Summary
The pigmentation of mammalian skin and hair develops through the interaction of two basic cell types — pigment donors and recipients. The pigment donors are melanocytes, which produce and distribute melanin through specialized structures. The pigment recipients are epithelial cells, which acquire melanin and put it to use, collectively yielding the pigmentation visible to the eye. This review will focus on the pigment recipients, the historically less understood cell type. These end-users of pigment are now known to exert a specialized control over the patterning of pigmentation, as they identify themselves as melanocyte targets, recruit pigment donors, and stimulate the transfer of melanin. As such, this review will discuss the evidence that the skin is like a coloring book: the pigment recipients create a “picture,” a blueprint for pigmentation, which is colorless initially but outlines where pigment should be placed. Melanocytes then melanize the recipients and “color in” the picture.
Keywords: pigment-recipient phenotype, Foxn1, melanosome transfer, pigmentary unit, tanning, hair, epidermis
Introduction
In mammals, pigmentation — the coloration used for light absorption or reflection — is principally a trait of epithelial cells. Epithelial cells contain most of an individual’s melanin, the pigment responsible for most pigmentation. Most pigmented cells — cells adapted for light absorption or reflection — are epithelial cells. And pigmented epithelial cells collectively provide most of the benefits of external pigmentation, such as thermoregulation, camouflage, protection from ultraviolet radiation (UVR), and the facilitation of reproductive and other social behaviors. But while some pigmented epithelial cells — in particular, those of the eye (reviewed in Hu et al., 2008) — produce their own melanin, most do not. Instead, they acquire their pigment from melanocytes, a cell type specializing in melanin synthesis. This division of activities — one cell producing pigment, another cell using it — is specific to the skin and represents an unusual mechanism of differentiation, as the pigmented epithelial cells differentiate (become pigmented) by importing a trait from others, not by manufacturing the trait themselves. The imported trait is intricate — whole organelles, termed melanosomes, or their complex contents — and produced by an extensive network of factors. As such, the epithelial cells differentiate without the expression of the requisite, complex differentiation program. This article will discuss the control of this unusual mechanism of differentiation and focus on a key control point, namely, the decision to turn an epithelial cell into a pigmented cell. Ultimately, this decision is in large part responsible for establishing the pattern of pigmentation.
Specificity and targeting in the development of pigmentation
In skin, pigmentation is precisely targeted. To generate epithelial pigmentation, each melanocyte extends multiple dendrites and contacts a defined group of epithelial cells, creating a pigmentary unit (reviewed in Wu and Hammer, 2014). Melanin-bearing melanosomes are subsequently transported from the melanocyte’s cell body along the lengths of its dendritic extensions to the dendrite tips. These melanosomes or their melanized contents are then transferred from the dendrites into the epithelial cells. The mechanism of transfer is the subject of debate, as epithelial cells have been proposed to acquire pigment via: 1) mechanisms based on intercellular bridges, in which melanosomes travel through pores or channels that directly connect the melanocyte’s cytoplasm to the epithelial cell’s cytoplasm, or 2) mechanisms based on endocytosis, in which epithelial cells engulf pigment-containing material that, depending on the study, consists of dendrite tips, melanocyte filopodia, melanosome-laden vesicles, secreted melanosomes, or the secreted contents of melanosomes (Wu and Hammer, 2014). Though controversial in means, pigment transfer is nonetheless clear-cut in its end, namely, the delivery of melanin to specific epithelial targets.
Pigmentation is also targeted on a broader scale, as melanocytes deliver pigment to specific types of cutaneous epithelial cells. For example, in the skin of humans and mice, most epithelial cell types —and accordingly most epithelial cells — do not form pigmentary units with melanocytes. Rather, the melanocytes form pigmentary units with a subset of cell types, which in mice are located mainly in hair follicles and in humans are located in the epidermis and a fraction of hair follicles, primarily the terminal hair follicles, which produce longer, thicker hair.
In pigmented hair follicles, melanocytes localize near the center of the hair bulb (Figure 1), where they intermingle with epithelial cells and attach to the basement membrane overlying the dermal papilla (a mesenchymal cell aggregate). At this central location, the melanocytes sit in close proximity to seven types of epithelial cells — three types of differentiating hair shaft cells (the precursors of the hair’s medulla, cortex, and cuticle), three types of differentiating inner root sheath cells (the precursors of the sheath’s cuticle, Huxley’s layer, and Henle’s layer), and the proliferating, undifferentiated pool of progenitor cells (matrix cells) from which the differentiating cells originate (reviewed in Chase, 1954). Yet, the melanocytes transfer pigment to just two of these cell types — the differentiating precursors of the hair cortex and medulla — that together generate the bulk of the hair. The other five types of epithelial cells remain unmelanized. Likewise, no pigment is placed in the hair bulb’s other components — the outer root sheath (the epithelium encasing the bulb) or dermal papilla. As such, melanocytes do not waste their pigment on follicular structures that stay hidden below the skin’s surface (e.g., the inner and outer root sheaths) and that would provide no benefit if pigmented. Rather, the melanocytes target their pigment to follicular cells with an obvious use for it — the hair’s cortical and medullary cells — which, when pigmented, confer clear advantages on their host organism.
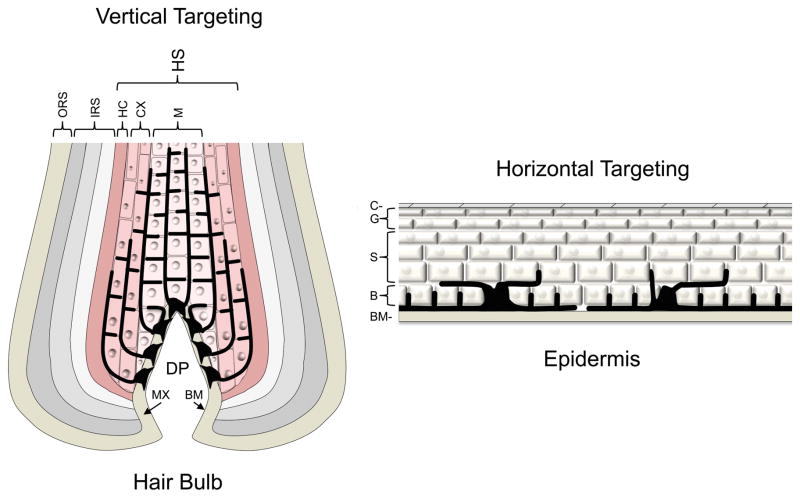
Figure 1.
Vertical versus horizontal targeting of pigment. The mammalian hair bulb (left diagram) and human epidermis (right diagram) are shown schematically. The hair bulb is organized into concentric columns of epithelial cells, while the epidermis is organized into layers of epithelial cells. For simplicity, the hair-bulb diagram indicates epithelial-cell borders in only the two innermost columns and does not label individually the three columns of the inner root sheath (IRS), which from outside to inside are Henle’s layer, Huxley’s layer, and the IRS cuticle. In the hair bulb and epidermis, melanocytes (shown in black) intermingle with epithelial cells and attach to the basement membrane (BM). In the hair bulb, melanocytes extend dendrites vertically upwards and provide pigment to the hair medulla (M) and cortex (CX). In the epidermis, melanocytes grow dendrites horizontally and provide pigment to the deepest two layers of epithelial cells, the basal (B) and first suprabasal layers. HS, hair shaft; HC, hair cuticle; ORS, outer root sheath; MX, matrix cells; DP, dermal papilla; S, spinous layer; G, granular layer; C, cornified layer.
To target pigment within the hair bulb, melanocytes develop and differentiate with directionality. After localizing near the bulb’s midpoint, the melanocytes extend their dendrites “upwards” (towards the skin’s surface), growing the dendrites to and along the rising columns of cortical and medullary cells (Figure 1). As such, the melanocytes collectively form a cone that points to the center of the hair shaft. They do not extend dendrites “horizontally” (laterally) into the precursors of the inner root sheath or “downwards” into the matrix cells or dermal papilla. The melanocytes thus avoid sending dendrites to cells where pigment would serve no purpose and instead project dendrites towards cells that “need” pigment, the cortical and medullary cells.
Similar targeting of pigment occurs in human epidermis, a stratified epithelium in which epithelial cells rise through the layers as they progress through four basic stages of differentiation — basal, spinous, granular, and cornified (Figure 1). In the epidermis, much as in the hair follicle, melanocytes form extensive contacts with two populations of epithelial cells and generate these contacts by developing and differentiating with directionality. But whereas melanocytes of the hair follicle send dendrites vertically upwards and provide pigment to two columns of epithelial cells, melanocytes of the epidermis attach to the basement membrane and extend their dendrites horizontally, connecting the dendrites primarily to two layers of epithelial cells — the basal and first suprabasal layers (Figure 1) (Fitzpatrick et al., 1967; Gilchrest et al., 1996; Han et al., 2002; Holbrook, 1991). These horizontal connections result in the transfer of pigment to basal keratinocytes and most likely to basal-like or early spinous keratinocytes, which comprise the first suprabasal layer. Melanocytes appear to make few if any contacts with granular cells or late-stage spinous cells, though such vertical connections should be physically possible, as melanocytes extend long vertical distances in the hair follicle. As such, the melanocytes preferentially form pigmentary units with the least differentiated epidermal cell types. This preference is clearly advantageous: the basal layer and (to a lesser extent) first suprabasal layer contain keratinocytes that are able to proliferate (Weinstein et al., 1984 and references therein) and hence are responsible for renewing or regenerating the epidermis. The remaining living layers consist of keratinocytes that are non-dividing, terminally differentiating, nearing death, and ultimately shed. Thus, the melanocytes target their melanin to progenitor keratinocytes and thereby protect this essential population from damage by UV radiation, damage that sooner or later would yield injured or diseased skin. In fact, after acquiring melanin, the epidermal keratinocyte concentrates the pigment over the apical side of its nucleus, forming a cap or “parasol” clearly built to shield the nucleus from sunlight (Wu and Hammer, 2014). In sum therefore, in the epidermis as in the hair follicle, melanocytes grow their dendrites to specific types of targets and thereby deposit pigment in a beneficial pattern.
As melanocytes provide melanin to some cells and not others, the skin must possess mechanisms responsible for this specificity and targeting. How then do melanocytes “know” where to place pigment? Historically, this question has been little investigated. While the transfer of pigment per se has been a recurring subject of study, the concomitant targeting of pigment — the direction of melanin to the cells where it has the greatest benefit — has received limited attention or recognition. Despite this lack of explicit study, the existing literature provides insight into the subject of targeting and its various basic questions. In particular, why is pigment transferred to one epithelial cell and not its neighbor? What determines the targets for pigmentation? What controls the formation of pigmentary units?
The instructive role of pigment recipients
Early evidence
An early clue to the mechanisms of pigment targeting came from the effects in vitro of different types of epithelial cells on the behavior of melanocytes. When human melanocytes were placed into culture with human epidermal keratinocytes, the melanocytes localized to the basal layer, just as they do in human epidermis (De Luca et al., 1988). In contrast, when the same melanocyte strains were cultivated with human oral epithelial cells, the melanocytes appeared intermittently in all layers and seemed randomly distributed (De Luca et al., 1988), as if the epithelial cells had carried the melanocytes to arbitrary positions while piling up. The effects of each epithelial cell type on pigment transfer were not assessed in the study. Nonetheless, their effects on pigment cell positioning clearly differed. Epidermal keratinocytes provided directional (vectorial) signals and targeted the melanocytes to specific positions in the epithelium, thereby generating an obvious pattern. In contrast, the oral epithelium, which normally lacks melanocytes, did not appear to provide vectorial signals. As a result, the melanocytes seemed unpatterned, at least along the vertical axis, as if they did not “know” what to do.
While absent from oral epithelium, melanocytes are normally found in locations outside the cutaneous epithelium, most prominently in the eye, where they are major components of the iris, ciliary body, and choroid (reviewed in Hu et al., 2008). Melanocytes are also present in the dermis of the skin sporadically (Fitch et al., 2003; Markert and Silvers, 1956) and at several sites protected from light, where pigmentation per se cannot be their major function, such as the valves and septa of the heart (Brito and Kos, 2008 and references therein), the stria vascularis of the inner ear (Hilding and Ginzberg, 1977), and the meninges of the brain (Markert and Silvers, 1956). Presumably, all of these sites provide melanocytes with pro-proliferation and/or pro-survival signals, as melanocytes appear to be maintained at these sites for long periods of time or throughout life. Yet at most if not all of these sites, melanocytes transfer little if any pigment to other cells and instead melanize only themselves. In these environments, which include the dermis of the skin, melanocytes clearly do not behave as they do in the epithelium of the skin, where they methodically (and often vigorously) melanize particular targets. Thus, cells that provide support to melanocytes do not necessarily obtain pigment from melanocytes. The skin’s epithelial environment is the only melanocyte-supporting environment in which pigment is transferred routinely. In animals and culture therefore, cutaneous epithelial cells appear to do something unique, on top of their support for melanocytes, so as to target or pattern pigmentation.
Foxn1 and the pigment-recipient phenotype
What then is this distinctive targeting mechanism in the cutaneous epithelium? As revealed by mutations in Foxn1, the answer lies in part with the pigment recipients, the epithelial cells that receive pigment from melanocytes. Foxn1 is a transcription factor that is found in a subset of epithelial cells and is well conserved in mammals, as the human and murine Foxn1 proteins are 85% identical (reviewed in Schlake, 2001). In murine skin, Foxn1 is present mainly in the epithelial cells of the hair cortex, one of the two main targets of murine melanocytes (Schlake, 2001; Weiner et al., 2007). When Foxn1 is ablated by mutation in mice, melanocytes localize to their normal position in the hair bulb and transfer melanin to the medullary cells of the hair shaft, which are normally Foxn1-negative. But the cortical cells fail to acquire pigment, as the melanocytes seemingly ignore them, resulting in the development of an unmelanized cortex next to a well-melanized medulla (Weiner et al., 2007). Thus, when cortical cells lose Foxn1, they do not become pigment recipients. As a consequence, the skin loses melanin from an array of repetitive structures (the cortexes of hair shafts), and the pattern of pigmentation becomes simpler.
