354. doi: 10.1111/j.1469-7610.2006.01701.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Greenbaum CW. Affect regulation and synchrony in mother-infant play as precursors to the development of symbolic competence. Infant Mental Health Journal. 1997;18:4–23. [Google Scholar]
- Ferrari PF, Paukner A, Ionica C, Suomi SJ. Reciprocal face-to-face communication between rhesus macaque mothers and their newborn infants. Current Biology. 2009a;19:1768–1772. doi: 10.1016/j.cub.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari PF, Paukner A, Ruggiero A, Darcey L, Unbehagen S, Suomi SJ. Interindividual differences in neonatal imitation and the development of action chains in rhesus macaques. Child Development. 2009b;80:1057–1068. doi: 10.1111/j.1467-8624.2009.01316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari PF, Visalberghi E, Paukner A, Fogassi L, Ruggiero A, Suomi S. Neonatal imitation in rhesus macaques. PLoS Biology. 2006;4:e302. doi: 10.1371/journal.pbio.0040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins S. Cultural perspectives on infant-caregiver interaction. In: Enfield NJ, Levinson S, editors. The roots of human sociality: Culture, cognition, and human interaction. Oxford, UK: Berg; 2006. pp. 279–298. [Google Scholar]
- Gothard KM, Erickson CA, Amaral DG. How do rhesus monkeys (Macaca mulatta) scan faces in a visual paired comparison task? Animal Cognition. 2004;7:25–36. doi: 10.1007/s10071-003-0179-6. [DOI] [PubMed] [Google Scholar]
- Heimann M. Neonatal imitation, gaze aversion, and mother-infant interaction. Infant Behavioral Development. 1989;12:495–505. [Google Scholar]
- Heimann M, Nelson KE, Schaller J. Neonatal imitation of tongue protrusion and mouth opening: methodological aspects and evidence of early individual differences. Scandinavian Journal of Psychology. 1989;30:90–101. doi: 10.1111/j.1467-9450.1989.tb01072.x. [DOI] [PubMed] [Google Scholar]
- Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Archives of General Psychiatry. 2008;65:946–954. doi: 10.1001/archpsyc.65.8.946. [DOI] [PubMed] [Google Scholar]
- Klin A, Warren J, Schultz R, Volkmar F, Cohen D. Defining and quantifying the social phenotype in autism. American Journal of Psychiatry. 2002;159:895–908. doi: 10.1176/appi.ajp.159.6.895. [DOI] [PubMed] [Google Scholar]
- Lewkowicz D, Hansen-Tift AM. Infants deploy selective attention to the mouth of a talking face when learning speech. Proceedings National Academy of Sciences USA. 2012;109:1431–1436. doi: 10.1073/pnas.1114783109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine RA, Dixon S, LeVine S, Richman A, Leiderman PH, Keefer CH, et al. Child care and culture: Lessons from Africa. New York: Cambridge University Press; 1994. [Google Scholar]
- Mendelson MJ, Haith MM, Goldman-Rakic PS. Face scanning and responsiveness to social cues in infant rhesus monkeys. Developmental Psychology. 1982;18:222–228. [Google Scholar]
- Meltzoff AN, Moore MK. Imitation of facial and manual gestures by human neonates. Science. 1977;178:75–78. doi: 10.1126/science.198.4312.75. [DOI] [PubMed] [Google Scholar]
- Merin N, Young GS, Ozonoff S, Rogers SJ. Visual fixation patterns during reciprocal social interaction distinguish a subgroup of 6-month-old infants at-risk for autism from comparison infants. Journal of Autism and Developmental Disorders. 2007;37:108–121. doi: 10.1007/s10803-006-0342-4. [DOI] [PubMed] [Google Scholar]
- Mesman J, van Ijzendoorn MH, Bakermans-Kranenburg MJ. The many faces of the Still-Face Paradigm: A review and meta-analysis. Developmental Review. 2009;29:120–162. [Google Scholar]