Conversely, the production of Foxn1 in new locations induces new sites of pigmentation. When a transgene misexpresses Foxn1 in epithelial cells of the murine epidermis and hair canals — sites that are normally unpigmented in mice — melanocytes localize to the new Foxn1-positive cells and transfer melanin to them (Weiner et al., 2007). Hence, the expansion of Foxn1’s expression domain converts the epidermis and adjacent canals to pigmented tissues. As these new sites are melanized, melanocytes continue to localize to the hair bulbs and to transfer normal quantities of melanin to the hair cortexes and medullas (Weiner et al., 2007). Thus, a gain in Foxn1-positive cells leads to a gain in pigment recipients and a gain in pigmented structures, as new skin components acquire pigment alongside the normal ones. The pattern of pigmentation accordingly becomes more complex, like the complexity of the Foxn1-positive epithelial cell population. The pattern of pigmentation also becomes more human-like, as the transgenic skin, like human skin, acquires a melanized epidermis and hair coat.
As the Foxn1 transgenics mature, the expression pattern of the transgene changes, as the number of transgene-expressing cells dwindles, and Foxn1-positive epithelial cells become scattered in the largely Foxn1-negative epidermis and hair canals. In parallel, the pattern of melanocytes also changes, as melanocytes disappear from Foxn1-negative epithelial regions but localize to and melanize the remaining Foxn1-positive keratinocytes (Weiner et al., 2007). In other words, melanocytes follow the Foxn1-positive cells — increasing, decreasing, or migrating with this epithelial subset. Thus, the melanocyte population mirrors the Foxn1-positive population. As transgene expression shrinks to islands of keratinocytes, the pattern of pigmentation, like that of Foxn1, becomes spotty.
In humans, FOXN1 is likewise associated with pigment recipients, as this transcription factor is present in the differentiating hair cortex, first suprabasal layer of the epidermis, and, sporadically, the basal layer of the epidermis (Weiner et al., 2007). Consistent with this association, FOXN1 is produced at high levels in seborrheic keratoses (Mandinova et al., 2009), benign skin tumors that are formed by epidermal keratinocytes and are often hyperpigmented by melanocytes. Thus, in humans as in mice, a gain of FOXN1 is linked to a gain in pigmentation.
Taken together, the results suggest that Foxn1 confers distinct properties on epithelial cells, collectively designated a “pigment-recipient phenotype” (Weiner et al., 2007). A cell with this phenotype attracts melanocytes, stimulates pigment transfer, and thereby engineers its own pigmentation. As such, the recipients constitute a specialized, epithelial counterpart to the melanocytes (the pigment donors). As recipient cells determine where pigment is placed, the pattern of recipients ultimately determines the pattern of pigmentation. In essence, recipients render the skin analogous to the coloring books of children. As recipient cells develop, they collectively form a “picture” — a blueprint for pigmentation —that lacks pigment initially but defines where pigment belongs. Melanocytes then transfer pigment to the recipients and add color to the picture. The recipients thus provide a template that melanocytes follow as they pigment the skin.
Fgf2 and pigment-recipient signaling in normal and diseased skin
To induce their own pigmentation, pigment recipients presumably emit signals that identify themselves as melanocyte targets and stimulate melanocytes to supply them with pigment. One such signal is most likely Fgf2 (Weiner et al., 2007). Fgf2 is a secreted factor that typically acts over short distances, given its affinity for heparan sulfate proteoglycans of the extracellular matrix and cell surface. In culture, keratinocytes secrete Fgf2 (Halaban et al., 1988b), whereas melanocytes possess Fgfr1 (Becker et al., 1992), an Fgf2 receptor and transmembrane tyrosine kinase. The secretion of Fgf2 by keratinocytes strongly stimulates the proliferation and survival of melanocytes in vitro (Halaban et al., 1987; Halaban et al., 1988b), suggesting that Fgf2 promotes the colonization of cutaneous epithelium by melanocytes. Depending on the experimental context, Fgf2 also enhances melanocyte chemotaxis (Horikawa et al., 1995), differentiation (Stocker et al., 1991), or melanogenic activity (Puri et al., 1996). Consistent with these pro-pigmentation activities, Fgf2 is upregulated by Foxn1, and when Fgf2 is neutralized, Foxn1-positive epithelial cells lose much of their ability to attract, expand, or sustain a neighboring set of melanocytes (Weiner et al., 2007). Most likely therefore, pigment recipients emit Fgf2 to recruit pigment donors and as such use a recruiting mechanism based at least in part on positive signaling.
The signals of pigment recipients appear to affect melanocytes at multiple levels and consequently should affect the health of the skin in multiple ways. When the number of Foxn1-positive epithelial cells rises or falls in the skin (via modulation of a Foxn1 transgene), the number of melanocytes rises or falls in tandem (Weiner et al., 2007). This finding suggests a simple rule: that the size of the target for pigmentation determines the size of the mature melanocyte population. Hence, as one of their functions, pigment recipients most likely establish the number of melanocytes needed by the skin and instruct melanocytes to attain this number, thus keeping the melanocyte population within a beneficial range. Notably, when melanocytes leave this beneficial range and become tumorigenic, they often appear to upregulate FGF2 (Becker et al., 1989; Halaban et al., 1988a), thus converting a likely recipient signal into an autocrine one and thereby stimulating their own expansion. Additionally, as melanocytes appear to follow Foxn1-positive cells and hence to mirror recipient cell locations (as well as number), recipient signals appear to promote the colonization of tissues by melanocytes (Weiner et al., 2007). As such, when pigment cells reach abnormal numbers or colonize abnormal sites, these behaviors may result in part from: 1) the abnormal transmission of pigment-recipient signals to melanocytes, or 2) the hijacking of recipient signaling by melanocytes, as melanocytes generate this signaling themselves and drive their own proliferation or spread.
Pigment recipients in tanning
The pigmentation of the epidermis can be divided into two basic categories — constitutive, which is self-induced and yields the baseline skin color of humans, and facultative, which is induced by UV radiation and is also known as tanning. Tanning increases the amount of melanin in the epithelial (non-melanocytic) component of the epidermis, but questions exist as to how this increase is achieved. For example, during tanning, do pigment recipients simply receive more melanin per cell? Or does UVR also increase the number of recipient cells in the skin, either by stimulating pre-existing recipients to multiply or by inducing new epithelial cells to become pigment recipients? Unfortunately, these questions have been difficult to answer definitively, as it is difficult to pinpoint the keratinocytes receiving pigment directly from melanocytes in intact epidermis (with or without exposure to UVR). Nonetheless, as the number and dendricity of melanocytes increases with UVR exposure (Gilchrest et al., 1996; Hacker et al., 2013), there is reason to think that pigment is transferred to a greater number of epithelial cells during tanning and that some types of epithelial cells (e.g., certain suprabasal keratinocytes) receive pigment only during tanning, making these cells strictly facultative targets for pigmentation. Potentially therefore, UV radiation induces a pigment-recipient phenotype in certain epithelial cells, and the population of self-defined melanocyte targets expands or contracts, together with the melanocyte population, based on the need of the individual for photoprotection.
Anti-pigment-donation signals?
While positive signals appear to be essential for pigment targeting, they may not be sufficient or may be inefficient by themselves. As such, we predict that negative signals are also needed for the precise patterning of pigmentation. For example, the normally unpigmented cells of the hair bulb (Figure 1) may keep themselves unpigmented by emitting signals that repel melanocyte dendrites or that block pigment transfer to themselves. Likewise, the melanocytes outside the cutaneous epithelium, which produce melanin but rarely if ever donate it, may be stopped from melanizing their neighbors by anti-pigment-donation signals, which suppress transfer mechanisms and cause melanocytes to hold their pigment. As the development of most animal traits appears to be guided by positive and negative extracellular signals, precedent suggests that pigment is targeted to the correct cells through at least two forces: the pigment-attracting actions of pigment recipients and the pigment-repelling actions of nonrecipient cells.
Components of the pigment-recipient phenotype
Foxn1-independent pigment recipients
Foxn1 was the first defined activator of the pigment recipient phenotype, but presumably, other activators exist as well. In mice and humans, Foxn1 is normally present in ~50% of the epithelial cells that receive pigment, as in murine and human hair follicles, Foxn1 is abundant in the cortex but absent from the medulla, and in human epidermis, FOXN1 is found throughout the first suprabasal layer but mostly missing from the basal layer (Weiner et al., 2007). Thus, Foxn1 appears to promote the development of roughly half of the skin’s epithelial pigmentation. This raises the question as to what promotes the other half and how pigment is targeted to Foxn1-negative epithelial cells. Likewise, while Fgf2 appears to recruit melanocytes to Foxn1-positive cells, pigment recipients — both Foxn1-positive and Foxn1-negative — presumably emit other signals as well to engineer their own pigmentation. What then are these targeting signals? At present, these questions have not been definitively answered. Nonetheless, a variety of studies have identified good candidates to serve as activating or signaling components of the pigment-recipient phenotype.
p53
One potential activating component of the pigment-recipient phenotype is p53 (Cui et al., 2007; Fell et al., 2014; McGowan et al., 2008). p53 clearly functions as a strong promoter of facultative pigmentation, as p53-knockout mice fail to exhibit a tanning response present in wild-type mice, namely, the tanning of the ear and tail epidermis with repeated exposure to UV radiation (Cui et al., 2007; Fell et al., 2014). This tanning response, though slower than human tanning, results ultimately in the robust melanization of epidermal keratinocytes (Cui et al., 2007), correlates with strong activation of p53 in keratinocytes (Cui et al., 2007), and requires the activation of keratinocyte p53, as the epidermis fails to tan when p53 is ablated from either keratinocytes specifically (Fell et al., 2014) or the germline (Cui et al., 2007). Thus, epithelial cells use p53 to induce their own tanning.
Epithelial p53 is also capable of promoting pigmentation in other circumstances, as mice display a darkening of the footpads, ears, tail, and hair when cutaneous epithelial cells specifically acquire a dominant p53 mutation (McGowan et al., 2008). This conditional p53 mutation — a tandem missense mutation (L25Q, W26S) generated, in the case of the dark-skinned mice, by a keratin-5-promoter-driven Cre recombinase — impairs certain activities of p53, such as its transactivation activity, but also stabilizes p53, causing it to accumulate to abnormally high levels (McGowan et al., 2008). In contrast to this dominant mutation, a germline p53 knockout mutation mitigates when heterozygous, and prevents when homozygous, a darkening of the footpad, ears, tail, and hair observed when cutaneous epithelial cells carry a dominantly-acting, conditional knockout mutation in Rps6 (McGowan et al., 2008), a component of the small ribosomal subunit. In other words, a loss of p53 inhibits the hyperpigmentation caused by a deleterious epithelial mutation, while a gain in epithelial p53 (albeit a mutated p53) induces epithelial hyperpigmentation (McGowan et al., 2008).
Moreover, epithelial p53 appears to possess a pro-pigmentation activity in the context of human basal cell carcinoma (BCC), a cancer of cutaneous epithelial cells in which p53 is often mutated. Specifically, the colonization of BCC tumors by melanocytes tightly correlates with the status of epithelial p53, as tumors with wild-type p53 possess melanocytes, while tumors with mutated p53 lack melanocytes (Cui et al., 2007). Thus, epithelial tumors appear to require the unimpaired activity of their own p53 to recruit pigment donors. Lastly, as suggested by several studies and summarized in subsequent sections, p53 is most likely a direct positive regulator of several genes known to encode pro-pigmentation intercellular signals, including FGF2 (Wei et al., 2006), providing a mechanism through which p53 can facilitate epithelial melanization. Taken together, the preceding studies show that p53 stimulates the pigmentation of its host epithelial cells and thus promotes the conversion of epithelial cells to pigment recipients, especially for the purpose of protection from UV radiation.
Types of pigment-recipient signals
The targeting of pigment is clearly a multi-step process, as melanocytes must: 1) migrate to the vicinity of their targets, 2) reach a population size suitable to provide all targets with pigment, 3) extend dendrites towards their targets, 4) connect their dendrites to specific target cells, and 5) synthesize pigment and transfer it with precision. Given these multiple steps, pigment recipients seem likely to direct the targeting of pigment through at least two types of signals — diffusible and cell-bound (Figure 2). Initially, pigment recipients may emit diffusible signals to attract melanocytes to their location in the skin and to induce the extension of dendrites towards themselves. Recipients may then use cell-bound signals to distinguish themselves from their neighbors, to lock connections with melanocytes into place, and to induce pigment transfer. Logically, either type of signal may promote melanocyte survival, proliferation, melanosome biogenesis, or melanogenesis. Notably, signals of both types are known to exhibit properties consistent with a role in the pigment-recipient phenotype.
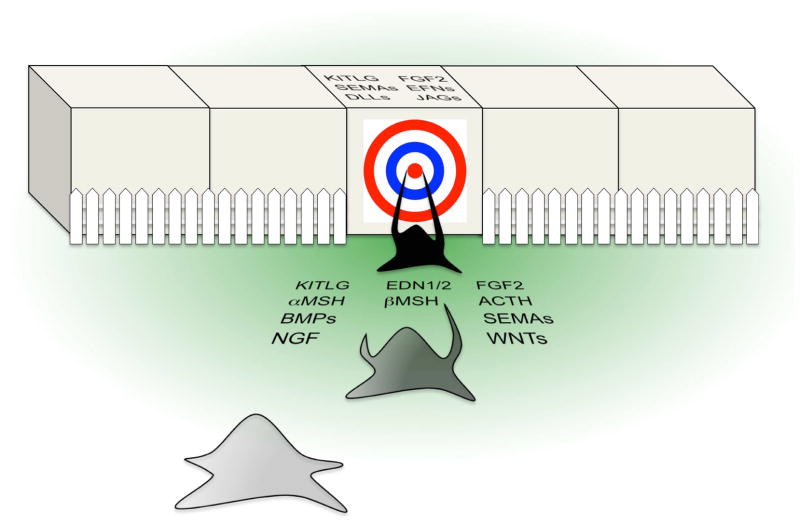
Figure 2.