- Morrill RJ, Paukner A, Ferrari PF, Ghazanfar AA. Monkey lipsmacking develops like the human speech rhythm. Developmental Science. 2012;15:557–586. doi: 10.1111/j.1467-7687.2012.01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy E. From imitation to conversation: The first dialogues with human neonates. Infant and Child Development. 2006;15:223–232. [Google Scholar]
- Nahm FK, Perret A, Amaral DG, Albright TD. How do monkeys look at faces? Journal of Cognitive Neuroscience. 1997;9:611–623. doi: 10.1162/jocn.1997.9.5.611. [DOI] [PubMed] [Google Scholar]
- Ordy JM, Latanick A, Samorajski T, Massopust LC. Visual acuity in newborn primate infants. Experimental Biology and Medicine. 1964;115:677–680. doi: 10.3181/00379727-115-29004. [DOI] [PubMed] [Google Scholar]
- Paukner A, Ferrari PF, Suomi SJ. Delayed imitation of lipsmacking by infant rhesus macaques (Macaca mulatta) PLoS One. 2011;6:e28848. doi: 10.1371/journal.pone.0028848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schietecatte I, Roeyers H, Warreyn P. Can infant orientation to social stimuli predict later joint attention skills? British Journal of Developmental Psychology. 2012;30:267–282. doi: 10.1111/j.2044-835X.2011.02039.x. [DOI] [PubMed] [Google Scholar]
- Shannon C, Champoux M, Suomi SJ. Rearing condition and plasma cortisol in rhesus monkey infants. American Journal of Primatology. 1998;46:311–321. doi: 10.1002/(SICI)1098-2345(1998)46:4<311::AID-AJP3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Simpson EA, Paukner A, Suomi SJ, Ferrari PF. Visual attention during neonatal imitation in macaque monkeys. Developmental Psychobiology. 2013 doi: 10.1002/dev.21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern DN. The interpersonal world of the infant: a view from psychoanalysis and developmental psychology. New York: Basic Books; 1985. [Google Scholar]
- Suddendorf T, Oostenbroek J, Nielsen M, Slaughter V. Is newborn imitation developmentally homologous to later social cognitive skills? Developmental Psychobiology. 2013;55:52–58. doi: 10.1002/dev.21005. [DOI] [PubMed] [Google Scholar]
- Trevarthen C. Conversation with a two-month-old. New Scientist. 1974;2:230–235. [Google Scholar]
- Trevarthen C. The foundations of intersubjectivity: Development of interpersonal and cooperative understanding in infants. In: Olson D, editor. The Social Foundation of Language and Thought. New York: Norton; 1980. pp. 316–342. [Google Scholar]
- Trevarthen C. The concept and foundations of infant intersubjectivity. In: Braten S, editor. Intersubjective communication and emotion in early ontogeny. Cambridge: Cambridge University Press; 1998. pp. 15–46. [Google Scholar]
- Tronick EZ. Emotions and emotional communication in infants. American Psychologist. 1989;44:112–119. doi: 10.1037//0003-066x.44.2.112. [DOI] [PubMed] [Google Scholar]
- Tronick EZ, Als H, Adamson L, Wise S, Brazelton TB. The infant’s response to entrapment between contradictory messages in face-to-face interaction. Journal of the American Academy of Child Psychiatry. 1978;17:1–13. doi: 10.1016/s0002-7138(09)62273-1. [DOI] [PubMed] [Google Scholar]
- Wagner JB, Luyster RJ, Yim JY, Tager-Flusberg H, Nelson CA. The role of early visual attention in social development. International Journal of Behavioral development. 2013;37:118–124. doi: 10.1177/0165025412468064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weikum WM, Vouloumanos A, Navarra J, Soto-Faraco S, Sebastiam-Galles N, Werker JF. Visual language discrimination in infancy. Science. 2007;316:1159. doi: 10.1126/science.1137686. [DOI] [PubMed] [Google Scholar]
- Young GS, Merin N, Rogers SJ, Ozonoff S. Gaze behaviour and affect at 6 months old: predicting clinical outcomes and language development in typically developing infants and infants at risk for autism. Developmental Science. 2009;12:798–814. doi: 10.1111/j.1467-7687.2009.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