The pigment-recipient phenotype. The illustration represents the pigment-recipient phenotype conceptually with epithelial cells appearing cubic and a migrating melanocyte appearing star-shaped. Upon acquiring the phenotype, an epithelial cell defines itself as a melanocyte target cell (indicated by a bull’s-eye; for simplicity, a single target cell is shown). The target cell then emits two types of signals: diffusible signals (green gradient; candidates listed), which induce the melanocyte to migrate towards the target and to extend dendrites, and cell-bound signals (candidates listed on the target cell; some candidates have diffusible forms as well), which distinguish the target from its neighbors, allow pigmentary connections to become hard-wired, and accordingly prompt the melanocyte to “hit the bull’seye.” As indicated by a fence, the melanocyte is prevented from connecting to the target cell’s neighbors, as the neighbors fail to emit pigment-recipient signals and may instead produce anti-pigment-donation signals, which block or repel pigmentary connections. With proper connections in place, the recipient’s signals induce melanogenesis and pigment transfer.
Kit ligand
Kitl (Kit ligand; also Scf, Mgf; in humans, KITLG) is an extracellular signaling protein that is essential for the development of melanocytes and is produced in diffusible and cell-bound forms (reviewed in Wehrle-Haller, 2003). In culture, these distinct forms have differing effects on melanocytic cells, as diffusible Kitl can stimulate cell chemotaxis, while cell-bound Kitl can affect cell positioning and promote cell survival and proliferation (Tabone-Eglinger et al., 2012). Consistent with these differing effects, murine embryos that produce diffusible Kitl only (KitlSl-d/Sl-d mutants) exhibit some migration of melanoblasts (melanocyte precursors) away from the neural crest (their point of origin), in particular, more migration than is observed in Kitl null mutants (Steel et al., 1992; Wehrle-Haller and Weston, 1995). But like Kitl null mutants, the KitlSl-d/Sl-d mutants rapidly lose their melanoblasts, in this case soon after the cells start their exodus from the neural crest, suggesting that diffusible Kitl is an inducer of melanoblast chemotaxis and that cell-bound Kitl is essential for melanoblast survival (Steel et al., 1992; Wehrle-Haller and Weston, 1995).
All Kitl isoforms bind to and activate Kit, a transmembrane receptor tyrosine kinase present on most cells of the melanocytic lineage (Botchkareva et al., 2001; Nishikawa et al., 1991; Yoshida et al., 1996; reviewed in Wehrle-Haller, 2003). But their precise effects on Kit can differ, as in culture, the cell-bound Kitl can induce longer Kit activation and slower Kit degradation than the diffusible Kitl (Miyazawa et al., 1995). Additionally, by binding to Kit in culture, cell-bound Kitl appears to promote cell-cell adhesion, an effect that does not depend on Kit kinase activity (Adachi et al., 1992; Kinashi and Springer, 1994), suggesting that these Kitl-Kit complexes are intrinsically adhesive or that they nucleate the assembly of adhesive complexes. As such, melanocytes might form stable attachments to other cells, such as their target cells, via the binding of their Kit protein to a neighbor’s cell-bound Kitl. Potentially therefore, the diffusible and cell-bound Kitls generate different amounts of Kit activity and/or functionally different Kit complexes, suggesting mechanisms by which they could induce different melanocyte responses.
During skin development, the cutaneous epithelium appears to use Kitl to recruit melanoblasts, as in mice, the developing epithelium expresses Kitl during its colonization by melanoblasts (which enter from the developing dermis) (Motro et al., 1991), and melanoblasts fail to colonize this epithelium when Kit signaling is inhibited (Aoki et al., 2011; Aoki et al., 2009; Nishikawa et al., 1991; Yoshida et al., 1996). This failure to colonize the epithelium is observed even when Kit-inhibited melanoblasts reach and occupy the superficial dermis in large numbers (Aoki et al., 2009) or are forcibly placed inside the epithelium by grafting (Aoki et al., 2011), suggesting that melanoblasts are not attracted to the epithelium, cannot survive within the epithelium, and may even be repelled from the epithelium without Kit-Kitl signaling. Similarly, the epidermis appears to use Kitl to retain the melanocyte lineage, as human epidermis exhibits lifelong production of KITLG (Longley et al., 1993) and colonization by melanocytes, while murine epidermis expresses Kitl transiently in early life (Motro et al., 1991; Sharov et al., 2003; Zeron-Medina et al., 2013) and likewise is colonized by the melanocytic lineage briefly as melanoblasts migrate to the developing hair follicles (Hirobe, 1984; Nishikawa et al., 1991; Nishimura et al., 2002). Additionally, the epidermis appears to use KITLG to promote tanning, as in skin and culture, human epidermal keratinocytes upregulate KITLG in response to UV radiation, and in guinea-pig skin, antibodies to Kit inhibit tanning (Hachiya et al., 2001). Like the epidermis, the hair bulb seemingly needs Kitl to acquire melanocytes, as melanocytes fail to colonize the hair bulbs of mice when Kit signaling is inhibited, even in cases where melanocyte progenitors stably occupy other parts of the hair follicle (Botchkareva et al., 2001; Nishikawa et al., 1991; Nishimura et al., 2002).
Moreover, in humans and mice, a gain of KITLG function leads to a gain of epithelial pigmentation. When KITLG is injected into humans, the injected sites exhibit an increase in the pigmentation of epidermal keratinocytes, together with increases in the number, dendricity, and melanogenic activity of epidermal melanocytes (Costa et al., 1996; Grichnik et al., 1995). Mutations in human KITLG can have a similar gain-of-pigmentation effect, as three KITLG missense mutations, all of which are dominant and were proposed to increase the affinity of KITLG for KIT, cause hyperpigmentation of epidermal keratinocytes (Amyere et al., 2011; Wang et al., 2009; Zanardo et al., 2004). When Kitl is produced in mice from transgenes using the keratin-14 (hk14) promoter, which should yield persistent Kitl expression in basal epidermal keratinocytes and other epithelial cells, keratinocytes become highly and persistently melanized, as melanocytes localize to the epidermis and transfer pigment (Kunisada et al., 1998a; Kunisada et al., 1998b). This epidermal melanization is observed whether transgenes are designed to produce diffusible and cell-bound Kitl simultaneously or cell-bound Kitl alone (Kunisada et al., 1998a; Kunisada et al., 1998b). Thus, the cell-bound Kitl appears to have potent effects on pigment deposition and to possess the ability to induce new patterns of epithelial pigmentation, provided the melanocytic lineage is positioned to detect this signal. Notably, at least one hk14-Kitl transgene — the one producing diffusible and cell-bound Kitl — induces the melanization of truly unusual epithelial sites, such as the gums, teeth, and nails (Kunisada et al., 1998b), which normally lack melanocytes. Thus, in mice, the gain of Kitl function resembles the gain of Foxn1 function, as Kitl converts epithelial tissues into pigmented tissues and its host cells (or source cells) into pigment recipients.
As yet, Kitl and Foxn1 have not been shown to be part of the same pathway, but Kitl is a downstream effector of p53, as p53 binds to a site in KITLG (Wei et al., 2006; Zeron-Medina et al., 2013) and upregulates Kitl in keratinocyte cultures (Murase et al., 2009; Zeron-Medina et al., 2013), UVR-treated and untreated skin (Murase et al., 2009; Zeron-Medina et al., 2013), and certain types of hyperpigmented skin (McGowan et al., 2008). Thus, p53 promotes epithelial pigmentation, at least in part, through the induction of Kitl. One key question remaining is whether pigment recipients require Kitl to engineer their own pigmentation. This question could be addressed in mice through the ablation of Kitl from specific epithelial cells, e.g., from hair-shaft cells only, an experiment recently made possible by the construction of a floxed Kitl allele. In sum, given its properties, Kitl is likely to serve as a component of the pigment-recipient phenotype and to target pigment, perhaps in parallel with Foxn1, to certain epithelial cells of the skin.
Endothelins
Endothelins are short, diffusible signaling peptides whose primary forms — Edn1, Edn2, and Edn3 — are derived from three different genes and in mammals are ligands of two basic types of G-protein-coupled transmembrane receptors, namely, Ednra, which binds mature Edn1 and Edn2 preferentially, and Ednrb, which binds all three mature endothelins with high affinity (reviewed in Saldana-Caboverde and Kos, 2010). Ednrb is essential for the development of most melanocytes in mice, is mutated in humans exhibiting melanocyte deficiencies (e.g., Waardenburg syndrome type IV), and appears to be present on melanoblasts, melanocytic progenitors, and differentiating/differentiated melanocytes, rendering the melanocytic lineage potentially responsive to all three mature endothelins (Saldana-Caboverde and Kos, 2010).
Edn2 appears to have abilities resembling those of Kitl and Foxn1, as Edn2, when expressed abnormally in murine skin, potentially induces a new site of epithelial pigmentation, namely, the secondary hair germ (Chang et al., 2013). In normal skin, hair is regenerated cyclically, and these “hair cycles” are divided into 3 phases: 1) anagen, in which the hair follicle develops a new hair bulb and grows a new hair; 2) catagen, in which the follicle completes the hair, destroys its hair bulb, and retracts, and 3) telogen, in which the shortened follicle holds its completed hair and maintains a relatively quiescent state, resting until the next anagen (reviewed in Chase, 1954). The secondary hair germ is unique to telogen and represents the deepest group of follicular epithelial cells, residing below the hair but above the dermal papilla. As a new anagen begins, the secondary hair germ and adjacent bulge region give rise to the new hair bulb and adjacent stalk. In telogen, the germ and/or bulge epithelial cells intermingle with quiescent, amelanotic melanocytic cells, which in anagen give rise to the melanogenic melanocytes of the new hair bulb (Nishimura et al., 2002; Rabbani et al., 2011). Thus, at the base of the telogen follicle, the progenitor/stem cells of the follicular epithelium normally rest side-by-side with the progenitor/stem cells of the melanocytes, awaiting the signal to produce a new, pigmented hair.
When the transcription factor Nfib is ablated from germ and bulge epithelial cells, these cells increase their expression of Edn2 (Chang et al., 2013). Concomitantly, the adjacent amelanotic melanocytic cells generate melanogenic melanocytes, which then transfer pigment to the normally unpigmented secondary hair germ, darkly melanizing it. This melanization of the hair germ appears to be reduced by an inhibitor of Ednrb and appears to be reproduced by the overexpression of Edn2 in the germ and bulge epithelium from a viral vector (Chang et al., 2013). Thus, when the deep epithelium of a telogen hair follicle overexpresses Edn2, the result is the targeting of pigment to the deepest epithelial cells.
While elevated Edn2 expression appears to stimulate the melanization of a novel site, it does not prompt the melanocytic lineage to colonize new sites, such as the epidermis, or to melanize new cells at times other than telogen (Chang et al., 2013). As such, Edn2 overexpression does not yield abnormal pigmentation during anagen, when pigment targeting normally occurs in murine skin, or catagen, when pigment targeting is normally terminated. Likewise, Edn2 overexpression does not cause hair follicles to lose the melanocytic lineage and thus to produce gray hair (Chang et al., 2013), which would occur if Edn2 simply induced melanocytic stem cells to differentiate (Nishimura et al., 2005; Rabbani et al., 2011). Rather, the gain of Edn2 function causes pigment donors to be generated at a specific place and time. The place is the epithelium overlying the dermal papilla, a place reminiscent of the site of pigment donation in the anagen hair bulb (Figure 1), suggesting that the papillary environment is conducive to pigment donation (Chang et al., 2013) and that Edn2 promotes donation in the context of this environment. The time is the part of the hair cycle when melanocytic progenitors normally wait for a signal or “green light” to produce pigment donors. Consistent with this timing, Edn2 is normally upregulated (along with Edn1) in the deep follicular epithelial cells at the time that the green light is given (Rabbani et al., 2011), namely, at the start of anagen, when the melanocytic progenitors generate new pigment donors. In all, Edn2 appears to confer on its source cells the ability to engineer their own pigmentation, provided melanocytic progenitors are permitted or primed to initiate the pigmentation of an epithelium, and provided the Edn2-secreting cells are located in an environment conducive to pigment donation. At present, the literature lacks a full picture as to what role Edn2 normally plays in cutaneous pigmentation, as the sites of cutaneous Edn2 expression have not been fully defined, and the existing Edn2 conditional-knockout mice have not been examined for pigmentation defects. Nonetheless, the preceding results suggest that Edn2 promotes the development of pigment donor/recipient units and that it does so, at least in part, by functioning as an initiator signal, which instructs melanocytic progenitors to “make a pigment donor.” The resulting donors then melanize targets generated by or derived from the Edn2-expressing epithelial population.
Like Edn2, EDN1 has potent pro-pigmentation properties, as in culture EDN1 increases melanocyte proliferation, migration, dendrite number, dendrite length, melanogenesis, and post-UVR survival (Hara et al., 1995; Horikawa et al., 1995; Imokawa et al., 1995; Imokawa et al., 1992; Kadekaro et al., 2005; Yada et al., 1991). In skin and culture, EDN1 is produced and secreted by epidermal keratinocytes (Ahn et al., 1998; Hachiya et al., 2004; Hara et al., 1995; Imokawa et al., 1995; Imokawa et al., 1992; Yohn et al., 1993), and its production in keratinocytes is directly activated in part by p53 (Hyter et al., 2013; Murase et al., 2009). Moreover, increases in EDN1 production/secretion correlate with increases in cutaneous pigmentation, as EDN1 is upregulated under conditions that darken skin, such as tanning/UVR exposure (Ahn et al., 1998; Hachiya et al., 2004; Hara et al., 1995; Imokawa et al., 1995; Imokawa et al., 1992), lentigo senilis (Kadono et al., 2001), and seborrheic keratosis (Teraki et al., 1996). Thus, EDN1 appears likely to promote the pigmentation of epithelial cells, particularly those of the epidermis.
While EDN1 is able to play a positive role in pigmentation, additional studies are needed to define this role precisely. In mice, the germline ablation of Edn1 yields no obvious defects in the pigmentary system but causes death shortly after birth (Reid et al., 1996), leaving it an open question as to whether Edn1 is required by the pigmentary system at a later time. So far, Edn1 conditional knockouts also have no reported defects in epithelial pigmentation, though the ablation of Edn1 in the cutaneous epithelium leads to modest decreases in the proliferation and survival of the epithelium’s melanocytes following the exposure of newborn mice to a single dose of UV radiation (Hyter et al., 2013). Edn3 germline knockouts exhibit a lack of hair pigmentation analogous to that of Ednrb germline knockouts, but this lack of pigmentation results from a lack of melanoblasts, a defect arising soon after these cells emerge from the neural crest and before the epidermis and hair follicles develop and differentiate (reviewed in Saldana-Caboverde and Kos, 2010). At later developmental stages, Edn3, as judged by its gain-of-function effects, appears to promote the development of melanocytes outside the cutaneous epithelium, such as the melanocytes of the dermis, eye, and heart (Aoki et al., 2009; Brito and Kos, 2008; Garcia et al., 2008; Kurita et al., 2005), effects similar to those of Hgf (hepatocyte growth factor) (Kunisada et al., 2000). In the epidermis and hair follicles of mice, the production of Edn3 from a transgene does not induce a gain of pigmented epithelial cells (Aoki et al., 2009; Brito and Kos, 2008; Garcia et al., 2008; Kurita et al., 2005), as the transgenic production of Kitl or Foxn1 does (Kunisada et al., 1998a; Kunisada et al., 1998b; Weiner et al., 2007). Taking all results together, epithelial cells appear likely to use Edn1 and Edn2, but not Edn3, to instruct melanocytic cells and to promote the melanization of themselves or their progeny. The work to date links Edn2 more strongly to pigmentation in the hair follicle and EDN1 more strongly to pigmentation of the epidermis. Potentially, when a mutation inactivates one of these two endothelins, the other compensates in the cutaneous epithelium.
Noggin and bone morphogenetic proteins
NOG (noggin) is a secreted protein, diffusible and probably cell-bound, whose best-characterized function is to block the actions of other secreted proteins, namely, BMPs (bone morphogenetic proteins) (reviewed in Krause et al., 2011), which regulate a wide variety of developmental processes. NOG inhibits BMP signaling by binding to BMPs and preventing them from binding to their receptors. Concomitantly, NOG appears selective as to which BMPs it antagonizes, as in vitro certain BMPs (BMPs 2, 4, 5, 7, 13, and 14) are efficiently inhibited by NOG while others (BMPs 3, 6, 9, 10, and 15) are relatively resistant to NOG (Krause et al., 2011).
When galline Nog is produced in mice from a transgene using the keratin-14 promoter (K14-Noggin), the normally unpigmented nails become pigmented, as melanocytes colonize the nail matrix (the root structure from which the nail develops) and transfer melanin to the matrix epithelial cells (Plikus et al., 2004). The nail matrix is generally analogous to the hair bulb, as it contains a proliferating epithelial cell population, which gives rise to a terminally differentiating epithelial population, which then forms a hard, keratinized cutaneous appendage. A gain of Nog function thus increases the similarity between the nail matrix and hair bulb, as Nog causes the nail matrix to acquire a melanocyte population, which then provides pigment to the precursor cells of the appendage. Additionally, this ability of Nog to target pigment to a new epithelial site bears a resemblance to the gain-of-function effects of Foxn1, Kitl, and most likely Edn2.
Concomitantly, the effects of Nog also differ with those of Foxn1, Kitl, and Edn2, as the K14-Noggin transgene does not alter the pigmentation of the skin or hair, despite its clear activity in the basal layer of the epidermis and hair follicles (Plikus et al., 2004). A similar murine transgene, K5-Noggin, which uses a keratin-5 promoter to produce murine Nog in basal epithelial cells of the skin, likewise does not change the cutaneous sites to which melanocytes localize or target pigment, though this transgene suppresses the synthesis of yellow melanin and thereby darkens the hair coat (Sharov et al., 2005). As the gain of Nog function does not change the distribution of pigment donors or recipients in the skin (their natural environment), Nog does not appear to be in itself a positive signal for the targeting of pigment, as for example Kitl appears to be.
Rather, as a known inhibitor of other proteins, Nog may inhibit the actions of anti-pigmentation factors, which normally prevent the nail matrix from behaving like a hair bulb, i.e., recruiting melanocytes and acquiring pigment. Consistent with this idea, NOG inhibits BMP4, a suppressor of melanocyte melanogenesis in culture and thus a likely anti-pigmentation factor (Park et al., 2009; Singh et al., 2012; Yaar et al., 2006). In contrast to BMP4, BMP6 is resistant to NOG and behaves as a pro-pigmentation factor, as it induces melanogenesis in cultured melanocytes and stimulates pigment transfer in melanocyte/keratinocyte co-cultures (Singh et al., 2012). Moreover, in culture, NOG binds to and inhibits the activity of SOST (sclerostin) (Winkler et al., 2004), a secreted protein that is an inhibitor of BMP6 and hence an anti-pigmentation factor in vitro (Singh et al., 2012). Taking the results together, Nog appears able to induce novel pigmentation via two mechanisms: by inhibiting anti-pigmentation Bmps (exemplified by BMP4) and by inhibiting the inhibitors of pro-pigmentation Bmps (e.g., by inhibiting Sost), thereby activating the pro-pigmentation Bmp class (exemplified by BMP6).
In mice, the germline knockout of Nog results in perinatal death and multiple morphogenetic defects, including a failure to develop most hair follicles (Botchkarev et al., 1999). As no conditional cutaneous knockouts have been reported, the normal role of Nog in hair and skin pigmentation is not yet clear.
On the other hand, Bmp signaling appears essential for cutaneous pigmentation, as the simultaneous ablation in murine melanocytes of two Bmp receptors — Bmpr2 and Acvr2a — causes gray hair (Han et al., 2012). The gray hair coat of Bmpr2/Acvr2a double knockouts does not result from a lack of melanocytes, as melanocytes colonize the hair bulbs in approximately normal numbers and occupy their normal location at the base of the hair shafts. Rather, the lack of pigmentation results from a defect in melanosome biogenesis, as the double-knockout melanocytes produce “miniaturized melanosomes,” abnormally small melanosomes that carry small amounts of melanin (Han et al., 2012). As the overproduction of Nog from the K14-Noggin or K5-Noggin transgenes does not likewise cause hypopigmentation or miniaturized melanosomes, the skin appears to require the signaling of Nog-resistant Bmps to construct melanosomes and generate melanin, an inference consistent with the stimulation of melanogenesis in culture by the NOG-resistant BMP6. In large part, BMP6 may be a promoter of the tanning response, as BMP6 and NOG are upregulated in cultures of human epidermal keratinocytes or melanocytes following exposure to UV radiation (Singh et al., 2012). Consistent with this tanning function, Bmp6 mutations do not yield obvious abnormalities in the baseline pigmentation of mice (Blessing et al., 1996; Solloway et al., 1998), suggesting that other Nog-resistant Bmps promote the development of the pigmentary system and/or hair pigmentation. So far, murine hair follicles were found to express Nog, Bmp2, Bmp3, Bmp4, Bmp6, Bmp7, Bmp8a, and Bmp8b (Bitgood and McMahon, 1995; Botchkarev et al., 1999; Clavel et al., 2012; Lyons et al., 1989; Takahashi and Ikeda, 1996; Zhao and Hogan, 1996) and may express additional Bmp family members. Further study is needed to elucidate the functions of the many Bmps in both constitutive and facultative pigmentation. Nonetheless taken together, the work to date suggests that epithelial pigmentation is promoted by Nog-resistant Bmps and inhibited by at least some Nog-sensitive Bmps.
Humans possess two basic types of hair follicles: terminal hair follicles, which are large and produce pigmented, thick hair (long, sturdy hair fibers, obvious to the eye); and vellus hair follicles, which are small and generate unpigmented, fine hair (short, thin, “fuzz-like” fibers). These follicular types are not fixed during follicle neogenesis, as follicles can switch from one type to the other post-development. For example, during puberty, vellus hair follicles transform into terminal hair follicles at a variety of body locations (e.g., in the axillar and pubic regions). Conversely, over time, terminal hair follicles of the scalp often become vellus or vellus-like, resulting in the condition commonly known as pattern baldness. Currently, the mechanisms responsible for hair-type specification and development are largely unknown. In this context, we note that Nog exhibits properties relevant to human hair-type control, as the production of Nog from murine transgenes leads to: 1) a switch of the nail matrix from melanocyte-negative to melanocyte-positive (Plikus et al., 2004), a switch analogous to that observed in the hair bulb during a vellus-to-terminal transformation, and 2) an increase in the thickness of hair shafts and size of hair follicles (Plikus et al., 2004; Sharov et al., 2006), a change again reminiscent of a vellus-to-terminal switch. Thus, Nog has abilities consistent with an anti-vellus/pro-terminal instructive signal. This idea suggests in turn that Nog-sensitve Bmps provide the opposite, namely, a pro-vellus/anti-terminal signal (Sharov et al., 2006). Clearly, such ideas are speculative at this point. Nonetheless, given the pro-pigmentation and hair-enlarging effects of Nog, there is reason to think that NOG and BMPs specify (at least in part) whether a hair becomes terminal or vellus and hence whether the hair epithelium becomes pigmented.
Wnts and Notch ligands
Wnts are extracellular signaling proteins that are diffusible (mostly short range), potentially cell-bound, and (like Bmps) regulators of a large variety of morphogenetic processes (reviewed in Yam and Charron, 2013). Wnts affect their target cells through an assortment of mechanisms, several of which use frizzled proteins as Wnt transmembrane receptors, and at least one of which, the canonical pathway, activates gene expression via the stabilization and nuclear localization of β-catenin.
Pigment-recipient cells express Wnt genes, as WNT3A and WNT5A are expressed in basal keratinocytes of human epidermis (Jia et al., 2008; Romanowska et al., 2009), and Wnt3 and Wnt4 are expressed in epithelial precursors of the medulla and probably cortex of murine hair follicles (Millar et al., 1999; Reddy et al., 2001). Concomitantly, pigment-donor cells appear to receive and to transduce Wnt signals, as the melanocytes of murine hair bulbs normally exhibit nuclear β-catenin (Rabbani et al., 2011). Moreover, Wnt signaling induces the differentiation and melanogenesis of pigment donors. In murine skin for example, when β-catenin is specifically mutated and thereby activated in melanocytic progenitors (thus mimicking Wnt signaling), these normally undifferentiated cells quickly differentiate and produce melanin (Rabbani et al., 2011). Likewise, when beads soaked with WNT7a are implanted into murine skin, the WNT protein induces the expression of differentiation markers in melanocytic progenitors (Rabbani et al., 2011). Conversely, when β-catenin is specifically ablated from melanocytic progenitors in murine skin, the production of differentiated melanocytes is impaired (Lang et al., 2005; Rabbani et al., 2011), resulting in the generation of gray hair. A gray hair coat (lightly melanized, not white) similarly results from the germline ablation of Fzd4 (frizzled-4) (Wang et al., 2001), suggesting a key role for this receptor in the transduction of Wnt signals in melanocytes. Thus, a gain of Wnt-pathway function promotes the development of melanogenic melanocytes, while a loss of Wnt-pathway function prevents the development of such cells. Though much remains unknown about the roles of individual Wnt family members in pigmentation, the work to date suggests that pigment recipients emit Wnt signals and thereby stimulate the differentiation of melanocytic cells into mature melanocytes, creating the donors needed to partner with the recipients.
The jagged and delta-like ligands are cell-bound (transmembrane) proteins that signal to neighboring cells by binding to the neighbors’ Notch receptors, which ultimately leads to the activation of gene expression via the transcription factor Rbpj (reviewed in Giniger, 2012). At least two Notch receptors — Notch1 and Notch2 — are present on melanocytes or their precursors in murine skin (Moriyama et al., 2006; Schouwey et al., 2007). Correspondingly, at least two NOTCH ligands — JAG1 (jagged-1) and DLL1 (delta-like 1) — are expressed in pigment-recipient cells, as the epithelial precursors of the murine hair cortex express Jag1 (Ambler and Watt, 2007; Estrach et al., 2006) and the basal keratinocytes of human epidermis express JAG1 and DLL1 (Thelu et al., 2002). In mice, Notch signaling promotes melanocyte development at multiple stages (Aubin-Houzelstein et al., 2008; Moriyama et al., 2006; Schouwey et al., 2007) and ultimately stimulates the formation and function of pigment donors. In particular, when Rbpj is ablated specifically from the melanocytic lineage, melanocytes partially colonize the hair bulbs but often produce little melanin and/or localize to the wrong site, namely, the dermal papilla (Aubin-Houzelstein et al., 2008). Thus, in the absence of canonical Notch signaling, bulbar melanocytes act as though they do not “see” their targets, as they migrate to the wrong cells and/or fail to synthesize melanin for the correct cells, which are located next to them. Taken together, these results implicate Notch ligands as signals used by pigment recipients to position melanocytes for pigment donation and to induce the differentiation of melanocytes into pigment donors.
Melanocyte stimulating hormones and adrenocorticotropic hormone
α-MSH (melanocyte stimulating hormone), β-MSH, and ACTH (adrenocorticotropic hormone) are secreted, diffusible peptides that are derived from the same precursor, POMC (pro-opiomelanocortin), and bind to the same G-protein-coupled transmembrane receptor, MC1R (melanocortin-1 receptor) (reviewed in Garcia-Borron et al., 2014). All three of these peptides can induce tanning-like responses in humans, as a gain of α-MSH, β-MSH, or ACTH function, due to peptide administration or disease, causes a gain in epidermal melanin and darkening of the skin (Lerner and McGuire, 1961; Lerner and McGuire, 1964; Levine et al., 1991; Pears et al., 1992). Additionally, in humans and mice, tanning can be impaired or blocked by loss-of-function mutations in MC1R (Beaumont et al., 2008; D’Orazio et al., 2006; Frandberg et al., 1998; Schioth et al., 1999; Valverde et al., 1995; reviewed in Garcia-Borron et al., 2014), which is normally present on melanocytes (Suzuki et al., 1996). Furthermore, in humans, a lack of POMC function affects pigmentation similarly to a lack of MC1R function, as loss-of-function mutations in POMC or MC1R can result in a red-haired, pale-skinned phenotype (Beaumont et al., 2008; D’Orazio et al., 2006; Frandberg et al., 1998; Krude et al., 1998; Krude et al., 2003; Schioth et al., 1999; Valverde et al., 1995). To date, the effects of POMC deficiency on tanning have not been reported, as human POMC mutations are rare and have received limited study. Nonetheless, as red-haired, pale-skinned individuals typically tan poorly, POMC deficiency seems likely to impair tanning as well. In culture, α-MSH, β-MSH, and ACTH promote melanocyte proliferation, dendrite formation, and melanogenesis (Abdel-Malek et al., 1995; De Luca et al., 1993; Hunt et al., 1994; Suzuki et al., 1996), consistent with their effects in skin. Finally, POMC production, while not specific to epidermal keratinocytes, is observed in keratinocytes, where it is stimulated by UV radiation and is directly upregulated by p53, particularly in response to UVR (Chakraborty et al., 1996; Cui et al., 2007; Fell et al., 2014; Wakamatsu et al., 1997). Taken together, the results suggest that epidermal keratinocytes use α-MSH, β-MSH, and/or ACTH to promote their melanization and, in particular, their tanning, making these peptides key for the facultative targeting of pigment.
Axon-guidance factors
Melanocytes bear a morphological resemblance to neurons, as both cell types radiate filamentous extensions, ultimately developing a tree-like appearance. Moreover, these cellular extensions share a functional resemblance, as they connect the melanocytes or neurons to specific targets and then transmit molecules to the targets with pinpoint precision. Given the similarities in the way melanocytes and neurons target molecules to particular cells, melanocytes may find and connect to their targets much as neurons find and connect to theirs, namely, through the influence of axon-guidance factors (Scott et al., 2008; Yang et al., 2006).
Axon-guidance factors are extracellular proteins that steer migrating neural cells or their developing extensions — both axons and dendrites — towards specific targets, ultimately determining the pattern of neural connections (reviewed in Kim and Chiba, 2004). These factors function as attractants or repellants and exert their effects by binding to transmembrane receptors, which then induce changes in the neural cell’s cytoskeleton as well as other molecular responses. Guidance factors are known to be diffusible or cell-bound and to fall into four major categories, namely, the semaphorins with their plexin/neuropilin receptors, Slits with their Robo receptors, netrins with their Unc5/Dcc receptors, and ephrins with their Eph receptors.
Melanocytes appear to possess representatives of all four major categories of axon-guidance receptors, as to date in culture or skin, melanocytes have been found to express PLXNB1 (plexin B1) (Argast et al., 2009; Soong et al., 2012), PLXNC1 (Scott et al., 2008), ROBO1 (Denk et al., 2011), ROBO2 (Denk et al., 2011; Yang et al., 2006), UNC5B (Yang et al., 2006), UNC5C (Kaufmann et al., 2009), UNC5D (Kaufmann et al., 2009), EPHA2 (Yang et al., 2006; Zhang et al., 2008), EPHA4 (Easty et al., 1997), EPHB3 (Easty et al., 1993), and EPHB6 (Hafner et al., 2003). Thus, melanocytes are likely to be responsive to all four major categories of axon-guidance factors. Correspondingly to date, in skin or culture, cutaneous epithelial cells have been found to express semaphorin and ephrin genes, specifically, SEMA3A (semaphorin 3A) (Kou et al., 2012; Kurschat et al., 2006), SEMA4D (Soong et al., 2012), SEMA7A (Scott et al., 2008), EFNA1 (ephrin-A1) (Guo et al., 2006), Efna3 (Yamada et al., 2008), Efna4 (Moss et al., 2005; Tumbar et al., 2004), Efnb1 (ephrin-B1) (Tumbar et al., 2004), Efnb2 (Egawa et al., 2009; Tumbar et al., 2004), and Efnb3 (Genander et al., 2010). Thus, cutaneous epithelial cells appear to emit at least two of the four major categories of axon-guidance signals.
A missense mutation in Sema3c, a diffusible semaphorin, results in the abnormal pigmentation of newborn mice, as compared to normal pups, the homozygous Sema3c mutants (Sema3cL605P/L605P) exhibit significantly less melanin in their skin but more melanin in noncutaneous locations such as the heart (Yu et al., 2004). To date, the cellular basis for this aberrant pigmentation has not been reported, and the perinatal death of the affected mutants has precluded analysis beyond the newborn stage. Nonetheless, the mutant phenotype implicates Sema3c in the guidance of the melanocytic lineage and its pigment to the skin and other locations.
Consistent with a role in the guidance of cellular extensions, PLXNB1 complexes are found on the dendrites of cultured melanocytes, and these dendritic complexes specifically bind SEMA4A (Soong et al., 2012), a semaphorin with diffusible and cell-bound forms. Moreover in culture, SEMA7A, a primarily cell-bound but potentially diffusible guidance signal, has an effect on melanocytes that resembles its effects on neurons, as it promotes the extension of melanocyte dendrites (Scott et al., 2008). Specifically, SEMA7A stimulates melanocytes to extend dendrites through a mechanism requiring β1 integrin and stimulates these dendritic extensions even more strongly when the melanocytes’ PLXNC1 is reduced (Scott et al., 2008). Thus, SEMA7A positively regulates dendrite formation via β1 integrin (and perhaps other receptors), and this positive regulation appears to be opposed by PLXNC1. Potentially, PLXNC1’s opposition represents negative regulation of dendrite formation by SEMA7A, as PLXNC1 is thought to be a SEMA7A receptor. Hence, SEMA7A may promote or prevent dendrite extension, depending on which receptors are present on a melanocyte. Such duality of function is observed for axon-guidance factors in the nervous system, as for example netrin-1 attracts neuronal extensions when it binds to the receptor DCC but repels neuronal extensions when it binds to the receptor UNC5 (or UNC5-DCC heteromeric receptors), making the receptor the key determinant of how the guidance signal is interpreted.
NGF (nerve growth factor) also has the ability to perform a positive guidance function, as this diffusible factor, known to promote axon extension in neurons, is produced by cutaneous epithelial cells (Di Marco et al., 1991; Tron et al., 1990; Yaar et al., 1991) and induces positive chemotaxis and dendrite extension in cultured melanocytes (Yaar et al., 1991). Furthermore, in the nervous system, Kitl, Fgf2, endothelins, Bmps, Wnts, and Notch ligands all appear to function as axon-guidance factors (Gore et al., 2008; Makita et al., 2008; Shirasaki et al., 2006; reviewed in Giniger, 2012; Yam and Charron, 2013), suggesting that melanocytes and neurons are primed to receive similar sets of signals and that their development is guided by similar sets of instructions. Currently, more work is needed to define the roles of axon-guidance systems in pigmentation. Nonetheless, given the work to date, there is reason to think that axon-guidance factors steer migrating melanocytes or their developing dendrites to specific epithelial cells and thereby shape the pattern of pigmentary connections, with pigment recipients using attractive factors to generate pigmentary units, and nonrecipient cells using repulsive factors to prevent such units from forming. In short, axon-guidance factors may ultimately prove to be melanocyte-guidance factors.
Transmembrane receptors
While producing pro-pigmentation signals to guide melanocytes, pigment-recipient cells also presumably produce their own pro-pigmentation receptors, i.e., receptors that facilitate the acquisition of pigment or a pigment-recipient phenotype. Two such receptors are likely to be PAR2 and Egfr. PAR2 (protease-activated receptor-2; also F2RL1) is a G-protein-coupled transmembrane receptor that is present on epidermal keratinocytes but not melanocytes and is activated when serine proteases cleave its extracellular N-terminal domain, which allows its new N-terminus to act as a tethered ligand (Lin et al., 2008; Seiberg et al., 2000 and references therein). The activation of PAR2 appears to promote the acquisition of melanin by keratinocytes, as peptide mimics of PAR2’s tethered ligand increase the melanization of epidermal keratinocytes in intact swine skin, human skin grafts (grafted onto mice) and human keratinocyte/melanocyte co-cultures (Lin et al., 2008; Seiberg et al., 2000). Conversely, serine-protease inhibitors (which inhibit the activation of PAR2 as one of their effects) decrease the melanization of epidermal keratinocytes in swine skin, human skin grafts, and human keratinocyte/melanocyte co-cultures (Lin et al., 2008; Seiberg et al., 2000). To promote keratinocyte melanization, PAR2 is thought to act in part as a facilitator of melanosome transfer or import, as PAR2 activity stimulates phagocytosis in keratinocyte cultures (Lin et al., 2008; Seiberg et al., 2000). At present, the role of PAR2 in the pigmentation of the hair shaft is not clear, as little is known about PAR2 activity in the hair follicle, and murine Par2 knockouts have no reported defects in coat coloration. As such, PAR2 may function as a pro-pigmentation receptor primarily in the epidermis.
Egfr (epidermal growth factor receptor) is a transmembrane receptor tyrosine kinase that has seven ligands (including Egf), is present on (but not limited to) cutaneous epithelial cells, is particularly prominent on basal keratinocytes of the epidermis, and exhibits increased activity in keratinocytes in response to UV radiation (reviewed in Schneider et al., 2008). Consistent with its UVR responsiveness, Egfr affects pigmentation, as a dominant missense mutation in murine Egfr (EgfrDsk5) induces the darkening of footpad epidermis (Fitch et al., 2003) and melanization of nails (Dackor et al., 2009). Most likely, the effects of EgfrDsk5 on pigmentation result from a gain of Egfr function, as footpad pigmentation becomes lighter or normal when the EgfrDsk5 allele is paired heterozygously with an Egfr loss-of-function allele (Fitch et al., 2003). To darken the footpad, EgfrDsk5 causes the normally limited melanocyte population of the footpad epidermis to expand and most likely to increase melanogenic activity (Fitch et al., 2003). The darkening of the footpad is preceded by an increase in epidermal keratinocytes and most likely the increased proliferation of basal keratinocytes. As such, the mutated Egfr arguably expands the number of melanocyte target cells prior to expanding the number of melanocytes. As the EgfrDsk5-induced darkening is limited to two specific epithelial sites, the darkening is likely to be driven by the two epithelial populations, which presumably emit pro-pigmentation signals and hence induce their own melanization. Accordingly, these results implicate Egfr in the creation of targets for pigmentation and the acquisition of a pigment-recipient phenotype.
Conclusions
To conclude, we return to a question with which we began: How do melanocytes know where to place pigment? The answer turns out to be simple: epithelial cells tell melanocytes where to place it. The recipients of pigment define themselves as targets for pigmentation and instruct melanocytes to become their pigment donors (Figure 2). Potentially, the normally unpigmented epithelial cells of the skin, particularly those in the vicinity of melanocytes, do the opposite, namely, define themselves as nonrecipients and repel or prevent pigment donation. While the morphological effects of pigment recipients are straightforward, their mechanisms of action are complex. As discussed in this article, many molecules appear to promote the targeting of pigment to epithelial cells and the differentiation of epithelial cells into pigmented cells. These pro-recipient molecules include transcription factors, extracellular signaling proteins, and transmembrane receptors. On the opposite side, candidates for nonrecipient, anti-pigment-donation factors include: 1) the SEMA7A/PLXNC1 signaling system, which appears to inhibit the extension of dendrites (Scott et al., 2008), and 2) the diffusible signals Edn3 and Hgf, which appear to promote the development of melanocytes that lie outside the cutaneous epithelium and do not donate pigment (Aoki et al., 2009; Brito and Kos, 2008; Garcia et al., 2008; Kunisada et al., 2000; Kurita et al., 2005). Presumably, many more molecular regulators of pigment targeting, donation, and reception await discovery. In sum, cutaneous epithelial cells differentiate into pigmented cells much as they differentiate into epidermal or follicular cells, namely, by deciding to acquire a trait (pigmentation) and activating a genetic program to procure it. By dedicating themselves to the acquisition of pigment, the pigment recipients become distinct from other epithelial cells and represent specialized partners for the melanocytes. By determining where pigment is ultimately placed, the recipients in essence sketch out a design that melanocytes follow as they color in the skin.
Acknowledgments
We are grateful to Miriam H. Feuerman for comments on the manuscript and to the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the U.S. National Institutes of Health for financial support (grants AR045284 and AR055218 to J.L.B.).
References
- Abdel-Malek Z, Swope VB, Suzuki I, Akcali C, Harriger MD, Boyce ST, Urabe K, Hearing VJ. Mitogenic and melanogenic stimulation of normal human melanocytes by melanotropic peptides. Proc Natl Acad Sci USA. 1995;92:1789–1793. doi: 10.1073/pnas.92.5.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi S, Ebi Y, Nishikawa S, Hayashi S, Yamazaki M, Kasugai T, Yamamura T, Nomura S, Kitamura Y. Necessity of extracellular domain of W (c-kit) receptors for attachment of murine cultured mast cells to fibroblasts. Blood. 1992;79:650–656. [PubMed] [Google Scholar]
- Ahn GY, Butt KI, Jindo T, Yaguchi H, Tsuboi R, Ogawa H. The expression of endothelin-1 and its binding sites in mouse skin increased after ultraviolet B irradiation or local injection of tumor necrosis factor alpha. J Dermatol. 1998;25:78–84. doi: 10.1111/j.1346-8138.1998.tb02354.x. [DOI] [PubMed] [Google Scholar]
- Ambler CA, Watt FM. Expression of Notch pathway genes in mammalian epidermis and modulation by beta-catenin. Dev Dyn. 2007;236:1595–1601. doi: 10.1002/dvdy.21151. [DOI] [PubMed] [Google Scholar]
- Amyere M, Vogt T, Hoo J, Brandrup F, Bygum A, Boon L, Vikkula M. KITLG mutations cause familial progressive hyper- and hypopigmentation. J Invest Dermatol. 2011;131:1234–1239. doi: 10.1038/jid.2011.29. [DOI] [PubMed] [Google Scholar]
- Aoki H, Hara A, Motohashi T, Osawa M, Kunisada T. Functionally distinct melanocyte populations revealed by reconstitution of hair follicles in mice. Pigment Cell Melanoma Res. 2011;24:125–135. doi: 10.1111/j.1755-148X.2010.00801.x. [DOI] [PubMed] [Google Scholar]
- Aoki H, Yamada Y, Hara A, Kunisada T. Two distinct types of mouse melanocyte: differential signaling requirement for the maintenance of non-cutaneous and dermal versus epidermal melanocytes. Development. 2009;136:2511–2521. doi: 10.1242/dev.037168. [DOI] [PubMed] [Google Scholar]
- Argast GM, Croy CH, Couts KL, Zhang Z, Litman E, Chan DC, Ahn NG. Plexin B1 is repressed by oncogenic B-Raf signaling and functions as a tumor suppressor in melanoma cells. Oncogene. 2009;28:2697–2709. doi: 10.1038/onc.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin-Houzelstein G, Djian-Zaouche J, Bernex F, Gadin S, Delmas V, Larue L, Panthier JJ. Melanoblasts’ proper location and timed differentiation depend on Notch/RBP-J signaling in postnatal hair follicles. J Invest Dermatol. 2008;128:2686–2695. doi: 10.1038/jid.2008.120. [DOI] [PubMed] [Google Scholar]
- Beaumont KA, Shekar SN, Cook AL, Duffy DL, Sturm RA. Red hair is the null phenotype of MC1R. Hum Mutat. 2008;29:E88–94. doi: 10.1002/humu.20788. [DOI] [PubMed] [Google Scholar]
- Becker D, Lee PL, Rodeck U, Herlyn M. Inhibition of the fibroblast growth factor receptor 1 (FGFR-1) gene in human melanocytes and malignant melanomas leads to inhibition of proliferation and signs indicative of differentiation. Oncogene. 1992;7:2303–2313. [PubMed] [Google Scholar]
- Becker D, Meier CB, Herlyn M. Proliferation of human malignant melanomas is inhibited by antisense oligodeoxynucleotides targeted against basic fibroblast growth factor. Embo J. 1989;8:3685–3691. doi: 10.1002/j.1460-2075.1989.tb08543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol. 1995;172:126–138. doi: 10.1006/dbio.1995.0010. [DOI] [PubMed] [Google Scholar]
- Blessing M, Schirmacher P, Kaiser S. Overexpression of bone morphogenetic protein-6 (BMP-6) in the epidermis of transgenic mice: inhibition or stimulation of proliferation depending on the pattern of transgene expression and formation of psoriatic lesions. J Cell Biol. 1996;135:227–239. doi: 10.1083/jcb.135.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Roth W, Nakamura M, Chen LH, Herzog W, Lindner G, McMahon JA, Peters C, Lauster R, et al. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat Cell Biol. 1999;1:158–164. doi: 10.1038/11078. [DOI] [PubMed] [Google Scholar]
- Botchkareva NV, Khlgatian M, Longley BJ, Botchkarev VA, Gilchrest BA. SCF/c-kit signaling is required for cyclic regeneration of the hair pigmentation unit. Faseb J. 2001;15:645–658. doi: 10.1096/fj.00-0368com. [DOI] [PubMed] [Google Scholar]
- Brito FC, Kos L. Timeline and distribution of melanocyte precursors in the mouse heart. Pigment Cell Melanoma Res. 2008;21:464–470. doi: 10.1111/j.1755-148X.2008.00459.x. [DOI] [PubMed] [Google Scholar]
- Chakraborty AK, Funasaka Y, Slominski A, Ermak G, Hwang J, Pawelek JM, Ichihashi M. Production and release of proopiomelanocortin (POMC) derived peptides by human melanocytes and keratinocytes in culture: regulation by ultraviolet B. Biochim Biophys Acta. 1996;1313:130–138. doi: 10.1016/0167-4889(96)00063-8. [DOI] [PubMed] [Google Scholar]
- Chang CY, Pasolli HA, Giannopoulou EG, Guasch G, Gronostajski RM, Elemento O, Fuchs E. NFIB is a governor of epithelial-melanocyte stem cell behaviour in a shared niche. Nature. 2013;495:98–102. doi: 10.1038/nature11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HB. Growth of the hair. Physiol Rev. 1954;34:113–126. doi: 10.1152/physrev.1954.34.1.113. [DOI] [PubMed] [Google Scholar]
- Clavel C, Grisanti L, Zemla R, Rezza A, Barros R, Sennett R, Mazloom AR, Chung CY, Cai X, Cai CL, et al. Sox2 in the dermal papilla niche controls hair growth by fine-tuning BMP signaling in differentiating hair shaft progenitors. Dev Cell. 2012;23:981–994. doi: 10.1016/j.devcel.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa JJ, Demetri GD, Harrist TJ, Dvorak AM, Hayes DF, Merica EA, Menchaca DM, Gringeri AJ, Schwartz LB, Galli SJ. Recombinant human stem cell factor (kit ligand) promotes human mast cell and melanocyte hyperplasia and functional activation in vivo. J Exp Med. 1996;183:2681–2686. doi: 10.1084/jem.183.6.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE, D’Orazio J, Fung CY, Schanbacher CF, Granter SR, et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128:853–864. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- D’Orazio JA, Nobuhisa T, Cui R, Arya M, Spry M, Wakamatsu K, Igras V, Kunisada T, Granter SR, Nishimura EK, et al. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature. 2006;443:340–344. doi: 10.1038/nature05098. [DOI] [PubMed] [Google Scholar]
- Dackor J, Li M, Threadgill DW. Placental overgrowth and fertility defects in mice with a hypermorphic allele of epidermal growth factor receptor. Mamm Genome. 2009;20:339–349. doi: 10.1007/s00335-009-9189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M, D’Anna F, Bondanza S, Franzi AT, Cancedda R. Human epithelial cells induce human melanocyte growth in vitro but only skin keratinocytes regulate its proper differentiation in the absence of dermis. J Cell Biol. 1988;107:1919–1926. doi: 10.1083/jcb.107.5.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M, Siegrist W, Bondanza S, Mathor M, Cancedda R, Eberle AN. Alpha melanocyte stimulating hormone (alpha MSH) stimulates normal human melanocyte growth by binding to high-affinity receptors. J Cell Sci. 1993;105(Pt 4):1079–1084. doi: 10.1242/jcs.105.4.1079. [DOI] [PubMed] [Google Scholar]
- Denk AE, Braig S, Schubert T, Bosserhoff AK. Slit3 inhibits activator protein 1-mediated migration of malignant melanoma cells. Int J Mol Med. 2011;28:721–726. doi: 10.3892/ijmm.2011.742. [DOI] [PubMed] [Google Scholar]
- Di Marco E, Marchisio PC, Bondanza S, Franzi AT, Cancedda R, De Luca M. Growth-regulated synthesis and secretion of biologically active nerve growth factor by human keratinocytes. J Biol Chem. 1991;266:21718–21722. [PubMed] [Google Scholar]
- Easty DJ, Ganz SE, Farr CJ, Lai C, Herlyn M, Bennett DC. Novel and known protein tyrosine kinases and their abnormal expression in human melanoma. J Invest Dermatol. 1993;101:679–684. doi: 10.1111/1523-1747.ep12371675. [DOI] [PubMed] [Google Scholar]
- Easty DJ, Mitchell PJ, Patel K, Florenes VA, Spritz RA, Bennett DC. Loss of expression of receptor tyrosine kinase family genes PTK7 and SEK in metastatic melanoma. Int J Cancer. 1997;71:1061–1065. doi: 10.1002/(sici)1097-0215(19970611)71:6<1061::aid-ijc24>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Egawa G, Osawa M, Uemura A, Miyachi Y, Nishikawa S. Transient expression of ephrin b2 in perinatal skin is required for maintenance of keratinocyte homeostasis. J Invest Dermatol. 2009;129:2386–2395. doi: 10.1038/jid.2009.105. [DOI] [PubMed] [Google Scholar]
- Estrach S, Ambler CA, Lo Celso C, Hozumi K, Watt FM. Jagged 1 is a beta-catenin target gene required for ectopic hair follicle formation in adult epidermis. Development. 2006;133:4427–4438. doi: 10.1242/dev.02644. [DOI] [PubMed] [Google Scholar]
- Fell GL, Robinson KC, Mao J, Woolf CJ, Fisher DE. Skin beta-endorphin mediates addiction to UV light. Cell. 2014;157:1527–1534. doi: 10.1016/j.cell.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch KR, McGowan KA, van Raamsdonk CD, Fuchs H, Lee D, Puech A, Herault Y, Threadgill DW, Hrabe de Angelis M, Barsh GS. Genetics of dark skin in mice. Genes Dev. 2003;17:214–228. doi: 10.1101/gad.1023703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick TB, Miyamoto M, Ishikawa K. The evolution of concepts of melanin biology. Arch Dermatol. 1967;96:305–323. [PubMed] [Google Scholar]
- Frandberg PA, Doufexis M, Kapas S, Chhajlani V. Human pigmentation phenotype: a point mutation generates nonfunctional MSH receptor. Biochem Biophys Res Commun. 1998;245:490–492. doi: 10.1006/bbrc.1998.8459. [DOI] [PubMed] [Google Scholar]
- Garcia RJ, Ittah A, Mirabal S, Figueroa J, Lopez L, Glick AB, Kos L. Endothelin 3 induces skin pigmentation in a keratin-driven inducible mouse model. J Invest Dermatol. 2008;128:131–142. doi: 10.1038/sj.jid.5700948. [DOI] [PubMed] [Google Scholar]
- Garcia-Borron JC, Abdel-Malek Z, Jimenez-Cervantes C. MC1R, the cAMP pathway, and the response to solar UV: extending the horizon beyond pigmentation. Pigment Cell Melanoma Res. 2014 doi: 10.1111/pcmr.12257. Epub May 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genander M, Holmberg J, Frisen J. Ephrins negatively regulate cell proliferation in the epidermis and hair follicle. Stem Cells. 2010;28:1196–1205. doi: 10.1002/stem.442. [DOI] [PubMed] [Google Scholar]
- Gilchrest BA, Park HY, Eller MS, Yaar M. Mechanisms of ultraviolet light-induced pigmentation. Photochem Photobiol. 1996;63:1–10. doi: 10.1111/j.1751-1097.1996.tb02988.x. [DOI] [PubMed] [Google Scholar]
- Giniger E. Notch signaling and neural connectivity. Curr Opin Genet Dev. 2012;22:339–346. doi: 10.1016/j.gde.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore BB, Wong KG, Tessier-Lavigne M. Stem cell factor functions as an outgrowth-promoting factor to enable axon exit from the midline intermediate target. Neuron. 2008;57:501–510. doi: 10.1016/j.neuron.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Grichnik JM, Crawford J, Jimenez F, Kurtzberg J, Buchanan M, Blackwell S, Clark RE, Hitchcock MG. Human recombinant stem-cell factor induces melanocytic hyperplasia in susceptible patients. J Am Acad Dermatol. 1995;33:577–583. doi: 10.1016/0190-9622(95)91274-6. [DOI] [PubMed] [Google Scholar]
- Guo H, Miao H, Gerber L, Singh J, Denning MF, Gilliam AC, Wang B. Disruption of EphA2 receptor tyrosine kinase leads to increased susceptibility to carcinogenesis in mouse skin. Cancer Res. 2006;66:7050–7058. doi: 10.1158/0008-5472.CAN-06-0004. [DOI] [PubMed] [Google Scholar]
- Hachiya A, Kobayashi A, Ohuchi A, Takema Y, Imokawa G. The paracrine role of stem cell factor/c-kit signaling in the activation of human melanocytes in ultraviolet-B-induced pigmentation. J Invest Dermatol. 2001;116:578–586. doi: 10.1046/j.1523-1747.2001.01290.x. [DOI] [PubMed] [Google Scholar]
- Hachiya A, Kobayashi A, Yoshida Y, Kitahara T, Takema Y, Imokawa G. Biphasic expression of two paracrine melanogenic cytokines, stem cell factor and endothelin-1, in ultraviolet B-induced human melanogenesis. Am J Pathol. 2004;165:2099–2109. doi: 10.1016/S0002-9440(10)63260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker E, Boyce Z, Kimlin MG, Wockner L, Pollak T, Vaartjes SA, Hayward NK, Whiteman DC. The effect of MC1R variants and sunscreen on the response of human melanocytes in vivo to ultraviolet radiation and implications for melanoma. Pigment Cell Melanoma Res. 2013;26:835–844. doi: 10.1111/pcmr.12157. [DOI] [PubMed] [Google Scholar]
- Hafner C, Bataille F, Meyer S, Becker B, Roesch A, Landthaler M, Vogt T. Loss of EphB6 expression in metastatic melanoma. Int J Oncol. 2003;23:1553–1559. [PubMed] [Google Scholar]
- Halaban R, Ghosh S, Baird A. bFGF is the putative natural growth factor for human melanocytes. In Vitro Cell Dev Biol. 1987;23:47–52. doi: 10.1007/BF02623492. [DOI] [PubMed] [Google Scholar]
- Halaban R, Kwon BS, Ghosh S, Delli Bovi P, Baird A. bFGF as an autocrine growth factor for human melanomas. Oncogene Res. 1988a;3:177–186. [PubMed] [Google Scholar]
- Halaban R, Langdon R, Birchall N, Cuono C, Baird A, Scott G, Moellmann G, McGuire J. Basic fibroblast growth factor from human keratinocytes is a natural mitogen for melanocytes. J Cell Biol. 1988b;107:1611–1619. doi: 10.1083/jcb.107.4.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han R, Baden HP, Brissette JL, Weiner L. Redefining the skin’s pigmentary system with a novel tyrosinase assay. Pigment Cell Res. 2002;15:290–297. doi: 10.1034/j.1600-0749.2002.02027.x. [DOI] [PubMed] [Google Scholar]
- Han R, Beppu H, Lee YK, Georgopoulos K, Larue L, Li E, Weiner L, Brissette JL. A pair of transmembrane receptors essential for the retention and pigmentation of hair. Genesis. 2012;50:783–800. doi: 10.1002/dvg.22039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M, Yaar M, Gilchrest BA. Endothelin-1 of keratinocyte origin is a mediator of melanocyte dendricity. J Invest Dermatol. 1995;105:744–748. doi: 10.1111/1523-1747.ep12325522. [DOI] [PubMed] [Google Scholar]
- Hilding DA, Ginzberg RD. Pigmentation of the stria vascularis. The contribution of neural crest melanocytes. Acta Otolaryngol. 1977;84:24–37. doi: 10.3109/00016487709123939. [DOI] [PubMed] [Google Scholar]
- Hirobe T. Histochemical survey of the distribution of the epidermal melanoblasts and melanocytes in the mouse during fetal and postnatal periods. Anat Rec. 1984;208:589–594. doi: 10.1002/ar.1092080414. [DOI] [PubMed] [Google Scholar]
- Holbrook KA. Structure and development of the skin. In: Soter NA, Baden H, editors. Pathophysiology of Dermatologic Diseases. 2. New York: McGraw-Hill, Inc; 1991. [Google Scholar]
- Horikawa T, Norris DA, Yohn JJ, Zekman T, Travers JB, Morelli JG. Melanocyte mitogens induce both melanocyte chemokinesis and chemotaxis. J Invest Dermatol. 1995;104:256–259. doi: 10.1111/1523-1747.ep12612795. [DOI] [PubMed] [Google Scholar]
- Hu DN, Simon JD, Sarna T. Role of ocular melanin in ophthalmic physiology and pathology. Photochem Photobiol. 2008;84:639–644. doi: 10.1111/j.1751-1097.2008.00316.x. [DOI] [PubMed] [Google Scholar]
- Hunt G, Donatien PD, Lunec J, Todd C, Kyne S, Thody AJ. Cultured human melanocytes respond to MSH peptides and ACTH. Pigment Cell Res. 1994;7:217–221. doi: 10.1111/j.1600-0749.1994.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Hyter S, Coleman DJ, Ganguli-Indra G, Merrill GF, Ma S, Yanagisawa M, Indra AK. Endothelin-1 is a transcriptional target of p53 in epidermal keratinocytes and regulates ultraviolet-induced melanocyte homeostasis. Pigment Cell Melanoma Res. 2013;26:247–258. doi: 10.1111/pcmr.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imokawa G, Miyagishi M, Yada Y. Endothelin-1 as a new melanogen: coordinated expression of its gene and the tyrosinase gene in UVB-exposed human epidermis. J Invest Dermatol. 1995;105:32–37. doi: 10.1111/1523-1747.ep12312500. [DOI] [PubMed] [Google Scholar]
- Imokawa G, Yada Y, Miyagishi M. Endothelins secreted from human keratinocytes are intrinsic mitogens for human melanocytes. J Biol Chem. 1992;267:24675–24680. [PubMed] [Google Scholar]
- Jia L, Zhou J, Peng S, Li J, Cao Y, Duan E. Effects of Wnt3a on proliferation and differentiation of human epidermal stem cells. Biochem Biophys Res Commun. 2008;368:483–488. doi: 10.1016/j.bbrc.2008.01.097. [DOI] [PubMed] [Google Scholar]
- Kadekaro AL, Kavanagh R, Kanto H, Terzieva S, Hauser J, Kobayashi N, Schwemberger S, Cornelius J, Babcock G, Shertzer HG, et al. alpha-Melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Res. 2005;65:4292–4299. doi: 10.1158/0008-5472.CAN-04-4535. [DOI] [PubMed] [Google Scholar]
- Kadono S, Manaka I, Kawashima M, Kobayashi T, Imokawa G. The role of the epidermal endothelin cascade in the hyperpigmentation mechanism of lentigo senilis. J Invest Dermatol. 2001;116:571–577. doi: 10.1046/j.1523-1747.2001.01296.x. [DOI] [PubMed] [Google Scholar]
- Kaufmann S, Kuphal S, Schubert T, Bosserhoff AK. Functional implication of Netrin expression in malignant melanoma. Cell Oncol. 2009;31:415–422. doi: 10.3233/CLO-2009-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Chiba A. Dendritic guidance. Trends Neurosci. 2004;27:194–202. doi: 10.1016/j.tins.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Kinashi T, Springer TA. Steel factor and c-kit regulate cell-matrix adhesion. Blood. 1994;83:1033–1038. [PubMed] [Google Scholar]
- Kou K, Nakamura F, Aihara M, Chen H, Seto K, Komori-Yamaguchi J, Kambara T, Nagashima Y, Goshima Y, Ikezawa Z. Decreased expression of semaphorin-3A, a neurite-collapsing factor, is associated with itch in psoriatic skin. Acta Derm Venereol. 2012;92:521–528. doi: 10.2340/00015555-1350. [DOI] [PubMed] [Google Scholar]
- Krause C, Guzman A, Knaus P. Noggin. Int J Biochem Cell Biol. 2011;43:478–481. doi: 10.1016/j.biocel.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19:155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- Krude H, Biebermann H, Schnabel D, Tansek MZ, Theunissen P, Mullis PE, Gruters A. Obesity due to proopiomelanocortin deficiency: three new cases and treatment trials with thyroid hormone and ACTH4-10. J Clin Endocrinol Metab. 2003;88:4633–4640. doi: 10.1210/jc.2003-030502. [DOI] [PubMed] [Google Scholar]
- Kunisada T, Lu SZ, Yoshida H, Nishikawa S, Mizoguchi M, Hayashi S, Tyrrell L, Williams DA, Wang X, Longley BJ. Murine cutaneous mastocytosis and epidermal melanocytosis induced by keratinocyte expression of transgenic stem cell factor. J Exp Med. 1998a;187:1565–1573. doi: 10.1084/jem.187.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisada T, Yamazaki H, Hirobe T, Kamei S, Omoteno M, Tagaya H, Hemmi H, Koshimizu U, Nakamura T, Hayashi SI. Keratinocyte expression of transgenic hepatocyte growth factor affects melanocyte development, leading to dermal melanocytosis. Mech Dev. 2000;94:67–78. doi: 10.1016/s0925-4773(00)00308-7. [DOI] [PubMed] [Google Scholar]
- Kunisada T, Yoshida H, Yamazaki H, Miyamoto A, Hemmi H, Nishimura E, Shultz LD, Nishikawa S, Hayashi S. Transgene expression of steel factor in the basal layer of epidermis promotes survival, proliferation, differentiation and migration of melanocyte precursors. Development. 1998b;125:2915–2923. doi: 10.1242/dev.125.15.2915. [DOI] [PubMed] [Google Scholar]
- Kurita K, Nishito M, Shimogaki H, Takada K, Yamazaki H, Kunisada T. Suppression of progressive loss of coat color in microphthalmia-vitiligo mutant mice. J Invest Dermatol. 2005;125:538–544. doi: 10.1111/j.0022-202X.2005.23861.x. [DOI] [PubMed] [Google Scholar]
- Kurschat P, Bielenberg D, Rossignol-Tallandier M, Stahl A, Klagsbrun M. Neuron restrictive silencer factor NRSF/REST is a transcriptional repressor of neuropilin-1 and diminishes the ability of semaphorin 3A to inhibit keratinocyte migration. J Biol Chem. 2006;281:2721–2729. doi: 10.1074/jbc.M507860200. [DOI] [PubMed] [Google Scholar]
- Lang D, Lu MM, Huang L, Engleka KA, Zhang M, Chu EY, Lipner S, Skoultchi A, Millar SE, Epstein JA. Pax3 functions at a nodal point in melanocyte stem cell differentiation. Nature. 2005;433:884–887. doi: 10.1038/nature03292. [DOI] [PubMed] [Google Scholar]
- Lerner AB, McGuire JS. Effect of alpha- and beta-melanocyte stimulating hormones on the skin colour of man. Nature. 1961;189:176–179. doi: 10.1038/189176a0. [DOI] [PubMed] [Google Scholar]
- Lerner AB, McGuire JS. Melanocyte-Stimulating Hormone and Adrenocorticotrophic Hormone. Their Relation to Pigmentation. N Engl J Med. 1964;270:539–546. doi: 10.1056/NEJM196403122701101. [DOI] [PubMed] [Google Scholar]
- Levine N, Sheftel SN, Eytan T, Dorr RT, Hadley ME, Weinrach JC, Ertl GA, Toth K, McGee DL, Hruby VJ. Induction of skin tanning by subcutaneous administration of a potent synthetic melanotropin. JAMA. 1991;266:2730–2736. [PubMed] [Google Scholar]
- Lin CB, Chen N, Scarpa R, Guan F, Babiarz-Magee L, Liebel F, Li WH, Kizoulis M, Shapiro S, Seiberg M. LIGR, a protease-activated receptor-2-derived peptide, enhances skin pigmentation without inducing inflammatory processes. Pigment Cell Melanoma Res. 2008;21:172–183. doi: 10.1111/j.1755-148X.2008.00441.x. [DOI] [PubMed] [Google Scholar]
- Longley BJ, Jr, Morganroth GS, Tyrrell L, Ding TG, Anderson DM, Williams DE, Halaban R. Altered metabolism of mast-cell growth factor (c-kit ligand) in cutaneous mastocytosis. N Engl J Med. 1993;328:1302–1307. doi: 10.1056/NEJM199305063281803. [DOI] [PubMed] [Google Scholar]
- Lyons KM, Pelton RW, Hogan BL. Patterns of expression of murine Vgr-1 and BMP-2a RNA suggest that transforming growth factor-beta-like genes coordinately regulate aspects of embryonic development. Genes Dev. 1989;3:1657–1668. doi: 10.1101/gad.3.11.1657. [DOI] [PubMed] [Google Scholar]
- Makita T, Sucov HM, Gariepy CE, Yanagisawa M, Ginty DD. Endothelins are vascular-derived axonal guidance cues for developing sympathetic neurons. Nature. 2008;452:759–763. doi: 10.1038/nature06859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandinova A, Kolev V, Neel V, Hu B, Stonely W, Lieb J, Wu X, Colli C, Han R, Pazin MJ, et al. A positive FGFR3/FOXN1 feedback loop underlies benign skin keratosis versus squamous cell carcinoma formation in humans. J Clin Invest. 2009;119:3127–3137. doi: 10.1172/JCI38543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert CL, Silvers WK. The Effects of Genotype and Cell Environment on Melanoblast Differentiation in the House Mouse. Genetics. 1956;41:429–450. doi: 10.1093/genetics/41.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan KA, Li JZ, Park CY, Beaudry V, Tabor HK, Sabnis AJ, Zhang W, Fuchs H, de Angelis MH, Myers RM, et al. Ribosomal mutations cause p53-mediated dark skin and pleiotropic effects. Nat Genet. 2008;40:963–970. doi: 10.1038/ng.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar SE, Willert K, Salinas PC, Roelink H, Nusse R, Sussman DJ, Barsh GS. WNT signaling in the control of hair growth and structure. Dev Biol. 1999;207:133–149. doi: 10.1006/dbio.1998.9140. [DOI] [PubMed] [Google Scholar]
- Miyazawa K, Williams DA, Gotoh A, Nishimaki J, Broxmeyer HE, Toyama K. Membrane-bound Steel factor induces more persistent tyrosine kinase activation and longer life span of c-kit gene-encoded protein than its soluble form. Blood. 1995;85:641–649. [PubMed] [Google Scholar]
- Moriyama M, Osawa M, Mak SS, Ohtsuka T, Yamamoto N, Han H, Delmas V, Kageyama R, Beermann F, Larue L, et al. Notch signaling via Hes1 transcription factor maintains survival of melanoblasts and melanocyte stem cells. J Cell Biol. 2006;173:333–339. doi: 10.1083/jcb.200509084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss A, Alvares D, Meredith-Middleton J, Robinson M, Slater R, Hunt SP, Fitzgerald M. Ephrin-A4 inhibits sensory neurite outgrowth and is regulated by neonatal skin wounding. Eur J Neurosci. 2005;22:2413–2421. doi: 10.1111/j.1460-9568.2005.04452.x. [DOI] [PubMed] [Google Scholar]
- Motro B, van der Kooy D, Rossant J, Reith A, Bernstein A. Contiguous patterns of c-kit and steel expression: analysis of mutations at the W and Sl loci. Development. 1991;113:1207–1221. doi: 10.1242/dev.113.4.1207. [DOI] [PubMed] [Google Scholar]
- Murase D, Hachiya A, Amano Y, Ohuchi A, Kitahara T, Takema Y. The essential role of p53 in hyperpigmentation of the skin via regulation of paracrine melanogenic cytokine receptor signaling. J Biol Chem. 2009;284:4343–4353. doi: 10.1074/jbc.M805570200. [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Kusakabe M, Yoshinaga K, Ogawa M, Hayashi S, Kunisada T, Era T, Sakakura T. In utero manipulation of coat color formation by a monoclonal anti-c-kit antibody: two distinct waves of c-kit-dependency during melanocyte development. Embo J. 1991;10:2111–2118. doi: 10.1002/j.1460-2075.1991.tb07744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 2005;307:720–724. doi: 10.1126/science.1099593. [DOI] [PubMed] [Google Scholar]
- Nishimura EK, Jordan SA, Oshima H, Yoshida H, Osawa M, Moriyama M, Jackson IJ, Barrandon Y, Miyachi Y, Nishikawa S. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854–860. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- Park HY, Wu C, Yaar M, Stachur CM, Kosmadaki M, Gilchrest BA. Role of BMP-4 and Its Signaling Pathways in Cultured Human Melanocytes. Int J Cell Biol. 2009;2009:750482. doi: 10.1155/2009/750482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pears JS, Jung RT, Bartlett W, Browning MC, Kenicer K, Thody AJ. A case of skin hyperpigmentation due to alpha-MSH hypersecretion. Br J Dermatol. 1992;126:286–289. doi: 10.1111/j.1365-2133.1992.tb00660.x. [DOI] [PubMed] [Google Scholar]
- Plikus M, Wang WP, Liu J, Wang X, Jiang TX, Chuong CM. Morpho-regulation of ectodermal organs: integument pathology and phenotypic variations in K14-Noggin engineered mice through modulation of bone morphogenic protein pathway. Am J Pathol. 2004;164:1099–1114. doi: 10.1016/S0002-9440(10)63197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri N, van der Weel MB, de Wit FS, Asghar SS, Das PK, Ramaiah A, Westerhof W. Basic fibroblast growth factor promotes melanin synthesis by melanocytes. Arch Dermatol Res. 1996;288:633–635. doi: 10.1007/BF02505269. [DOI] [PubMed] [Google Scholar]
- Rabbani P, Takeo M, Chou W, Myung P, Bosenberg M, Chin L, Taketo MM, Ito M. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell. 2011;145:941–955. doi: 10.1016/j.cell.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy S, Andl T, Bagasra A, Lu MM, Epstein DJ, Morrisey EE, Millar SE. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech Dev. 2001;107:69–82. doi: 10.1016/s0925-4773(01)00452-x. [DOI] [PubMed] [Google Scholar]
- Reid K, Turnley AM, Maxwell GD, Kurihara Y, Kurihara H, Bartlett PF, Murphy M. Multiple roles for endothelin in melanocyte development: regulation of progenitor number and stimulation of differentiation. Development. 1996;122:3911–3919. doi: 10.1242/dev.122.12.3911. [DOI] [PubMed] [Google Scholar]
- Romanowska M, Evans A, Kellock D, Bray SE, McLean K, Donandt S, Foerster J. Wnt5a exhibits layer-specific expression in adult skin, is upregulated in psoriasis, and synergizes with type 1 interferon. PLoS One. 2009;4:e5354. doi: 10.1371/journal.pone.0005354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldana-Caboverde A, Kos L. Roles of endothelin signaling in melanocyte development and melanoma. Pigment Cell Melanoma Res. 2010;23:160–170. doi: 10.1111/j.1755-148X.2010.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schioth HB, Phillips SR, Rudzish R, Birch-Machin MA, Wikberg JE, Rees JL. Loss of function mutations of the human melanocortin 1 receptor are common and are associated with red hair. Biochem Biophys Res Commun. 1999;260:488–491. doi: 10.1006/bbrc.1999.0935. [DOI] [PubMed] [Google Scholar]
- Schlake T. The nude gene and the skin. Exp Dermatol. 2001;10:293–304. doi: 10.1034/j.1600-0625.2001.100501.x. [DOI] [PubMed] [Google Scholar]
- Schneider MR, Werner S, Paus R, Wolf E. Beyond wavy hairs: the epidermal growth factor receptor and its ligands in skin biology and pathology. Am J Pathol. 2008;173:14–24. doi: 10.2353/ajpath.2008.070942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouwey K, Delmas V, Larue L, Zimber-Strobl U, Strobl LJ, Radtke F, Beermann F. Notch1 and Notch2 receptors influence progressive hair graying in a dose-dependent manner. Dev Dyn. 2007;236:282–289. doi: 10.1002/dvdy.21000. [DOI] [PubMed] [Google Scholar]
- Scott GA, McClelland LA, Fricke AF. Semaphorin 7a promotes spreading and dendricity in human melanocytes through beta1-integrins. J Invest Dermatol. 2008;128:151–161. doi: 10.1038/sj.jid.5700974. [DOI] [PubMed] [Google Scholar]
- Seiberg M, Paine C, Sharlow E, Andrade-Gordon P, Costanzo M, Eisinger M, Shapiro SS. The protease-activated receptor 2 regulates pigmentation via keratinocyte-melanocyte interactions. Exp Cell Res. 2000;254:25–32. doi: 10.1006/excr.1999.4692. [DOI] [PubMed] [Google Scholar]
- Sharov AA, Fessing M, Atoyan R, Sharova TY, Haskell-Luevano C, Weiner L, Funa K, Brissette JL, Gilchrest BA, Botchkarev VA. Bone morphogenetic protein (BMP) signaling controls hair pigmentation by means of cross-talk with the melanocortin receptor-1 pathway. Proc Natl Acad Sci USA. 2005;102:93–98. doi: 10.1073/pnas.0408455102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharov AA, Li GZ, Palkina TN, Sharova TY, Gilchrest BA, Botchkarev VA. Fas and c-kit are involved in the control of hair follicle melanocyte apoptosis and migration in chemotherapy-induced hair loss. J Invest Dermatol. 2003;120:27–35. doi: 10.1046/j.1523-1747.2003.12022.x. [DOI] [PubMed] [Google Scholar]
- Sharov AA, Sharova TY, Mardaryev AN, Tommasi di Vignano A, Atoyan R, Weiner L, Yang S, Brissette JL, Dotto GP, Botchkarev VA. Bone morphogenetic protein signaling regulates the size of hair follicles and modulates the expression of cell cycle-associated genes. Proc Natl Acad Sci USA. 2006;103:18166–18171. doi: 10.1073/pnas.0608899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaki R, Lewcock JW, Lettieri K, Pfaff SL. FGF as a target-derived chemoattractant for developing motor axons genetically programmed by the LIM code. Neuron. 2006;50:841–853. doi: 10.1016/j.neuron.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Singh SK, Abbas WA, Tobin DJ. Bone morphogenetic proteins differentially regulate pigmentation in human skin cells. J Cell Sci. 2012;125:4306–4319. doi: 10.1242/jcs.102038. [DOI] [PubMed] [Google Scholar]
- Solloway MJ, Dudley AT, Bikoff EK, Lyons KM, Hogan BL, Robertson EJ. Mice lacking Bmp6 function. Dev Genet. 1998;22:321–339. doi: 10.1002/(SICI)1520-6408(1998)22:4<321::AID-DVG3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Soong J, Chen Y, Shustef EM, Scott GA. Sema4D, the ligand for Plexin B1, suppresses c-Met activation and migration and promotes melanocyte survival and growth. J Invest Dermatol. 2012;132:1230–1238. doi: 10.1038/jid.2011.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel KP, Davidson DR, Jackson IJ. TRP-2/DT, a new early melanoblast marker, shows that steel growth factor (c-kit ligand) is a survival factor. Development. 1992;115:1111–1119. doi: 10.1242/dev.115.4.1111. [DOI] [PubMed] [Google Scholar]
- Stocker KM, Sherman L, Rees S, Ciment G. Basic FGF and TGF-beta 1 influence commitment to melanogenesis in neural crest-derived cells of avian embryos. Development. 1991;111:635–645. doi: 10.1242/dev.111.2.635. [DOI] [PubMed] [Google Scholar]
- Suzuki I, Cone RD, Im S, Nordlund J, Abdel-Malek ZA. Binding of melanotropic hormones to the melanocortin receptor MC1R on human melanocytes stimulates proliferation and melanogenesis. Endocrinology. 1996;137:1627–1633. doi: 10.1210/endo.137.5.8612494. [DOI] [PubMed] [Google Scholar]
- Tabone-Eglinger S, Wehrle-Haller M, Aebischer N, Jacquier MC, Wehrle-Haller B. Membrane-bound Kit ligand regulates melanocyte adhesion and survival, providing physical interaction with an intraepithelial niche. Faseb J. 2012;26:3738–3753. doi: 10.1096/fj.12-206045. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Ikeda T. Transcripts for two members of the transforming growth factor-beta superfamily BMP-3 and BMP-7 are expressed in developing rat embryos. Dev Dyn. 1996;207:439–449. doi: 10.1002/(SICI)1097-0177(199612)207:4<439::AID-AJA8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Teraki E, Tajima S, Manaka I, Kawashima M, Miyagishi M, Imokawa G. Role of endothelin-1 in hyperpigmentation in seborrhoeic keratosis. Br J Dermatol. 1996;135:918–923. doi: 10.1046/j.1365-2133.1996.d01-1095.x. [DOI] [PubMed] [Google Scholar]
- Thelu J, Rossio P, Favier B. Notch signalling is linked to epidermal cell differentiation level in basal cell carcinoma, psoriasis and wound healing. BMC Dermatol. 2002;2:7. doi: 10.1186/1471-5945-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tron VA, Coughlin MD, Jang DE, Stanisz J, Sauder DN. Expression and modulation of nerve growth factor in murine keratinocytes (PAM 212) J Clin Invest. 1990;85:1085–1089. doi: 10.1172/JCI114539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde P, Healy E, Jackson I, Rees JL, Thody AJ. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat Genet. 1995;11:328–330. doi: 10.1038/ng1195-328. [DOI] [PubMed] [Google Scholar]
- Wakamatsu K, Graham A, Cook D, Thody AJ. Characterisation of ACTH peptides in human skin and their activation of the melanocortin-1 receptor. Pigment Cell Res. 1997;10:288–297. doi: 10.1111/j.1600-0749.1997.tb00688.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Huso D, Cahill H, Ryugo D, Nathans J. Progressive cerebellar, auditory, and esophageal dysfunction caused by targeted disruption of the frizzled-4 gene. J Neurosci. 2001;21:4761–4771. doi: 10.1523/JNEUROSCI.21-13-04761.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZQ, Si L, Tang Q, Lin D, Fu Z, Zhang J, Cui B, Zhu Y, Kong X, Deng M, et al. Gain-of-function mutation of KIT ligand on melanin synthesis causes familial progressive hyperpigmentation. Am J Hum Genet. 2009;84:672–677. doi: 10.1016/j.ajhg.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrle-Haller B. The role of Kit-ligand in melanocyte development and epidermal homeostasis. Pigment Cell Res. 2003;16:287–296. doi: 10.1034/j.1600-0749.2003.00055.x. [DOI] [PubMed] [Google Scholar]
- Wehrle-Haller B, Weston JA. Soluble and cell-bound forms of steel factor activity play distinct roles in melanocyte precursor dispersal and survival on the lateral neural crest migration pathway. Development. 1995;121:731–742. doi: 10.1242/dev.121.3.731. [DOI] [PubMed] [Google Scholar]
- Wei CL, Wu Q, Vega VB, Chiu KP, Ng P, Zhang T, Shahab A, Yong HC, Fu Y, Weng Z, et al. A global map of p53 transcription-factor binding sites in the human genome. Cell. 2006;124:207–219. doi: 10.1016/j.cell.2005.10.043. [DOI] [PubMed] [Google Scholar]
- Weiner L, Han R, Scicchitano BM, Li J, Hasegawa K, Grossi M, Lee D, Brissette JL. Dedicated epithelial recipient cells determine pigmentation patterns. Cell. 2007;130:932–942. doi: 10.1016/j.cell.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Weinstein GD, McCullough JL, Ross P. Cell proliferation in normal epidermis. J Invest Dermatol. 1984;82:623–628. doi: 10.1111/1523-1747.ep12261462. [DOI] [PubMed] [Google Scholar]
- Winkler DG, Yu C, Geoghegan JC, Ojala EW, Skonier JE, Shpektor D, Sutherland MK, Latham JA. Noggin and sclerostin bone morphogenetic protein antagonists form a mutually inhibitory complex. J Biol Chem. 2004;279:36293–36298. doi: 10.1074/jbc.M400521200. [DOI] [PubMed] [Google Scholar]
- Wu X, Hammer JA. Melanosome transfer: it is best to give and receive. Curr Opin Cell Biol. 2014;29C:1–7. doi: 10.1016/j.ceb.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaar M, Grossman K, Eller M, Gilchrest BA. Evidence for nerve growth factor-mediated paracrine effects in human epidermis. J Cell Biol. 1991;115:821–828. doi: 10.1083/jcb.115.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaar M, Wu C, Park HY, Panova I, Schutz G, Gilchrest BA. Bone morphogenetic protein-4, a novel modulator of melanogenesis. J Biol Chem. 2006;281:25307–25314. doi: 10.1074/jbc.M600580200. [DOI] [PubMed] [Google Scholar]
- Yada Y, Higuchi K, Imokawa G. Effects of endothelins on signal transduction and proliferation in human melanocytes. J Biol Chem. 1991;266:18352–18357. [PubMed] [Google Scholar]
- Yam PT, Charron F. Signaling mechanisms of non-conventional axon guidance cues: the Shh, BMP and Wnt morphogens. Curr Opin Neurobiol. 2013;23:965–973. doi: 10.1016/j.conb.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Midorikawa T, Oura H, Yoshino T, Ohdera M, Kubo Y, Arase S. Ephrin-A3 not only increases the density of hair follicles but also accelerates anagen development in neonatal mice. J Dermatol Sci. 2008;52:178–185. doi: 10.1016/j.jdermsci.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Yang G, Zhang G, Pittelkow MR, Ramoni M, Tsao H. Expression profiling of UVB response in melanocytes identifies a set of p53-target genes. J Invest Dermatol. 2006;126:2490–2506. doi: 10.1038/sj.jid.5700470. [DOI] [PubMed] [Google Scholar]
- Yohn JJ, Morelli JG, Walchak SJ, Rundell KB, Norris DA, Zamora MR. Cultured human keratinocytes synthesize and secrete endothelin-1. J Invest Dermatol. 1993;100:23–26. doi: 10.1111/1523-1747.ep12349932. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Kunisada T, Kusakabe M, Nishikawa S, Nishikawa SI. Distinct stages of melanocyte differentiation revealed by anlaysis of nonuniform pigmentation patterns. Development. 1996;122:1207–1214. doi: 10.1242/dev.122.4.1207. [DOI] [PubMed] [Google Scholar]
- Yu Q, Shen Y, Chatterjee B, Siegfried BH, Leatherbury L, Rosenthal J, Lucas JF, Wessels A, Spurney CF, Wu YJ, et al. ENU induced mutations causing congenital cardiovascular anomalies. Development. 2004;131:6211–6223. doi: 10.1242/dev.01543. [DOI] [PubMed] [Google Scholar]
- Zanardo L, Stolz W, Schmitz G, Kaminski W, Vikkula M, Landthaler M, Vogt T. Progressive hyperpigmentation and generalized lentiginosis without associated systemic symptoms: a rare hereditary pigmentation disorder in south-east Germany. Acta Derm Venereol. 2004;84:57–60. doi: 10.1080/00015550310005780. [DOI] [PubMed] [Google Scholar]
- Zeron-Medina J, Wang X, Repapi E, Campbell MR, Su D, Castro-Giner F, Davies B, Peterse EFP, Sacilotto N, Walker GJ, et al. A polymorphic p53 response element in KIT ligand influences cancer risk and has undergone natural selection. Cell. 2013;155:410–422. doi: 10.1016/j.cell.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Njauw CN, Park JM, Naruse C, Asano M, Tsao H. EphA2 is an essential mediator of UV radiation-induced apoptosis. Cancer Res. 2008;68:1691–1696. doi: 10.1158/0008-5472.CAN-07-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao GQ, Hogan BL. Evidence that mouse Bmp8a (Op2) and Bmp8b are duplicated genes that play a role in spermatogenesis and placental development. Mech Dev. 1996;57:159–168. doi: 10.1016/0925-4773(96)00543-6. [DOI] [PubMed] [Google Scholar]